New Biomarkers of Hymenoptera Venom Allergy in a Group of Inflammation Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Groups and Serum Samples
2.2. Measurement of Inflammation Panel
2.3. Data Analysis
3. Results
3.1. Alterations in Serum Concentration of Inflammatory Factors in Hymenoptera Venom Allergy
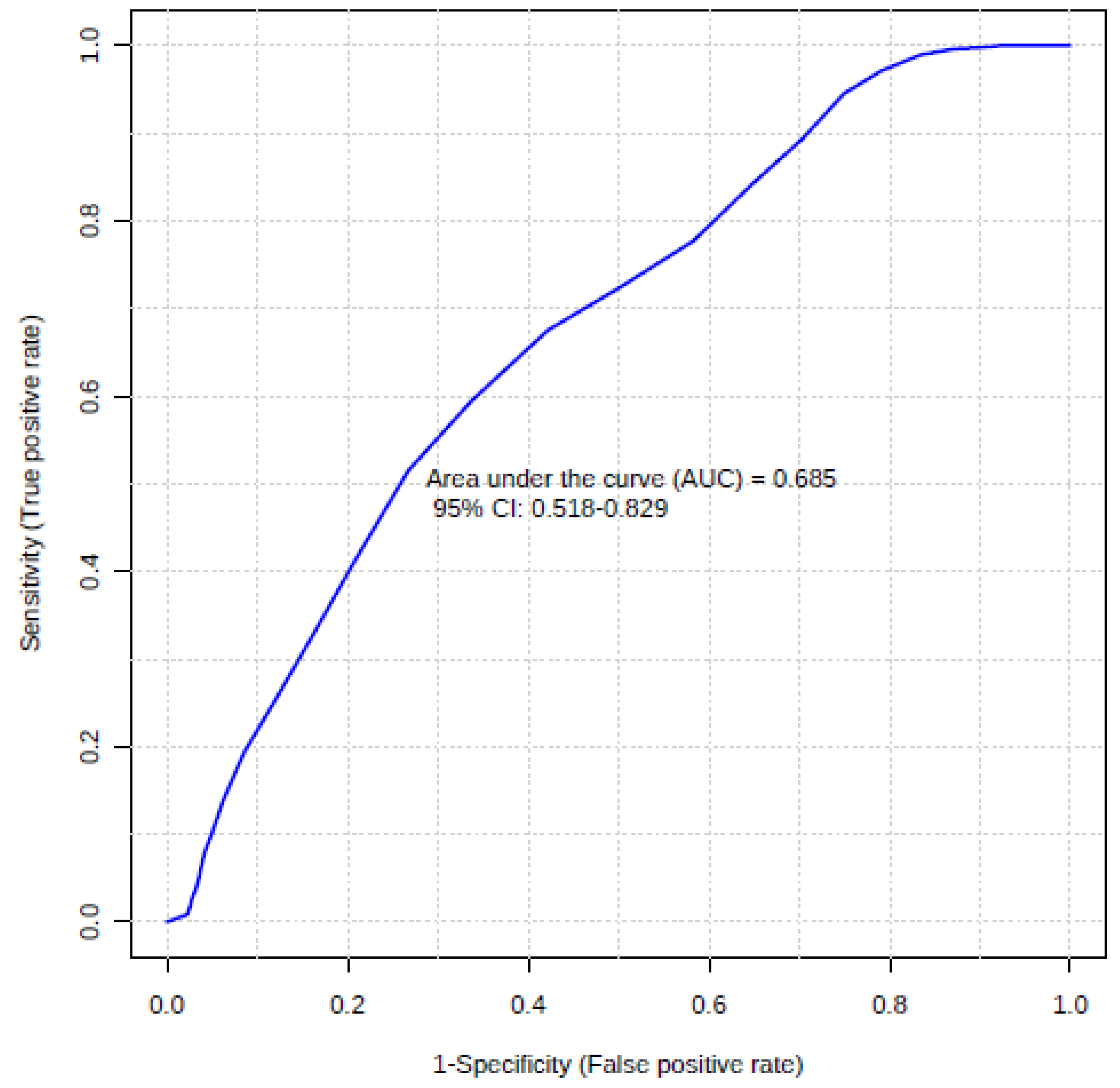
3.2. Usefulness of Inflammation Factors in Diagnosis of Hymenoptera Venom Allergy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Moerbeke, D.E. European allergy white paper: Allergic diseases as a public health problem in Europe. In Regional Environmental Change; UCB Institute of Allergy: Brussels, Belgium, 1997. [Google Scholar]
- I Asher, M.; Weiland, S.K. The International Study of Asthma and Allergies in Childhood (ISAAC). Clin. Exp. Allergy 1998, 28, 52–66. [Google Scholar] [CrossRef]
- Matuszewska, E.; Matysiak, J.; Bręborowicz, A.; Olejniczak, K.; Kycler, Z.; Kokot, Z.J.; Matysiak, J. Proteomic features characterization of Hymenoptera venom allergy. Allergy Asthma Clin. Immunol. 2019, 15, 1–8. [Google Scholar] [CrossRef]
- Matysiak, J.; Matysiak, J.; Kokot, Z.J.; Breborowicz, A. Alergia na jad owadów błonkoskrzydłych ze szczególnym uwzglednieniem pszczoły miodnej (Apis mellifera)—Aktualny stan wiedzy. Alerg. Astma Immunol. 2011, 16, 163–171. [Google Scholar]
- Pesek, R.D.; Lockey, R.F. Treatment of Hymenoptera venom allergy: An update. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Ollert, M.; Blank, S. Anaphylaxis to Insect Venom Allergens: Role of Molecular Diagnostics. Curr. Allergy Asthma Rep. 2015, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Novembre, E.; Cianferoni, A.; Bernardini, R.; Veltroni, M.; Ingargiola, A.; Lombardi, E.; Vierucci, A. Vierucci Epidemiology of insect venom sensitivity in children and its correlation to clinical and atopic features. Clin. Exp. Allergy 1998, 28, 834–838. [Google Scholar] [CrossRef]
- Grigoreas, C.; Galatas, I.D.; Kiamouris, C.; Papaioannou, D. Insect-venom allergy in Greek adults. Allergy 1997, 52, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, C.; Mauro, M.; Pastorello, E.A. Hymenoptera stings in conscripts. Allergy 1997, 52, 680–681. [Google Scholar] [CrossRef]
- Charpin, D.; Birnbaum, J.; Lanteaume, A.; Vervloet, D. Prevalence of allergy to hymenoptera stings in different samples of the general population. J. Allergy Clin. Immunol. 1992, 90, 331–334. [Google Scholar] [CrossRef]
- Fernandez, J.; Blanca, M.; Soriano, V.; Sanchez, J.; Juarez, C. Epidemiological study of the prevalence of allergic reactions to Hymenoptera in a rural population in the Mediterranean area. Clin. Exp. Allergy 1999, 29, 1069–1074. [Google Scholar] [CrossRef]
