Abstract
Background: Poor glycemic control is associated with chronic life-threatening complications. Several studies have revealed that sleep status is associated with glycemic control. Aim: to examine the association between sleep duration, quality and glycemic control among adults with diabetes. Methods: Data on 2500 participants aged 18–60 years were collected from the Qatar Biobank (QBB). Sleep duration and quality were assessed by a self-completed health and lifestyle questionnaire, and glycemic control was assessed using HbA1c. Logistic regression was used to assess the association between sleep duration, napping, snoring and poor glycemic control. Results: After adjusting for age and gender, sleep duration was not associated with poor glycemic control. Lack of association persisted after controlling for smoking, physical activity, education, BMI, fruit and vegetable intake, insulin and medication use. However, sleeping for long hours at night (≥8 h) had a trend in increasing the risk of poor glycemic control (OR = 1.28; 95% CI: 0.94–1.74). Napping was positively associated with poor glycemic control. After adjusting for age and gender, patients who reported “sometimes, frequently, or always” napping had more than 30% increased risk of poor control as compared to patients who reported “never/rarely” napping. Snoring was not associated with poor glycemic control among the study sample when adjusted for age and gender (p = 0.61). Other factors were found to be associated with a better glycemic control such as female, high educational and high physical activity level. Conclusions: our results suggest that napping may be an independent risk factor for a poor glycemic control in diabetes; further investigations are required.
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by an increase in blood glucose levels resulting from pancreatic defects in insulin production, insulin function, or both. DM has become one of the major public health problems worldwide with more than 350 million estimated to have diabetes and a further eight million people are assessed as prediabetic [1]. The prevalence of diabetes has been increasing rapidly in the Middle East countries for both genders, reaching 14.6% with 81 million individuals in the region being diabetic [2]. The prevalence of diabetes in Qatar among adults has reached 16.5% and the burden is higher among Qataris compared to non-Qataris. The most affected age groups were between 38 and 47 years old (27.9%) followed by 28 to 37 years old (25.5%) [3].
Multiple life-threatening complications are associated with diabetes [4]. Uncontrolled diabetes affects patients’ quality of life [4]. However, it has been shown in previously published studies that regular blood glucose monitoring and sustainable glycemic control can prevent and/or delay diabetes complications [5]. Huge body of research documented the impact of multiple lifestyle factors on glycemic control including diet, physical activity and sleeping pattern [6,7,8]. Sleep hygiene is important for the overall health, which implies adequate duration, appropriate timing, good quality, regularity and lack of sleep disturbances [9,10]. Along with the rising epidemic of DM and obesity, the prevalence of sleep disorder has been increasing dramatically worldwide and is considered now as a public health epidemic [11,12,13]. In fact, insufficient sleep time and poor sleeping patterns were associated with attention, behavior and learning issues, with a higher risk of accidents, injuries, hypertension, obesity, diabetes, and depression [10]. Several studies elucidated the interrelation between sleep conditions and some chronic diseases such as diabetes, kidney disease, metabolic syndrome, hypertension, and inflammatory bowel disease [14,15,16,17,18]. Recent meta-analysis indicated that short sleep duration was significantly associated with increased prevalence of MetS (OR 1.11, 95% CI 1.05–1.18) and incident of MetS (RR 1.28, 95% CI 1.07–1.53) in cross-sectional and longitudinal studies, respectively [17]. Another meta-analysis reported a U-shaped association between sleep duration and high risk of hypertension [19]
The finding of diverse studies shows that sleep disturbances, including insomnia, obstructive sleep apnea (OSA), and restless legs syndrome (RLS), are highly prevalent in people with diabetes and ranges from 38 to 97% among people with type 2 diabetes mellitus (T2DM) [13,20]. Moreover, various studies investigated the association between sleep (duration and quality), and glycemic control in diabetic patients [12,15,16,21,22,23].
Sleep duration is suggested to be related to glycemic control in people with diabetes. The recommended duration for healthy sleep for adults is 7 or more hours [24]. The bidirectional relationship between sleep disorders and metabolic disease has been reported by several studies [14,25,26]. Among sleep disorder, Obstructive sleep apnea (OSA) has received a particular attention due to the fact of its relationship with T2DM. Recent studies have elucidated the bidirectional association between OSA and T2DM [25,27]. Results of the population-based study reported that participants with OSA had an estimated hazard ratio (HR, 95% CI) of 2.06 (1.86, 2.28) for diabetes compared to participants without OSA. The same study revealed that the multivariable HR (95% CI) for OSA was 1.53 (1.32, 1.77) in individuals with diabetes [27] (Huang et al., 2018). Hypoxia was suggested to be one possibly underlying mechanism of the relationship between OSA and T2DM [25].
Shorter sleep duration was associated with poor glycemic control and higher glycated hemoglobin (HbA1c) [12,21,28]. Individuals with diabetes who slept less than 5 h/night had higher HbA1c than those who slept 7–8 h/night [28]. Besides, a very short and long sleep durations were positively associated with T2DM [12]. A study found that participants with a sleep duration of ≥9 h had 79% increased odds of having T2DM and an 88% increased odds of pre-diabetes [29]. Furthermore, controversial results were found between napping and diabetes. Some studies showed that daytime nap was associated with a higher risk of developing diabetes [22,23]. In contrast, recent data showed that napping is associated with a better glycemic control in T2DM [12]. In addition, in terms of sleep quality, sleep disturbances have been related to diabetes control. Poor quality of sleep was independently associated with poor glycemic control [30]. However, another study, including participants with type 1 diabetes mellitus (T1DM), showed that the average of glycemic control was negatively linked to sleep quality [21].
