Efficacy of Phytocannabinoids in Epilepsy Treatment: Novel Approaches and Recent Advances
Abstract
1. Introduction
2. The Endocannabinoid System and Its Modulation by Phytocannabinoids
3. Anticonvulsant Effects of Phytocannabinoids
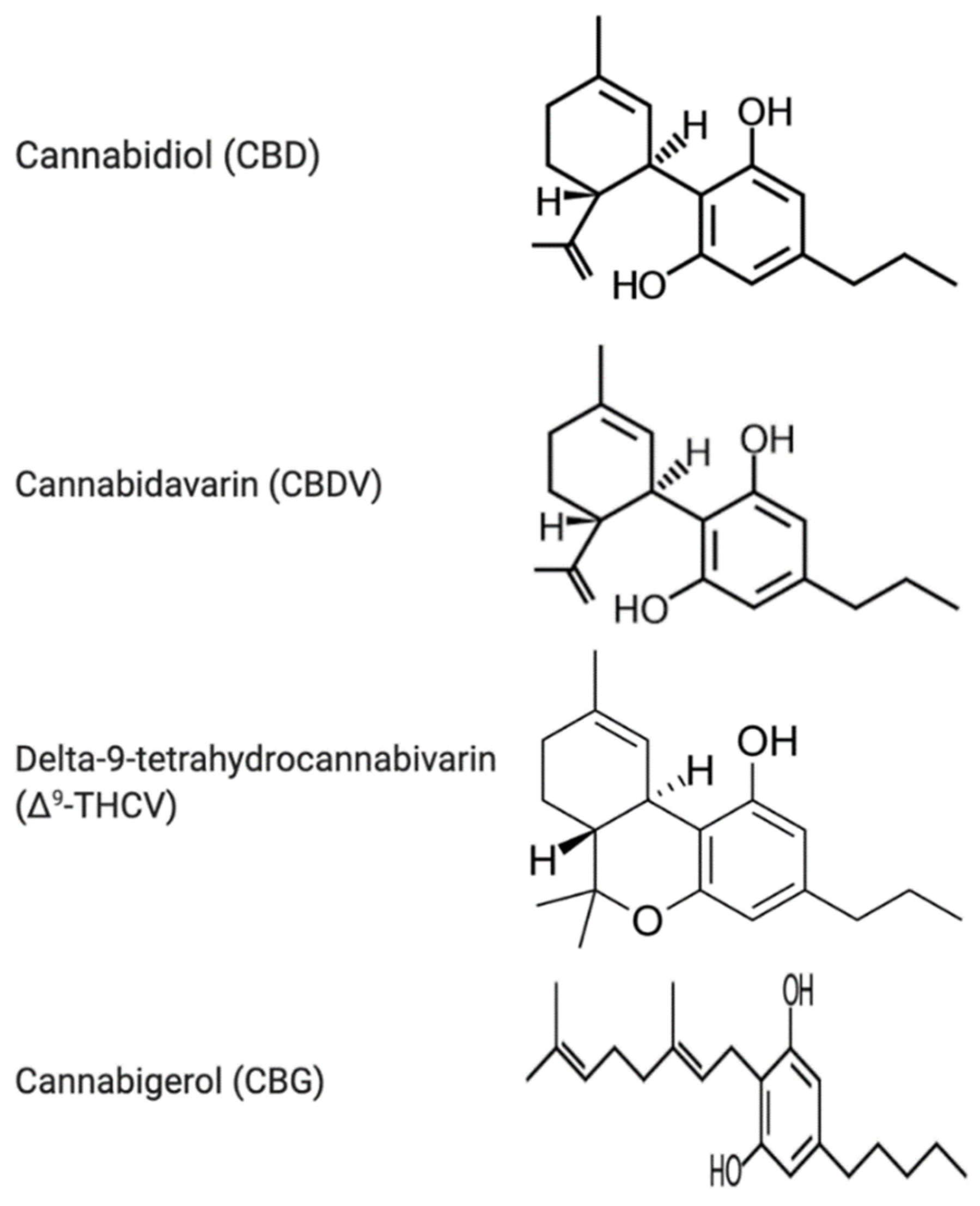
3.1. Cannabidiol
3.1.1. Molecular Targets of CBD
3.1.2. Pre-Clinical Evidence of CBD Efficacy
3.1.3. Clinical Evidence of CBD Efficacy
3.2. Cannabidivarin (CBDV)
3.2.1. Molecular Targets of CBDV
3.2.2. Pre-Clinical Evidence of CBDV Efficacy
3.2.3. Clinical Evidence of CBDV Efficacy
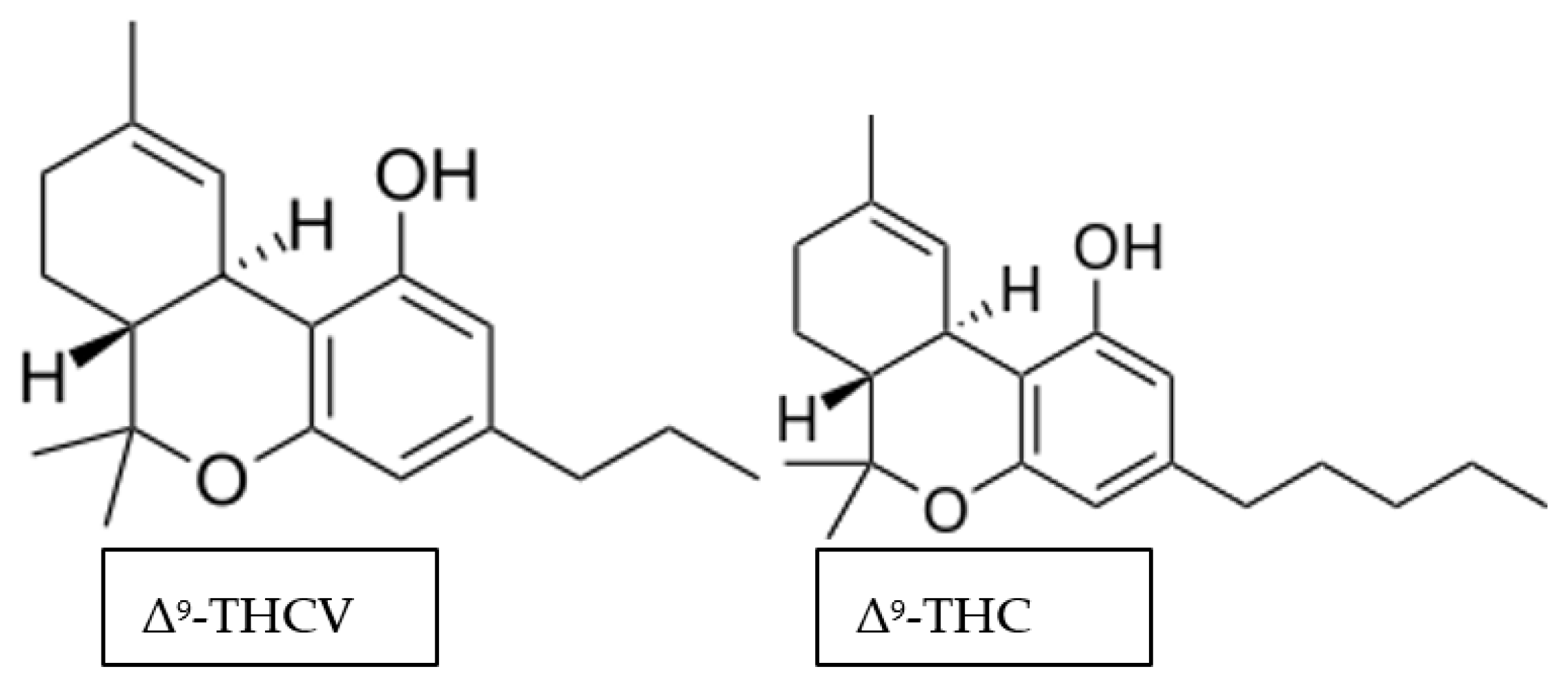
3.3. Delta-9-Tetrahydrocannabivarin (Δ9-THCV)
3.3.1. Molecular Targets of Δ9-THCV
3.3.2. Pre-Clinical Evidence of Δ9-THCV Efficacy
3.4. Cannabigerol (CBG)
3.4.1. Molecular Targets of CBG
3.4.2. Preclinical Evidence of CBG Efficacy
3.5. Overview of Phytocannabinoids
4. Neurogenerative/Neuroprotective Potential of Phytocannabinoids
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, E.C.; Tsien, R.W.; Whalley, B.J.; Devinsky, O. Cannabinoids and Epilepsy. Neurotherapeutics 2015, 12, 747–768. [Google Scholar] [CrossRef] [PubMed]
- Broicher, S.; Jokeit, H. Emotional Agnosis and Theory of Mind. In The Neuropsychiatry of Epilepsy; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Perucca, P.; Scheffer, I.E.; Kiley, M. The Management of Epilepsy in Children and Adults. Med. J. Aust. 2018, 208, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S. The New Classification of Seizures by the International League Against Epilepsy 2017. Curr. Neurol. Neurosci. Rep. 2017, 17, 48. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Raspall-Chaure, M.; Neville, B.G.; Scott, R.C. The medical management of the epilepsies in children: Conceptual and practical considerations. Lancet Neurol. 2008, 7, 57–69. [Google Scholar] [CrossRef]