- Müller, U.R. Bee venom allergy in beekeepers and their family members. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 343–347. [Google Scholar] [CrossRef]
- Münstedt, K.; Hellner, M.; Winter, D.; Von Georgi, R. Allergy to bee venom in beekeepers in Germany. J. Investig. Allergol. Clin. Immunol. 2008, 18, 100–105. [Google Scholar]
- Annila, I.T.; Karjalainen, E.S.; A Annila, P.; A Kuusisto, P. Bee and Wasp Sting Reactions in Current Beekeepers. Ann. Allergy Asthma Immunol. 1996, 77, 423–427. [Google Scholar] [CrossRef]
- De la Torre-Morin, F.; García-Robaina, J.C.; Vázquez-Moncholí, C.; Fierro, J.; Bonnet-Moreno, C. Epidemiology of allergic reactions in beekeepers: A lower prevalence in subjects with more than 5 years exposure. Allergol. Immunopathol. 1995, 23, 127–132. [Google Scholar]
- Fujita, H.; Soyka, M.B.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy. Clin. Transl. Allergy 2012, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, C.M.; Urra, J.M.; Alfaya, T.; De La Roca, F.; Feo-Brito, F. Expression of Th1, Th2, lymphocyte trafficking and activation markers on CD4+ T-cells of Hymenoptera allergic subjects and after venom immunotherapy. Mol. Immunol. 2014, 62, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, A.; Justo-Jacomini, D.L.; Zollner, R.D.L.; Brochetto-Braga, M.R. Facing Hymenoptera Venom Allergy: From Natural to Recombinant Allergens. Toxins 2015, 7, 2551–2570. [Google Scholar] [CrossRef]
- Sturm, G.J.; Varga, E.-M.; Roberts, G.; Mosbech, H.; Bilò, M.B.; Akdis, C.A.; Antolín-Amérigo, D.; Cichocka-Jarosz, E.; Gawlik, R.; Jakob, T.; et al. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy 2018, 73, 744–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.G. Clinical features and severity grading of anaphylaxis. J. Allergy Clin. Immunol. 2004, 114, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Antolin-Amerigo, D.; Ruiz-Leon, B.; Boni, E.; Alfaya-Arias, T.; Alvarez-Mon, M.; Barbarroja-Escudero, J.; González-de-Olano, D.; Moreno-Aguilar, C.; Rodríguez-Rodríguez, M.; Sánchez-González, M.J.; et al. Component-resolved diagnosis in hymenoptera allergy. Allergol. Immunopathol. 2018, 46, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Schiener, M.; Graessel, A.; Ollert, M.; Schmidt-Weber, C.B.; Blank, S. Allergen-specific immunotherapy of Hymenoptera venom allergy—Also a matter of diagnosis. Hum. Vaccines Immunother. 2017, 13, 2467–2481. [Google Scholar] [CrossRef] [Green Version]
- E Reisman, R. Unusual reactions to insect stings. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 355–358. [Google Scholar] [CrossRef]