To our best knowledge, no study has been conducted in Qatar, where the climate and lifestyle are different, investigating the relationship between sleep patterns and glycemic control. Hence, the present study aims to investigate the association between sleep duration, sleep quality, and glycemic control (i.e., HbA1c) among adult patients with diabetes in Qatar.
2. Methods
2.1. Study Population
Participants’ data were collected from Qatar Biobank (QBB). QBB is an initiative launched to make vital health research possible by gathering biological samples and information on the health and lifestyle from large numbers of Qatar’s population.
The study population included a case-control of 2500 Qatari adults (men and women) and long-term residents (individuals living in the country for ≥15 years) from 18–60 years of age with a history of type 2 diabetes. Exclusion criteria were pregnant women and patients with terminating illnesses. Participants were categorized into two groups. The control group included participants with good glycemic control (HbA1c < 7%), while the case group included participants with poor glycemic control (HbA1c ≥ 7%). Pregnant women and people with terminating illnesses were excluded. Biochemical, clinical and anthropometric data were obtained from Qatar biobank. It included (HbA1c), fasting plasma glucose tests, blood pressure measurement, and patient’s anthropometrics. In addition to a computer-administered health and lifestyle questionnaire that evolved detailed questions on socio-demographic factors, current and past health, family history of health conditions, current and past smoking habits, occupational information, mobile phone use, physical activity levels, sleeping patterns, reproductive health (women), and cognitive and psychological state. Data of food frequency questionnaire was also provided which included how often participants consume various foods and beverages and any pat modifications to their diet over preceding year. The Qatar Biobank study was approved by the Institutional Review Board from the Hamad Medical Corporation Ethics Committee. The current analysis was approved under the IRB exempted category.
2.2. Study Variables
2.2.1. Dependent Variable (Glycemic Control)
The dependent variable of this study was the glycemic control among patients diagnosed with diabetes. The HbA1c was used to assess the glycemic control among the participants in this study. Blood samples were withdrawal from participants and analyzed by biobank in Doha. Based on the American Diabetes Association, the criteria for diabetes was a level of HbA1c ≥ 6.5% (48 mmol/mol) [31]. In the current study, concentrations of HbA1c ≥ 7% were used as an indicator of poor glycemic control.
2.2.2. Independent Variable (Sleep Duration and Quality)
Sleep duration refers to the total amount of sleep obtained either during the nocturnal sleep episode or across a period of 24-h. Sleep duration was determined by the following question “In a typical week during the last year, approximately how many hours of sleep did you get in 24 h? (include naps)”. The answers were categorized into 4 categories: Less than 5 h, between 5 and less than 7 h, between 7 and less than 8 h, 8 h or more. Naps frequency was also assessed and categorized as never/rarely, sometimes, frequently, or always. Snoring was assessed by asking the participants “During the last year, has your spouse or a relative mentioned your snoring? (1) Yes (2) No”.
2.3. Covariates
Covariant factors include age, gender, educational level, body mass index (BMI), physical activity level, coffee intake, smoking status, and use of medication. The educational level was divided into three categories: Low level of education (up to secondary school), medium education (technical or professional school), and high education (university and above). BMI was calculated as weight/height squared (kg/m2). Participants were categorized into three groups: obese, overweight, and non-obese. Subjects with BMI between 18.5 and 24.9 kg/m2 were considered normal, subjects with BMI between 25 and 29.9 kg/m2 were considered overweight, and subjects with BMI > 30 kg/m2 were considered obese. In terms of lifestyle factors such as physical activity, participants were classified into subgroups (Tertiles) based on their metabolic equivalent (MET) calculation. Qatar biobank provided the participants with self-completed health and lifestyle questionnaires to complete information about their physical activities as well as other factors [32]. After that, Qatar biobank calculated the MET of each participant according to the type and time spent exercising. Only leisure time of physical activity was considered in the calculations. Participant identification of smoking status was provided by Qatar biobank through self-completed health and lifestyle questionnaires that were provided to the participant [32]. In our study, participants were divided into groups as current smokers, history of smoking (ex- smokers) or non-smokers. In terms of dietary intake, coffee consumption was assessed through the self-completed diet questionnaire that was provided by Qatar biobank, participants determined the type of drink (tea/coffee), amount of sugar added, and frequency [32]. Diabetes treatment was assessed by the following question: “Are you being treated for your diabetes?”. Participants can select more than one answer. Answers included no, tablets, insulin, diet, or increased physical activity.
2.4. Statistical Analysis
Sample characteristics were presented as mean (SD) or percentage by sleep duration. Chi-square test or ANOVA were used to test the differences between groups for categorical or continuous variables. Logistic regression was used to assess the association between sleep duration, napping, snore and glycemic control. A set of models were used. The first model was adjusted for age and gender; the second model further adjusted for smoking, physical activity, education, BMI, fruit and vegetable intake; the final model further adjusted for insulin use, other diabetes medication, and hypertension medication. We used ordinal number of sleep related measures to test linear trend in multivariable models. All the analyses were conducted using STATA 16 (StataCorp, College Station, TX, USA). Statistical significance was considered when p < 0.05.
3. Results
3.1. Sample Characteristics
Table 1 shows the study sample characteristics by sleep duration. About half of the sample had poor glycemic control and an average age of 51 years. About 41% of the sample were men with about 40% of the study population had either low or high education. The majority of the study sample (74%) were non- smokers. Intake of fruits and vegetables and BMI did not differ according to sleep duration. About 60.6% of the study sample were obese with 25.4% under insulin use and more than half reported taking diabetes medication other than insulin and hypertension medication (34.5%). Around half of the study population snore. Napping was common among the participants with 19.2% and 19.4% reported napping frequent and always. Only gender, educational level and napping were associated with sleep duration (p < 0.001). The majority of participants who slept < 5 h or > 8 h had a low educational level (47.5% and 42%, respectively) and those who slept > 8 h always napped (36.6%). The majority of participants that slept between 5 and less than 8 h had higher educational level.

Table 1.
Sample characteristics by sleep duration.
3.1.1. Sleep Characteristic and Poor Glycemic Control
Table 2 represents the logistics regression models for the association between sleep characteristic and poor glycemic control among people with diabetes. After adjusting for age and gender, sleep duration was not associated with poor glycemic control. Lack of association persisted after controlling for smoking, physical activity, education, BMI, fruit and vegetable intake, insulin and medication use. Napping seemed to be positively associated with poor glycemic control. After adjusting for age and gender, patients who reported “always” napping had 37% increased risk of poor control as compared to patients who reported “never/rarely” napping (CI: 1.05–1.78). Results were attenuated after adjusting for smoking, physical activity, education, BMI, fruit and vegetable intake, insulin and medication use. Snoring was not associated with poor glycemic control among the study sample.

Table 2.
Association (OR 95% CI) between sleep characteristic and poor glycemic control among people with diabetes.
3.1.2. Sleep and Other Lifestyle Factors in Relation to with Poor Glycemic Control
Figure 1 shows the association between sleep and other lifestyles with poor glycemic control. Sleeping for long hours at night (≥8 h) has a trend in increasing the risk of poor glycemic control. Frequent napping was associated to an increased risk of poor glycemic control compared to those who never/rarely napped. Snoring and smoking were not associated with poor glycemic control. Women were less likely to have poor glycemic control than men. Physical activity was inversely associated with glycemic control. Education was inversely but obesity and insulin users were positively associated with poor glycemic control.
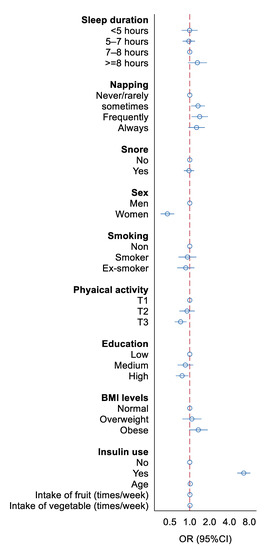
Figure 1.
Association between sleep and other lifestyles with poor glycemic control. All the variables were mutually adjusted in the model.
4. Discussion
The present study demonstrated that diabetic patients napping showed significantly higher HbA1c levels than those who never napped. Additionally, educational level, gender, and napping were associated with sleep duration. These results are consistent with previous findings and indicate that napping has an impact on the glycemic control [22,33,34,35,36].
Several studies had consistently shown that naps could be associated with higher risk of developing diabetes. Napping and long daytime napping were positively associated with diabetes [22,33,34]. The present study found a positive association between napping and poor glycemic control. Similar results were reported by other studies, showing a linear relationship [22,34]. In a prospective Cohort study, Li et al. reported that daytime napping >30 min was associated with an elevated HbA1c level. In addition, no sleep deprivation combined with napping >30 min carries a risk of abnormal glucose metabolism. Whereas, sleep deprivation combined with brief daytime napping <30 min was not associated with an elevated HbA1c level [37]. In contrast, a study showed that naps could relieve the side effects of short nighttime sleep on glycemic control in T2DM [38]. Some studies did not find an association between napping and glycemic control among type 2 diabetes cases [12,38]. Findings revealed several potential inter-related sleep and circadian mechanisms in which napping could affect the glycemic control [35,36]. Both sleep homeostatic and circadian processes have profound influences on multiple physiological functions including the release of pancreatic insulin, insulin sensitivity and sympathetic-parasympathetic balance [39,40]. Increasing sympathetic activity upon awakening from naps, mainly prolonged naps, could results in disruption of the sympathovagal balance, activation of the renin-angiotensin system, which can minimize pancreatic beta-cell insulin secretion, insulin resistance and associated hyperglycemia [36,41,42]. Another proposed mechanism stated that people who nap might be partly less active. This results in reciprocal changes in circulating levels of leptin and ghrelin, which might increase appetite and caloric intake, reduce energy expenditure, and lead to impaired glycemic control [43]. In our study, the correlation demonstrated between napping and poor glycemic control was still significant after adjusting for the confounding factors. Worthwhile mentioning, napping may be a surrogate marker for obstructive sleep apnea which is an established risk factor for poor glycemic control in patients with diabetes. No data about sleep apnea was available; hence, the association between napping, sleep apnea and glycemic control was not possible.
In addition, our results showed that the majority of participants who slept for <5 h or from 5–8 h napped less compared to participants who slept longer at night (>8 h). The majority of participants who slept >8 h always napped (36.6%). Napping is considered desirable to compensate for the negative impact of insufficient nighttime sleep on health, especially in short sleepers (<5 h) [44,45,46]. Our findings need further investigation about the quality of sleep of those that slept longer at night especially that the proportion of participants snoring was not different than the other groups.