- Jakob, T.; Rafei-Shamsabadi, D.; Spillner, E.; Müller, S. Diagnostik der Hymenopteren-giftallergie: Aktuelle Konzepte und Entwicklungen mit besonderem Fokus auf die molekulare Allergiediagnostik. Allergy J. 2017, 26, 33–48. [Google Scholar] [CrossRef]
- Niedoszytko, M.; De Monchy, J.; Van Doormaal, J.J.; Jassem, E.; Elberink, J.N.G.O. Mastocytosis and insect venom allergy: Diagnosis, safety and efficacy of venom immunotherapy. Allergy 2009, 64, 1237–1245. [Google Scholar] [CrossRef]
- Ferreira, V.L.; Borba, H.H.; Bonetti, A.D.F.; Leonart, L.P.; Pontarolo, R. Cytokines and Interferons: Types and Functions. In Autoantibodies and Cytokines; IntechOpen: London, UK, 2019; ISBN 978-1-83962-130-7. [Google Scholar]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A. Historical Review of Cytokines. Eur. J. Immunol. 2007, 37, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horala, A.; Swiatly, A.; Matysiak, J.; Banach, P.; Nowak-Markwitz, E.; Kokot, Z.J. Diagnostic Value of Serum Angiogenesis Markers in Ovarian Cancer Using Multiplex Immunoassay. Int. J. Mol. Sci. 2017, 18, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voronov, E.; Apte, R.N.; Sofer, S. THE systemic inflammatory response syndrome related to the release of cytokines following severe envenomation. J. Venom. Anim. Toxins 1999, 5, 5–33. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Durham, S.R. Hymenoptera Venom Allergy: How Does Venom Immunotherapy Prevent Anaphylaxis From Bee and Wasp Stings? Front. Immunol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Broniarczyk-Dyła, G.; Prusińska-Bartoś, M.; Grzegorczyk, J.; Jarzebska, M.; Wawrzycka-Kaflik, A. Soluble receptors CD30 and CD26 in serum of patients with atopic dermatitis as markers of disease activity. Adv. Dermatol. Allergol. Dermatol. Alergol. 2005, 22, 219–226. [Google Scholar]
- Hansen, H.P.; Kisseleva, T.; Kobarg, J.; Horn-Lohrens, O.; Havsteen, B.; Lemke, H. A zinc metalloproteinase is responsible for the release of cd30 on human tumor cell lines. Int. J. Cancer 1995, 63, 750–756. [Google Scholar] [CrossRef]
- Kampa, F.; Mitteldorf, C. A review of CD30 expression in cutaneous neoplasms. J. Cutan. Pathol. 2021, 48, 495–510. [Google Scholar] [CrossRef]
- Croft, M. The TNF family in T cell differentiation and function--unanswered questions and future directions. Semin. Immunol. 2014, 26, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Van der Weyden, C.A.; Pileri, S.A.; Feldman, A.L.; Whisstock, J.; Prince, H.M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017, 7, e603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Anrooij, B.; Kluin, P.M.; Oude Elberink, J.N.G.; Kluin-Nelemans, J.C. CD30 in systemic mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.; Orazi, A. Mastocytosis and related disorders. Semin. Diagn. Pathol. 2012, 29, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Sotlar, K.; Horny, H.-P. Aberrant expression of CD30 in aggressive systemic mastocytosis and mast cell leukemia: A differential diagnosis to consider in aggressive hematopoietic CD30-positive neoplasms. Leuk. Lymphoma 2011, 52, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Fölster-Holst, R.; Henseler, T.; Wehde, J.; Lemke, H.; Weichenthal, M.; Christophers, E.; Hansen, H.P. Soluble CD30 plasma concentrations correlate with disease activity in patients with atopic dermatitis. Acta Derm. Venereol. 2002, 82, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polte, T.; Behrendt, A.; Hansen, G. Direct evidence for a critical role of CD30 in the development of allergic asthma. J. Allergy Clin. Immunol. 2006, 118, 942–948. [Google Scholar] [CrossRef]
- Fuchiwaki, T.; Sun, X.; Fujimura, K.; Yamada, H.; Shibata, K.; Muta, H.; Podack, E.R.; Kawauchi, H.; Yoshikai, Y. The central role of CD30L/CD30 interactions in allergic rhinitis pathogenesis in mice. Eur. J. Immunol. 2011, 41, 2947–2954. [Google Scholar] [CrossRef]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Hassan, L.; Medenwald, D.; Tiller, D.; Kluttig, A.; Ludwig-Kraus, B.; Kraus, F.B.; Greiser, K.H.; Mikolajczyk, R. The association between change of soluble tumor necrosis factor receptor R1 (sTNF-R1) measurements and cardiovascular and all- cause mortality—Results from the population- based (Cardiovascular Disease, Living and Ageing in Halle) CARLA study 2002–2016. PLoS ONE 2020, 15, e0241213. [Google Scholar] [CrossRef] [PubMed]
- Pergola, V.; Di Salvo, G.; Martiniello, A.R.; Irace, L.; Tedesco, M.A.; Scialdone, A.; Iacono, A. TNF alpha and heart failure. Minerva Cardioangiol. 2000, 48, 475–484. [Google Scholar] [PubMed]
- Zheng, C.; Yan, W.; Luo, Y.; Wang, T.; Shu, Y. Value of sTNF-R1 and linc0597 as indicators for disease activity and diagnosis of lupus nephritis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5582–5591. [Google Scholar] [PubMed]
- Becke, F.M.; Hehlgans, T.; Brockhoff, G.; Männel, D.N. Development of allergic contact dermatitis requires activation of both tumor necrosis factor-receptors. Eur. Cytokine Netw. 2001, 12, 45–50. [Google Scholar] [PubMed]