The accumulated evidence suggests that sleep disturbances as well as altered sleep duration is associated with higher HbA1c levels [47,48,49]. In the present study, sleep duration did not seem to significantly affect HbA1c levels. However, participants sleeping more than 8 h tend to have a higher HbA1c. Previous studies reported that short and long sleep durations were related to high HbA1c levels in diabetic patients [47,48]. Lee SWH, et al. ([9]) found similar results in T2DM participants, with a U-shaped dose-response relationship. The mechanism is still not well understood but some studies suggest that the correlation is mediated by the impact of sleep duration on appetite-regulating hormones [50]. Short sleep duration is believed to be related to higher levels of ghrelin hormone and decreased leptin hormone level, which could potentially lead to increased appetite, food intake, obesity, and impaired glucose tolerance [47,48]. However, the mechanism of long sleep duration and high glycemia is still unclear and needs further investigation [48].
Furthermore, habitual snoring is highly prevalent, especially among diabetic patients [51,52,53]. Obstructive sleep apnea (OSA) including snoring was independently associated with diabetes and higher HbA1c values [34,54]. In our study, we did not find any relation between snoring and glycemic control. Other studies have found similar results with an association on the borderline of statistical significance [18]. The mean HbA1c among Type 1 diabetes miletus (T1DM) patients was similar between people who snore and those who did not [55]. Nevertheless, studies revealed that the severity of obstructive sleep apnea syndrome (OSAS) was associated with glycemic control. A higher snoring intensity and frequency were positively associated with glycemic control [56]. The association between snoring and T2DM is biologically plausible [57]. Possible mechanisms were discussed. Since snoring inhibits good quality sleep through oxygen desaturation and upper airway obstruction, it could lead to insulin resistance and disruption of glucose metabolism [57,58]. Increased sleep fragmentation and frequent arousals trigger pro-inflammatory cascade by elevating interleukin-6, C-reactive protein, and fibrinogen levels and lowering albumin levels, thereby systematically damaging glucose stability and beta-cell function [58]. Another mechanism proposed in several studies, that snoring activates the sympathetic nervous system, resulting in catecholamine elevation and hypothalamic-pituitary-adrenal axis activation. This combination of elevated cortisol, formation of reactive oxygen species, and increased oxidative stress alter sleep thereby leads to impaired glucose metabolism [56,59,60,61,62]. Moreover, the intermittent hypoxia and marked sleep fragmentation often cause excessive daytime sleepiness [56]. Thus, in the current study, snoring intensity and frequency may have confounded the association between nighttime sleep duration, midday naps, and glycemic control.
A number of factors such as educational level, body mass index (BMI), gender, physical activity, smoking, and insulin use may confound the association between sleep patterns and glycemic control. In terms of education, several studies found a significant impact of the educational level on the glycemic control [63]. Participants with higher educational level had a better awareness of the complications and a high rate of adherence to diet [63]. In the present study, we did not find a significant correlation between the educational level and glycemic control. However, participants with a high educational level tend to have better glycemic control. These results are consistent with other studies [64,65]. Educational level was significantly associated with better attitudes and practices that helps in controlling diabetes [64,65,66,67,68]. A recent study revealed that people with educational qualifications had good knowledge regarding diabetes compared to those who could not read and write [66]. The HbA1c levels were highest among low-educated adults, and death risks associated with uncontrolled HbA1c were significantly greater [67]. Good glycemic control was observed among type 2 diabetic Pakistani patients who scored higher for diabetes knowledge [68]. Conversely, another study found that disease knowledge was not significantly correlated with HbA1c levels [69].
Body mass index (BMI) is one of the important factors for measuring obesity. In our study, obesity had a trend to increase the risk of poor glycemic control. The majority of the study population were obese. A study showed a positive association between obesity and having a suboptimal glycemic control (HbA1c level ≥ 7%) in both T1DM and T2DM [70]. However, a case-control study analyzing the association between BMI and glycemic control showed that having higher BMI (>25 kg/m2) was not associated with greater odds of having a higher HbA1c. They compared patients who had suboptimal glycemic control defined by an HbA1c value >7% with patients who had optimal glycemic control defined by an HbA1c value <7% [71]. Also, our analysis showed that physically active participants tend to have better glycemic control. The latter can be explained by the process of glucose uptake by skeletal muscles during exercise. Glucose is transported from the capillary bed via facilitated diffusion through the GLUT4 transporter as an energy substrate for the working muscle during exercise [72].
The present study revealed that women tend to have less risk of poor glycemic control compared to men. In contrast, a cross-sectional study including 9418 patients with T2D found that women with T2DM had worse glycemic control than men due to the differences in glucose homeostasis, treatment response, and psychological factors [73]. In 2015, a study including 602 adult patients with T2DM shows that gender was not significantly associated with poor glycemic control in either unadjusted or adjusted analyses [74]. Furthermore, there was no association between smoking and poor glycemic control. However, in other studies smoking was positively associated with poor glycemic control due to the presence of neuronal nicotinic acetylcholine receptors (nAChRs) in the β cells of pancreatic islets, which affects the insulin secretion [75,76]. Moreover, in the present study, insulin use was associated to a higher risk of poor glycemic control. Similarly, a cross-sectional study including 1253 patients with T2DM revealed an increased risk for poor glycemic control in patients using insulin. Possible reasons were storage conditions and improper usage of insulin. They found that only 37.6% of the participants used self-monitoring blood glucose devices, which indicates a lack of proper monitoring [77]. However, studies have shown that intensive insulin therapy in type 2 diabetic patients results in excellent glycemic control as it improves insulin sensitivity and regulates blood glucose levels [78].
This study offers several strengths. The sample size is large and multiple variables were taken into consideration such as age, gender, education, smoking, and BMI. Nevertheless, our study has some limitations. First, the present study had qualitative data about coffee consumption and naps but not quantitative. We did not ask for the details of napping such as whether the nap was voluntary or involuntary, the nap environment (e.g., light and noise level), sleepiness after awakening, and sleep satisfaction. Second, the characteristics of the study population including male/female ratio among the study sample may be a source of discrepancy in results interpretation as women were predominant. In addition, more than half of the population were obese and this could also affect the results interpretation. Another limitation of the current study may be the assessment of the sleep quality without dedicated questionnaire (PSQI). Future study may need to verify the results of this study using objective sleep assessment tool such as actigraphy. Finally, there was insufficient information regarding napping time and snoring intensity and frequency, which may affect the association between napping and glycemic control. Despite the limitations of the study, we found a significant association between napping and the risk of poor glycemic control.
5. Conclusions
In conclusion, our results suggest that sleep health is an important modifiable risk factor for improving glycemic control in diabetes. Napping may be an independent risk for poorer glycemic control. Further investigation is definitively necessary to determine whether napping is beneficial or not for improving glycemic control, taking into account the frequency and intensity of snoring. Further research is needed to establish the causal link between sleep and impaired glucose metabolism. These findings may open up new strategies for targeted intervention to improve the duration and quality of sleep.
Author Contributions
Conceptualization, H.B.; methodology H.B.; formal analysis, Z.S.; investigation, A.A.S., N.A.M., and S.A.M.; writing—original draft preparation, A.A.S., N.AM., and S.A.M.; writing—review and editing, A.H., A.K., and H.B.; supervision, H.B. and A.K. and A.H.; funding acquisition, H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Qatar University, grant number QUST-1-CHS-2021-6.
Institutional Review Board Statement
The IRB was obtained from Qatar BioBank (EX-2021-QF-QBB-RES-ACC-00004-0153).
Informed Consent Statement
This project is based on secondary data collected by Qatar BioBank study. When data was collected, informed written consent was obtained from all participants.
Data Availability Statement
Data is owned by Qatar BioBank and is not an open source data.
Acknowledgments
We would like to thank Qatar Biobank for providing the data. We are also grateful to all participants and members of the current study. The publication of this manuscript was funded by Qatar National Library.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jia, G.; Aroor, A.R.; Martinez-Lemus, L.A.; Sowers, J.R. Overnutrition, mTOR signaling, and cardiovascular diseases. Am. J. Physiol. Regul Integr. Comp. Physiol. 2014, 307, R1198–R1206. [Google Scholar] [CrossRef]
- Farmanfarma, K.K.; Ansari-Moghaddam, A.; Zareban, I.; Adineh, H.A. Prevalence of type 2 diabetes in Middle–East: Systematic review& meta-analysis. Sci. Direct 2020, 14, 297–304. [Google Scholar]
- Al Abdulla, S.A.; Hassan, D.M.; Mohammed, A.M.; Bevington, J. SMART Population Screening and Management in Qatar. Int. J. Diabetes Clin. Res. 2019, 6, 99. [Google Scholar]
- Nickerson, H.D.; Dutta, S. Diabetic complications: Current challenges and opportunities. J. Cardiovasc. Transl. Res. 2012, 5, 375–379. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Smith, D.O. Monitoring glycemic control: The cornerstone ofdiabetes care. Clin. Ther. 2005, 27, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.; Matsukawa, M.; Iijima, H. Associations between eating habits and glycemic control and obesity in Japanese workers with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 647–658. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.; Yu, D.; Li, H. Impact of physical activity on glycemic control and insulin resistance: A study of community-dwelling diabetic patients in Eastern China. Intern. Med. 2016, 55, 1055–1060. [Google Scholar] [CrossRef]
- Azharuddin, M.; Kapur, P.; Adil, M.; Ghosh, P.; Sharma, M. The impact of sleep duration and sleep quality on glycaemic control in Asian population with type 2 diabetes mellitus: A systematic literature review and meta-analysis of observational studies. Clin. Epidemiol. Glob. Health 2020, 8, 967–975. [Google Scholar] [CrossRef]
- Lee, S.W.H.; Ng, K.Y.; Chin, W.K. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Med. Rev. 2017, 31, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.A.; Kotagal, S.; Lloyd, R.M.; Malow, B.A.; Maski, K.; Nichols, C.; Quan, S.F.; et al. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2016, 12, 785–786. [Google Scholar] [CrossRef]