- Ahmad, S.; Azid, N.A.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. The Key Role of TNF-TNFR2 Interactions in the Modulation of Allergic Inflammation: A Review. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, B.; Shute, J.; Howarth, P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in asthma. Thorax 1999, 54, 590–596. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, P.; Berghella, A.M.; Contasta, I.; Adorno, D. CD30 antigen: Not a physiological marker for TH2 cells but an important costimulator molecule in the regulation of the balance between TH1/TH2 response. Transpl. Immunol. 2003, 12, 49–61. [Google Scholar] [CrossRef]
- Horn-Lohrens, O.; Tiemann, M.; Lange, H.; Kobarg, J.; Hafner, M.; Hansen, H.; Sterry, W.; Parwaresch, R.M.; Lemke, H. Shedding of the soluble form of CD30 from the Hodgkin-analogous cell line L540 is strongly inhibited by a new CD30-specific antibody (Ki-4). Int. J. Cancer 1995, 60, 539–544. [Google Scholar] [CrossRef]
- Carballido, J.M.; Carballido-Perrig, N.; Terres, G.; Heusser, C.H.; Blaser, K. Bee venom phospholipase A2-specific T cell clones from human allergic and non-allergic individuals: Cytokine patterns change in response to the antigen concentration. Eur. J. Immunol. 1992, 22, 1357–1363. [Google Scholar] [CrossRef]
- Meiler, F.; Zumkehr, J.; Klunker, S.; Rückert, B.; Akdis, C.A.; Akdis, M. In vivo switch to IL-10–secreting T regulatory cells in high dose allergen exposure. J. Exp. Med. 2008, 205, 2887–2898. [Google Scholar] [CrossRef] [Green Version]
- Fallarino, F.; Grohmann, U. Using an ancient tool for igniting and propagating immune tolerance: IDO as an inducer and amplifier of regulatory T cell functions. Curr. Med. Chem. 2011, 18, 2215–2221. [Google Scholar] [CrossRef]
- Schmidt, S.V.; Nino-Castro, A.C.; Schultze, J.L. Regulatory dendritic cells: There is more than just immune activation. Front Immunol. 2012, 3, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oude Elberink, J.N.G.; Dubois, A.E.J. Quality of life in insect venom allergic patients. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Tusiimire, J.; Wallace, J.; Woods, N.; Dufton, M.J.; Parkinson, J.A.; Abbott, G.; Clements, C.J.; Young, L.; Park, J.K.; Jeon, J.W.; et al. Effect of Bee Venom and Its Fractions on the Release of Pro-Inflammatory Cytokines in PMA-Differentiated U937 Cells Co-Stimulated with LPS. Vaccines 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, D.-O.; Park, S.-Y.; Lee, K.-J.; Heo, M.-S.; Kim, K.-C.; Kim, M.-O.; Lee, J.-D.; Choi, Y.H.; Kim, G.-Y. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int. Immunopharmacol. 2007, 7, 1092–1101. [Google Scholar] [CrossRef]