- Sleep Disorders and Sleep Deprivation: An. Unmet Public Health Problem; Colten, H.R., Altevogt, B.M., Eds.; National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Gozashti, M.H.M.; Eslami, N.M.; Radfar, M.H.M.; Pakmanesh, H.M. Sleep Pattern, Duration and Quality in Relation with Glycemic Control in People with Type 2 Diabetes Mellitus. Iran. J. Med. Sci. 2016, 41, 531–538. [Google Scholar]
- Jemere, T.; Mossie, A.; Berhanu, H.; Yeshaw, Y. Poor sleep quality and its predictors among type 2 diabetes mellitus patients attending Jimma University Medical Center, Jimma, Ethiopia. BMC Res. Notes 2019, 12, 488. [Google Scholar] [CrossRef]
- Barone, M.T.; Menna-Barreto, L. Diabetes and sleep: A complex cause-and-effect relationship. Diabetes Res. Clin. Pract. 2011, 91, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sochal, M.; Małecka-Panas, E.; Gabryelska, A.; Talar-Wojnarowska, R.; Szmyd, B.; Krzywdzińska, M.; Białasiewicz, P. Determinants of sleep quality in inflammatory bowel diseases. J. Clin. Med. 2020, 9, 2921. [Google Scholar] [CrossRef]
- Hui, L.; Benca, R. The Bidirectional Relationship Between Obstructive Sleep Apnea and Chronic Kidney Disease. J. Stroke Cerebrovasc. Dis. 2021, 105652. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.; Chen, B.; Basta, M.; Vgontzas, A. 1035 Sleep Duration and Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Sleep 2020, 43, A393. [Google Scholar] [CrossRef]
- Wang, T.; Lu, J.; Wang, W.; Mu, Y.; Zhao, J.; Liu, C.; Chen, L.; Shi, L.; Li, Q.; Yang, T.; et al. Sleep duration and snoring associate with hypertension and glycaemic control in patients with diabetes. Diabet. Med. 2015, 32, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mei, H.; Jiang, Y.-R.; Sun, W.-Q.; Song, Y.-J.; Liu, S.-J.; Jiang, F. Relationship between duration of sleep and hypertension in adults: A meta-analysis. J. Clin. Sleep Med. 2015, 11, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Chasens, E.R.; Luyster, F.S.J.D.S. Effect of sleep disturbances on quality of life, diabetes self-care behavior, and patient-reported outcomes. Diabetes Spectr. 2016, 29, 20–23. [Google Scholar] [CrossRef]
- Perez, K.M.; Hamburger, E.R.; Lyttle, M.; Williams, R.; Bergner, E.; Kahanda, S.; Cobry, E.; Jaser, S.S. Sleep in Type 1 Diabetes: Implications for Glycemic Control and Diabetes Management. Curr. Diabetes Rep. 2018, 18, 5. [Google Scholar] [CrossRef]
- Xu, Q.; Song, Y.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Chen, H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care 2010, 33, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, C.Q.; Lam, T.H.; Liu, B.; Jin, Y.L.; Zhu, T.; Zhang, W.S.; Cheng, K.K.; Thomas, G.N. Short or long sleep duration is associated with memory impairment in older Chinese: The Guangzhou Biobank Cohort Study. Sleep 2011, 34, 575–580. [Google Scholar] [CrossRef]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Gabryelska, A.; Karuga, F.F.; Szmyd, B.; Białasiewicz, P. HIF-1α as a mediator of insulin resistance, T2DM, and its complications: Potential links with obstructive sleep apnea. Front. Physiol. 2020, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Nicholl, D.D.; Ahmed, S.B.; Loewen, A.H.; Hemmelgarn, B.R.; Sola, D.Y.; Beecroft, J.M.; Turin, T.C.; Hanly, P.J. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest 2012, 141, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, B.M.; Stampfer, M.J.; Tworoger, S.S.; Hu, F.B.; Redline, S. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective US cohorts. Diabetes Care 2018, 41, 2111–2119. [Google Scholar] [CrossRef]
- Whitaker, K.M.; Lutsey, P.L.; Ogilvie, R.P.; Pankow, J.S.; Bertoni, A.; Michos, E.D.; Punjabi, N.; Redline, S. Associations between polysomnography and actigraphy-based sleep indices and glycemic control among those with and without type 2 diabetes: The Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy172. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M.; Newman, A.B.; Resnick, H.E.; Redline, S.; Baldwin, C.M.; Nieto, F.J. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch. Intern. Med. 2005, 165, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Thakkinstian, A.; Anothaisintawee, T.; Chontong, S.; Borel, A.L.; Perfect, M.M.; Janovsky, C.C.; Kessler, R.; Schultes, B.; Harsch, I.A.; et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: Systematic review and meta-analysis. Sleep Med. 2016, 23, 26–45. [Google Scholar] [CrossRef]
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab. Syndr. Obes. 2017, 10, 345–361. [Google Scholar] [CrossRef]
- Al Kuwari, H.; Al Thani, A.; Al Marri, A.; Al Kaabi, A.; Abderrahim, H.; Afifi, N.; Qafoud, F.; Chan, Q.; Tzoulaki, I.; Downey, P.; et al. The Qatar Biobank: Background and methods. BMC Public Health 2015, 15, 1208. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, Q.; Wei, J.; Meng, X.; Jia, C. Association of daytime napping with prediabetes and diabetes in a Chinese population: Results from the baseline survey of the China Health and Retirement Longitudinal Study. J. Diabetes 2018, 10, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.B.; Jiang, C.Q.; Thomas, G.N.; Arora, T.; Zhang, W.S.; Taheri, S.; Adab, P.; Lam, T.H.; Cheng, K.K. Napping is associated with increased risk of type 2 diabetes: The Guangzhou Biobank Cohort Study. Sleep 2010, 33, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Laposky, A.D.; Bass, J.; Kohsaka, A.; Turek, F.W. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 2008, 582, 142–151. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Hermida, R.C.; Castriotta, R.J.; Portaluppi, F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007, 8, 668–680. [Google Scholar] [CrossRef]
- Li, X.; Pang, X.; Zhang, Q.; Qu, Q.; Hou, Z.; Liu, Z.; Lv, L.; Na, G.; Zhang, W.; Sun, C.; et al. Long-Term Single and Joint Effects of Excessive Daytime Napping on the HOMA-IR Index and Glycosylated Hemoglobin: A Prospective Cohort Study. Medicine 2016, 95, e2734. [Google Scholar] [CrossRef]
- Makino, S.; Hirose, S.; Kakutani, M.; Fujiwara, M.; Nishiyama, M.; Terada, Y.; Ninomiya, H. Association between nighttime sleep duration, midday naps, and glycemic levels in Japanese patients with type 2 diabetes. Sleep Med. 2018, 44, 4–11. [Google Scholar] [CrossRef]
- Spiegel, K.; Knutson, K.; Leproult, R.; Tasali, E.; Van Cauter, E. Sleep loss: A novel risk factor for insulin resistance and Type 2 diabetes. J. Appl. Physiol. 2005, 99, 2008–2019. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Rasch, B.; Dodt, C.; Molle, M.; Born, J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology 2007, 32, 884–891. [Google Scholar] [CrossRef]
- Papandreou, C.; Diaz-Lopez, A.; Babio, N.; Martinez-Gonzalez, M.A.; Bullo, M.; Corella, D.; Fito, M.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Long Daytime Napping Is Associated with Increased Adiposity and Type 2 Diabetes in an Elderly Population with Metabolic Syndrome. J. Clin. Med. 2019, 8, 1053. [Google Scholar] [CrossRef]
- Song, Q.; Liu, X.; Zhou, W.; Wang, X.; Wu, S. Short-term changes in sleep duration and risk of type 2 diabetes: Kailuan prospective study. Medicine 2016, 95, e5363. [Google Scholar] [CrossRef]
- Milner, C.E.; Cote, K.A. Benefits of napping in healthy adults: Impact of nap length, time of day, age, and experience with napping. J. Sleep Res. 2009, 18, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Lovato, N.; Lack, L. The effects of napping on cognitive functioning. Prog. Brain Res. 2010, 185, 155–166. [Google Scholar] [CrossRef]
- Lim, J.; Lo, J.C.; Chee, M.W. Assessing the benefits of napping and short rest breaks on processing speed in sleep-restricted adolescents. J. Sleep Res. 2017, 26, 219–226. [Google Scholar] [CrossRef]
- Sakamoto, R.; Yamakawa, T.; Takahashi, K.; Suzuki, J.; Shinoda, M.M.; Sakamaki, K.; Danno, H.; Tsuchiya, H.; Waseda, M.; Takano, T.; et al. Association of usual sleep quality and glycemic control in type 2 diabetes in Japanese: A cross sectional study. Sleep and Food Registry in Kanagawa (SOREKA). PLoS ONE 2018, 13, e0191771. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A.; Pan, C.; Lu, J.; Dou, J.; Lu, Z.; Ba, J.; Wang, B.; Mu, Y. Impact of night sleep duration on glycemic and triglyceride levels in Chinese with different glycemic status. J. Diabetes 2015, 7, 24–30. [Google Scholar] [CrossRef]
- Denic-Roberts, H.; Costacou, T.; Orchard, T.J. Subjective sleep disturbances and glycemic control in adults with long-standing type 1 diabetes: The Pittsburgh’s Epidemiology of Diabetes Complications study. Diabetes Res. Clin. Pract. 2016, 119, 1–12. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; L’Hermite-Balériaux, M.; Copinschi, G.; Penev, P.D.; Van Cauter, E. Leptin Levels Are Dependent on Sleep Duration: Relationships with Sympathovagal Balance, Carbohydrate Regulation, Cortisol, and Thyrotropin. J. Clin. Endocrinol. Metab. 2004, 89, 5762–5771. [Google Scholar] [CrossRef]
- Katsumata, K.; Okada, T.; Miyao, M.; Katsumata, Y. High incidence of sleep apnea syndrome in a male diabetic population. Diabetes Res. Clin. Pract. 1991, 13, 45–51. [Google Scholar] [CrossRef]
- Norton, P.G.; Dunn, E.V. Snoring as a risk factor for disease: An epidemiological survey. Br. Med. J. 1985, 291, 630–632. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Araujo, A.B.; McKinlay, J.B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006, 29, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Benedict, C. Sleep characteristics and HbA1c in patients with type 2 diabetes on glucose-lowering medication. BMJ Open Diabetes Res. Care 2020, 8, e001702. [Google Scholar] [CrossRef] [PubMed]