- Whitehead, G.S.; Thomas, S.Y.; Shalaby, K.H.; Nakano, K.; Moran, T.P.; Ward, J.M.; Flake, G.P.; Nakano, H.; Cook, D.N. TNF is required for TLR ligand–mediated but not protease-mediated allergic airway inflammation. J. Clin. Investig. 2017, 127, 3313–3326. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Wang, C.H.; Wu, P.W.; He, J.R.; Huang, C.C.; Chang, P.H.; Fu, C.H.; Lee, T.J. Increased nasal matrix metalloproteinase-1 and -9 expression in smokers with chronic rhinosinusitis and asthma. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.G.; Na Kim, M.; Hong, J.Y.; Lee, J.W.; Kim, S.Y.; Kim, K.W.; Lee, C.G.; Elias, J.A.; Song, T.W.; Sohn, M.H. Chitinase 3-Like 1 Contributes to Food Allergy via M2 Macrophage Polarization. Allergy, Asthma Immunol. Res. 2020, 12, 1012–1028. [Google Scholar] [CrossRef]
- Zhi, Y.; Gao, P.; Xin, X.; Li, W.; Ji, L.; Zhang, L.; Zhang, X.; Zhang, J. Clinical signifcance of sCD163 and its possible role in asthma (Review). Mol. Med. Rep. 2017, 15, 2931–2939. [Google Scholar] [CrossRef] [Green Version]
- Gowhari-Shabgah, A.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Ghasemi, A.; Ghoryani, M.; Mohammadi, M. A significant decrease of BAFF, APRIL, and BAFF receptors following mesenchymal stem cell transplantation in patients with refractory rheumatoid arthritis. Gene 2020, 732, 144336. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Kurokawa, M.; Uede, T.; Nishimura, M.; Huang, S.-K. Role of osteopontin, a multifunctional protein, in allergy and asthma. Clin. Exp. Allergy 2011, 41, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.L.; Kraft, M. Metalloproteinases as modulators of allergic asthma: Therapeutic perspectives. Met. Med. 2015, 2015, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Sage, E.H.; Reed, M.; Funk, S.E.; Truong, T.; Steadele, M.; Puolakkainen, P.; Maurice, D.H.; Bassuk, J.A. Cleavage of the Matricellular Protein SPARC by Matrix Metalloproteinase 3 Produces Polypeptides That Influence Angiogenesis. J. Biol. Chem. 2003, 278, 37849–37857. [Google Scholar] [CrossRef] [Green Version]
- Stone, A.V.; Vanderman, K.S.; Willey, J.S.; Long, D.L.; Register, T.C.; Shively, C.A.; Stehle, J.R., Jr.; Loeser, R.F.; Ferguson, C.M. Osteoarthritic changes in vervet monkey knees correlate with meniscus degradation and increased matrix metalloproteinase and cytokine secretion. Osteoarthr. Cartil. 2015, 23, 1780–1789. [Google Scholar] [CrossRef] [Green Version]
- Boruk, M.; Railwah, C.; Lora, A.; Nath, S.; Wu, D.; Chow, L.; Borhanjoo, P.; Dabo, A.J.; Chowdhury, S.; Kaiser, R.; et al. Elevated S100A9 expression in chronic rhinosinusitis coincides with elevated MMP production and proliferation in vitro. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hinks, T.S.; Brown, T.; Lau, L.C.; Rupani, H.; Barber, C.; Elliott, S.; Ward, J.A.; Ono, J.; Ohta, S.; Izuhara, K.; et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3–like protein 1. J. Allergy Clin. Immunol. 2016, 138, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Gearing, A.J.H.; Beckett, P.; Christodoulou, M.; Churchill, M.; Clements, J.; Davidson, A.H.; Drummond, A.H.; Galloway, W.A.; Gilbert, R.; Gordon, J.L.; et al. Processing of tumour necrosis factor-α precursor by metalloproteinases. Nat. Cell Biol. 1994, 370, 555–557. [Google Scholar] [CrossRef]
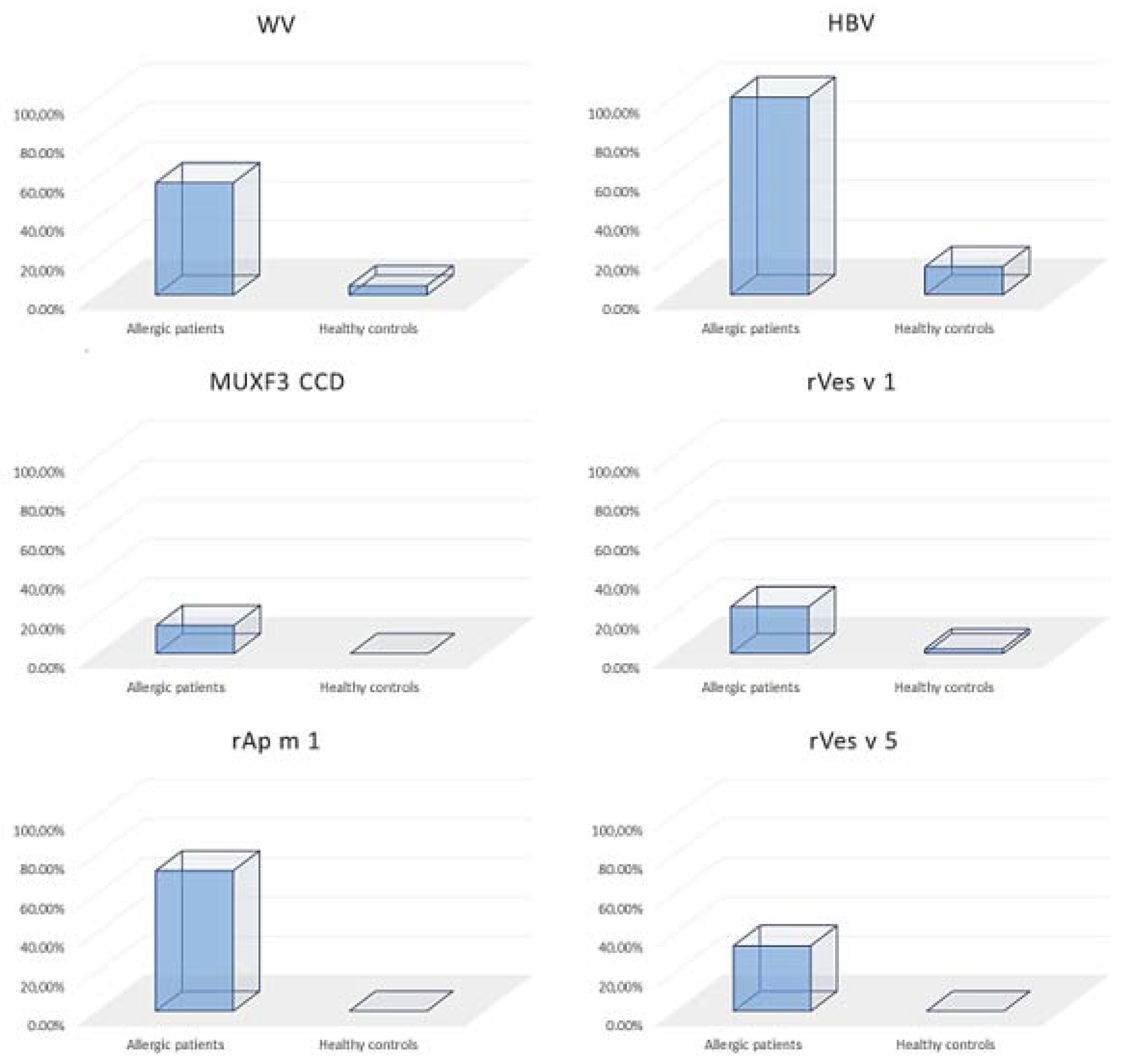


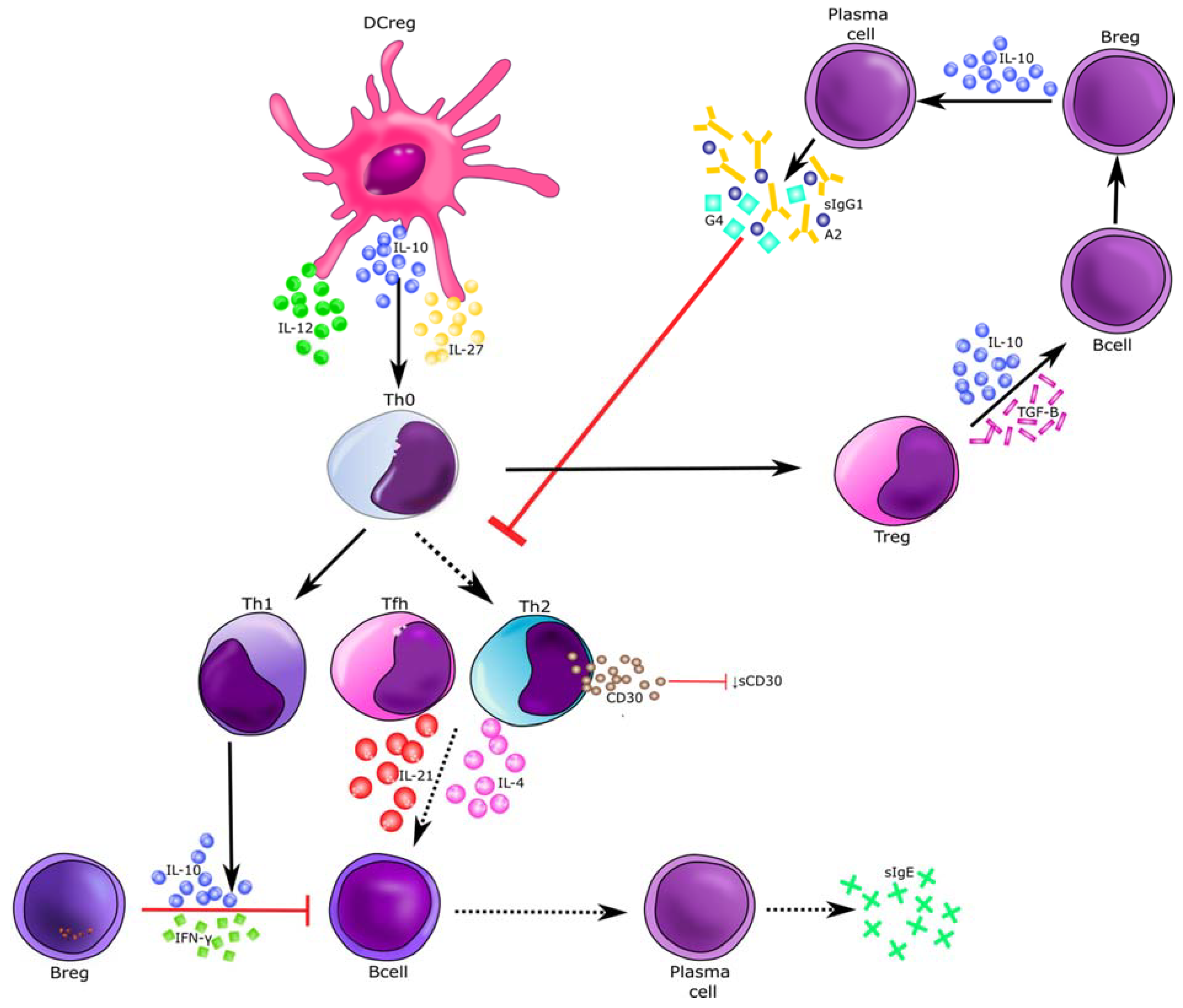
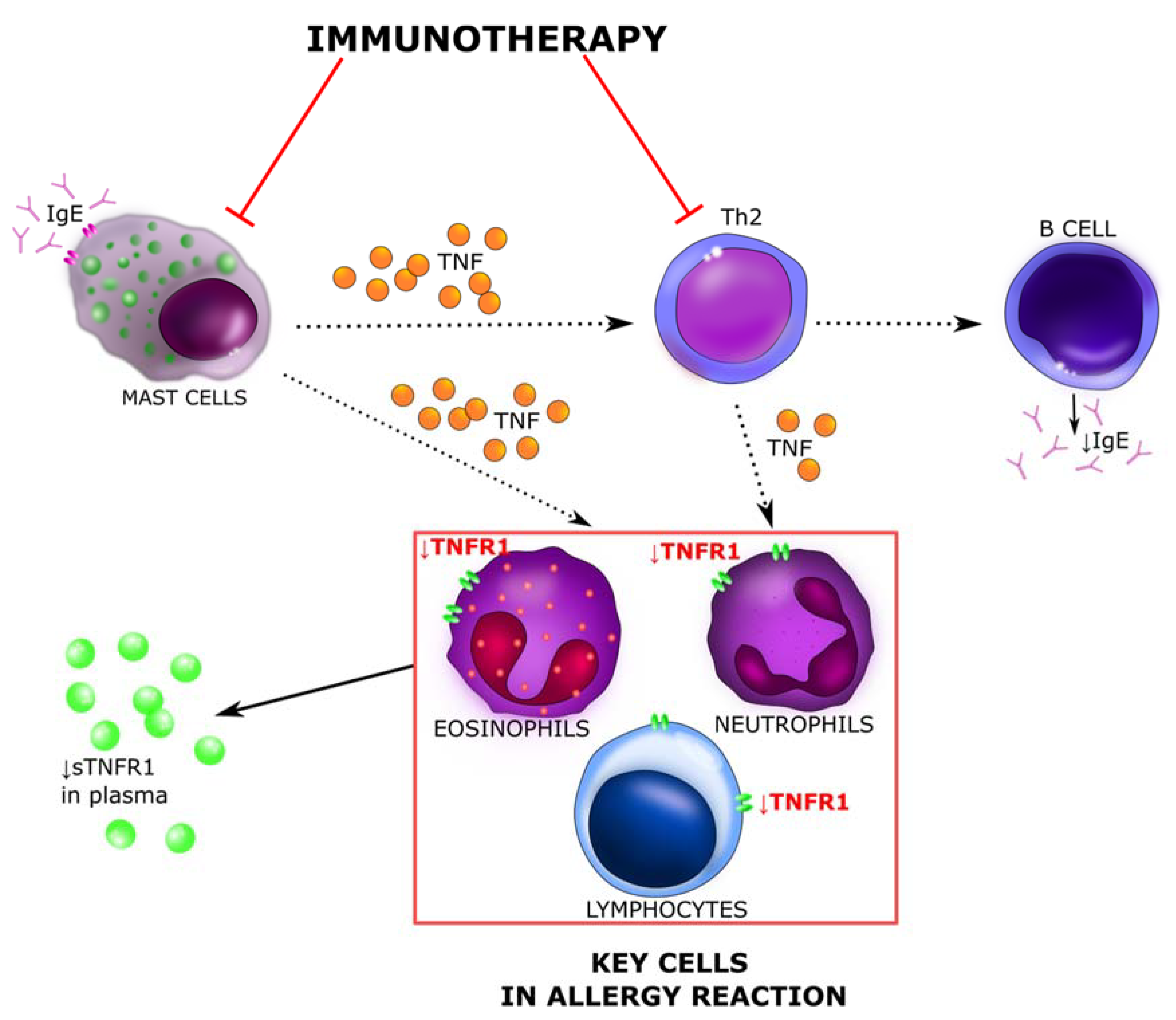
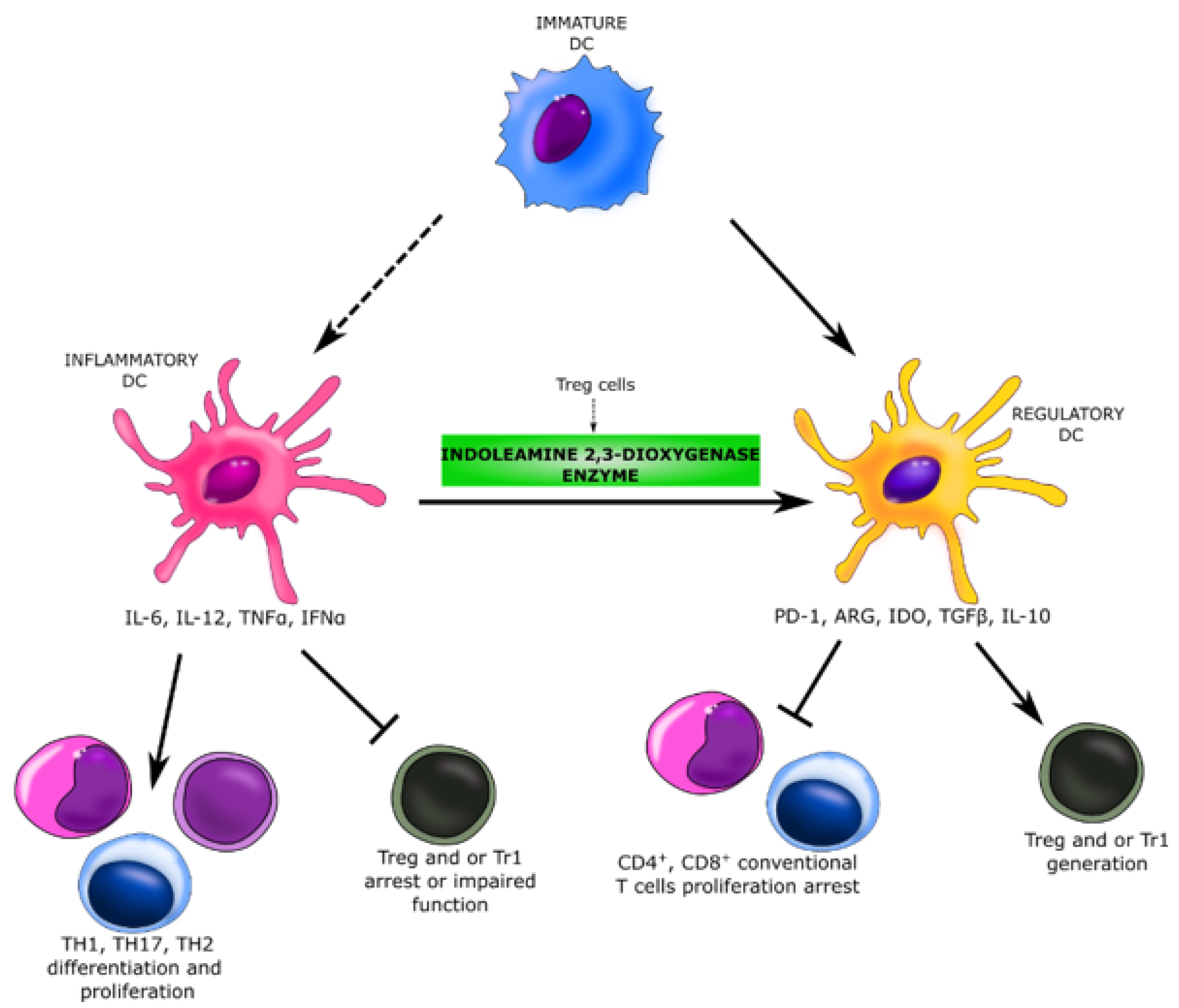
| Characteristics of the Participants | Study Group | Control Group |
|---|---|---|
| No. of subjects | 21 | 42 |
| Sex | ||
| Male | 12 (57.1%) | 26 (61.9%) |
| Female | 9 (42.9%) | 16 (38.1%) |
| Age | ||
| Median | 36 | 54 |
| Mean | 37 | 44 |
| Range | 7–67 | 3–74 |
| Comorbidities | ||
| Diabetes | 0 | 2 |
| Atopic dermatitis | 1 | 0 |
| Allergic rhinitis | 3 | 0 |
| Asthma | 2 | 2 |
| Hypertension | 2 | 5 |
| Atherosclerosis | 1 | 1 |
| Unstable angina | 0 | 1 |
| Medications | 9 | 11 |
| Beekeepers | 14 (67%) | 16 (38%) |
| Working period | 6 years | 28 years |
| No. of days in the apiary during the beekeeping season/week | 2.2 | 2.7 |
| Stings/day | 3 | 4 |
| Inflammation Marker | Allergy Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Median | Mean | SD | Median | Mean | SD | |
| APRIL/TNFSF13 | 26,517.5 | 25,487.8 | 16,344.1 | 17,305.0 | 24,700.7 | 21,523.6 |
| BAFF/TNFSF13B | 8451.2 | 8882.3 | 2076.4 | 8711.2 | 9095.3 | 2857.9 |
| sCD30/TNFRSF8 | 482.2 | 735.1 | 611.7 | 907.2 | 1051.6 | 675.8 |
| sCD163 | 152,431.4 | 156,666.3 | 37,598.5 | 145,661.2 | 163,804.5 | 60,273.9 |
| Chitinase 3-like 1 | 7308.5 | 8537.5 | 3371.8 | 8727.9 | 9334.5 | 4573.4 |
| gp130/sIL-6Rbeta | 54,221.1 | 57,332.8 | 12,140.3 | 58,621.7 | 60,935.2 | 17,034.5 |
| sIL-6Ralfa | 13,706.5 | 13,199.5 | 3246.8 | 12,583.9 | 12,843.0 | 4144.3 |
| IL-19 | 56.7 | 58.9 | 9.7 | 57.9 | 59.7 | 15.9 |
| IL-26 | 59.6 | 69.0 | 25.8 | 70.6 | 77.1 | 34.3 |
| MMP-1 | 1196.9 | 2091.3 | 2034.2 | 1361.2 | 2097.2 | 2703.7 |
| MMP-2 | 24,149.6 | 26,313.3 | 12,881.0 | 26,794.6 | 30,788.1 | 20,011.1 |
| MMP-3 | 5943.1 | 7032.5 | 5131.3 | 3251.1 | 5248.7 | 4970.4 |
| Osteocalcin | 3997.2 | 7244.4 | 7706.6 | 6967.8 | 10,220.4 | 8319.0 |
| Osteopontin | 15,388.0 | 21,273.0 | 16,831.9 | 24,705.8 | 26,392.9 | 15,668.4 |
| Pentraxin-3 | 720.0 | 856.8 | 459.2 | 829.1 | 1069.2 | 698.0 |
| sTNF-R1 | 3185.3 | 3176.3 | 843.0 | 3670.8 | 3860.8 | 1336.4 |
| sTNF-R2 | 3904.9 | 4384.9 | 1567.3 | 5167.0 | 5750.7 | 3009.7 |