- Borel, A.L.; Pepin, J.L.; Nasse, L.; Baguet, J.P.; Netter, S.; Benhamou, P.Y. Short sleep duration measured by wrist actimetry is associated with deteriorated glycemic control in type 1 diabetes. Diabetes Care 2013, 36, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.J.; Lee, H.; Shim, J.S.; Kim, H.C. Association of Snoring with Prediabetes and Type 2 Diabetes Mellitus: The Cardiovascular and Metabolic Diseases Etiology Research Center Cohort. Diabetes Metab. J. 2020, 45, 687. [Google Scholar] [CrossRef]
- Zhang, S.X.; Khalyfa, A.; Wang, Y.; Carreras, A.; Hakim, F.; Neel, B.A.; Brady, M.J.; Qiao, Z.; Hirotsu, C.; Gozal, D. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int. J. Obes. 2014, 38, 619–624. [Google Scholar] [CrossRef]
- Alam, I.; Lewis, K.; Stephens, J.W.; Baxter, J.N. Obesity, metabolic syndrome and sleep apnoea: All pro-inflammatory states. Obes. Rev. 2007, 8, 119–127. [Google Scholar] [CrossRef]
- Follenius, M.; Brandenberger, G.; Bandesapt, J.J.; Libert, J.P.; Ehrhart, J. Nocturnal cortisol release in relation to sleep structure. Sleep 1992, 15, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bloch-Damti, A.; Bashan, N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1553–1567. [Google Scholar] [CrossRef]
- Alberti, A.; Sarchielli, P.; Gallinella, E.; Floridi, A.; Floridi, A.; Mazzotta, G.; Gallai, V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: A preliminary study. J. Sleep Res. 2003, 12, 305–311. [Google Scholar] [CrossRef]
- Nonogaki, K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 2000, 43, 533–549. [Google Scholar] [CrossRef]
- Al-Rasheedi, A.A. The Role of Educational Level in Glycemic Control among Patients with Type II Diabetes Mellitus. Int. J. Health Sci. 2014, 8, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Khan, K.; Kidney, C.; Knowles, V.; Koo, A.; Lakhan, A.; Lalla, D.; Lalloo, C.; Lallo, S.A.; Singh, S. Demographic and lifestyle factors that affect HbA1c awareness amongst type II diabetic patients in Trinidad. Arch. Physiol. Biochem. 2018, 124, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Asmelash, D.; Abdu, N.; Tefera, S.; Baynes, H.W.; Derbew, C. Knowledge, Attitude, and Practice towards Glycemic Control and Its Associated Factors among Diabetes Mellitus Patients. J. Diabetes Res. 2019, 2019, 2593684. [Google Scholar] [CrossRef]
- Alemayehu, A.M.; Dagne, H.; Dagnew, B. Knowledge and associated factors towards diabetes mellitus among adult non-diabetic community members of Gondar city, Ethiopia 2019. PLoS ONE 2020, 15, e0230880. [Google Scholar] [CrossRef] [PubMed]
- Dupre, M.E.; Silberberg, M.; Willis, J.M.; Feinglos, M.N. Education, glucose control, and mortality risks among U.S. older adults with diabetes. Diabetes Res. Clin. Pract. 2015, 107, 392–399. [Google Scholar] [CrossRef]
- Bukhsh, A.; Khan, T.M.; Sarfraz Nawaz, M.; Sajjad Ahmed, H.; Chan, K.G.; Goh, B.H. Association of diabetes knowledge with glycemic control and self-care practices among Pakistani people with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2019, 12, 1409–1417. [Google Scholar] [CrossRef]
- Parimalakrishnan, S.; Dussa, K.; Sahay, R. Assessment of diabetes knowledge using diabetes knowledge questionnaire among people with type 2 diabetes mellitus. Asian J. Pharm. Clin. Res. 2015, 8, 254–256. [Google Scholar]
- Bae, J.P.; Lage, M.J.; Mo, D.; Nelson, D.R.; Hoogwerf, B.J. Obesity and glycemic control in patients with diabetes mellitus: Analysis of physician electronic health records in the US from 2009–2011. J. Diabetes Complicat. 2016, 30, 212–220. [Google Scholar] [CrossRef]
- Mut-Vitcu, G.; Hudrea, I.C.; Mosteoru, S.; Gaita, L.; Gaita, D. Body Mass Index and Glycaemic Control in Patients with Diabetes Mellitus: A Case-Control Study. Rom. J. Diabetes Nutr. Metab. Dis. 2017, 24, 119–125. [Google Scholar] [CrossRef][Green Version]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.G.; da Silva Moreira, S.; Almeida, M.; de Souza Teles, C.A.; Andrade, C.S.; Reingold, A.L.; Moreira, E.D., Jr. Sex differences and correlates of poor glycaemic control in type 2 diabetes: A cross-sectional study in Brazil and Venezuela. BMJ Open 2019, 9, e023401. [Google Scholar] [CrossRef]
- Reynolds, D.B.; Walker, R.J.; Campbell, J.A.; Egede, L.E. Differential effect of race, education, gender, and language discrimination on glycemic control in adults with type 2 diabetes. Diabetes Technol. 2015, 17, 243–247. [Google Scholar] [CrossRef]
- Śliwińska-Mossoń, M.; Milnerowicz, H. The impact of smoking on the development of diabetes and its complications. Diabetes Vasc. Dis. Res. 2017, 14, 265–276. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Hellström-Lindahl, E.; Grill, V. Evidence for functional nicotinic receptors on pancreatic beta cells. Metab. Clin. Exp. 2005, 54, 247–254. [Google Scholar] [CrossRef]
- Afroz, A.; Ali, L.; Karim, M.N.; Alramadan, M.J.; Alam, K.; Magliano, D.J.; Billah, B. Glycaemic Control for People with Type 2 Diabetes Mellitus in Bangladesh—An urgent need for optimization of management plan. Sci. Rep. 2019, 9, 10248. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999, 131, 281–303. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).