| TSLP | 19.7 | 19.0 | 4.2 | 17.3 | 19.5 | 6.0 |
| TWEAK/TNFSF12 | 442.9 | 485.1 | 126.8 | 459.8 | 514.4 | 185.1 |
| Inflammation Marker | p Value | ||||
|---|---|---|---|---|---|
| Shapiro–Wilk Test | Mann–Whitney U Test | Student’s t-Test | Welch Test | ||
| Allergy Group | Control Group | ||||
| APRIL/TNFSF13 | 0.0970 | <0.0001 | 0.488754 | ||
| BAFF/TNFSF13B | 0.0033 | 0.1595 | 0.890011 | ||
| sCD30/TNFRSF8 | 0.0001 | 0.0002 | 0.014876 | ||
| sCD163 | 0.7636 | 0.0004 | 0.738166 | ||
| Chitinase 3-like 1 | 0.2398 | 0.0101 | 0.644653 | ||
| gp130/sIL-6Rbeta | 0.1245 | 0.0260 | 0.309224 | ||
| sIL-6Ralfa | 0.6361 | 0.6943 | 0.7241 | ||
| IL-19 | 0.1561 | 0.7654 | 0.7939 * | ||
| IL-26 | 0.0008 | 0.0109 | 0.496046 | ||
| MMP-1 | 0.0001 | <0.0001 | 0.556371 | ||
| MMP-2 | 0.0021 | <0.0001 | 0.556371 | ||
| MMP-3 | 0.0143 | 0.0001 | 0.077366 | ||
| Osteocalcin | <0.0001 | <0.0001 | 0.083497 | ||
| Osteopontin | 0.0007 | 0.0062 | 0.1379 | ||
| Pentraxin-3 | 0.0022 | <0.0001 | 0.262069 | ||
| sTNF-R1 | 0.4389 | 0.0108 | 0.046427 | ||
| sTNF-R2 | 0.2228 | 0.0033 | 0.11198 | ||
| TSLP | 0.0834 | 0.0001 | 0.8177 | ||
| TWEAK/TNFSF12 | 0.0055 | 0.0482 | 0.556371 | ||
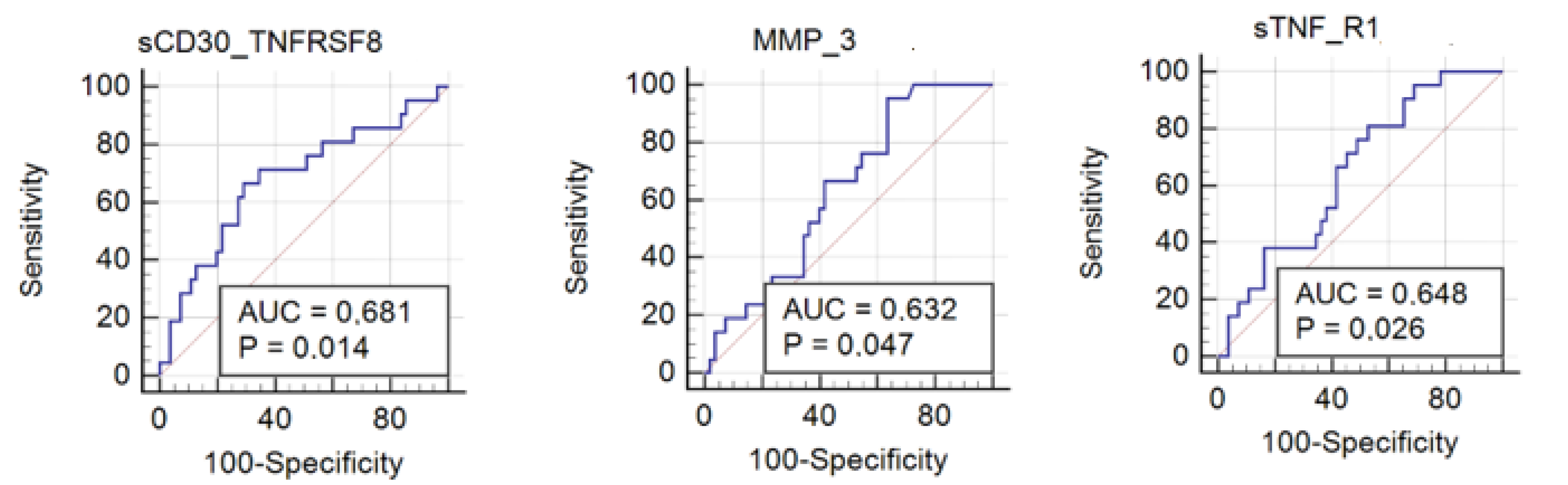
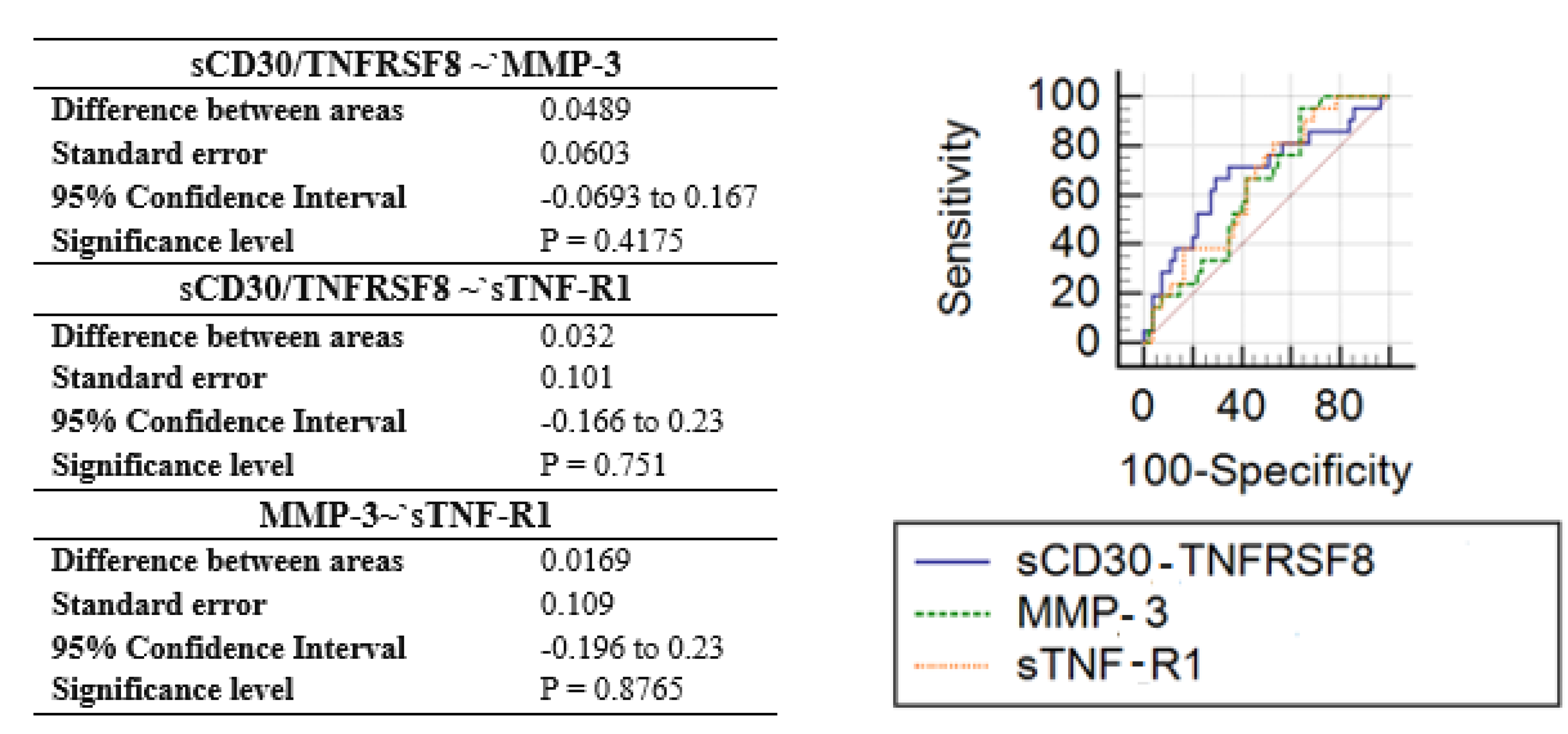
| Inflammation Marker | Study vs. Control Group | |
|---|---|---|
| p-Value | AUC | |
| sCD30/TNFRSF8 | 0.0135 | 0.68 |
| sTNF-R1 | 0.0258 | 0.65 |
| MMP-3 | 0.0468 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Packi, K.; Matysiak, J.; Matuszewska, E.; Bręborowicz, A.; Kycler, Z.; Matysiak, J. New Biomarkers of Hymenoptera Venom Allergy in a Group of Inflammation Factors. Int. J. Environ. Res. Public Health 2021, 18, 4011. https://doi.org/10.3390/ijerph18084011
Packi K, Matysiak J, Matuszewska E, Bręborowicz A, Kycler Z, Matysiak J. New Biomarkers of Hymenoptera Venom Allergy in a Group of Inflammation Factors. International Journal of Environmental Research and Public Health. 2021; 18(8):4011. https://doi.org/10.3390/ijerph18084011
Chicago/Turabian StylePacki, Kacper, Joanna Matysiak, Eliza Matuszewska, Anna Bręborowicz, Zdzisława Kycler, and Jan Matysiak. 2021. "New Biomarkers of Hymenoptera Venom Allergy in a Group of Inflammation Factors" International Journal of Environmental Research and Public Health 18, no. 8: 4011. https://doi.org/10.3390/ijerph18084011
APA StylePacki, K., Matysiak, J., Matuszewska, E., Bręborowicz, A., Kycler, Z., & Matysiak, J. (2021). New Biomarkers of Hymenoptera Venom Allergy in a Group of Inflammation Factors. International Journal of Environmental Research and Public Health, 18(8), 4011. https://doi.org/10.3390/ijerph18084011