- Perucca, E.; Tomson, T. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 2011, 10, 446–456. [Google Scholar] [CrossRef]
- Galanopoulou, A.S.; Buckmaster, P.S.; Staley, K.J.; Moshé, S.L.; Perucca, E.; Engel, J.; Löscher, W.; Noebels, J.L.; Pitkänen, A.; Stables, J.; et al. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia 2012, 53, 571–582. [Google Scholar] [CrossRef]
- Van Vliet, E.A.; Aronica, E.; Gorter, J.A. Blood-brain barrier dysfunction, seizures and epilepsy. Semin. Cell Dev. Biol. 2015, 38, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Schmidt, D. Modern antiepileptic drug development has failed to deliver: Ways out of the current dilemma. Epilepsia 2011, 52, 657–678. [Google Scholar] [CrossRef]
- Choi, H.; Hayat, M.J.; Zhang, R.; Hirsch, L.J.; Bazil, C.W.; Mendiratta, A.; Kato, K.; Javed, A.; Legge, A.W.; Buchsbaum, R.; et al. Drug-resistant epilepsy in adults: Outcome trajectories after failure of two medications. Epilepsia 2016, 57, 1152–1160. [Google Scholar] [CrossRef]
- Wirrell, E. Infantile, Childhood, and Adolescent Epilepsies. Contin. Lifelong Learn. Neurol. 2016, 22, 60–93. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J. Outcomes in newly diagnosed epilepsy in adolescents and adults: Insights across a generation in Scotland. Seizure 2017, 44, 206–210. [Google Scholar] [CrossRef]
- Berg, A.T.; Zelko, F.A.; Levy, S.R.; Testa, F.M. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: A prospective cohort study. Neurology 2012, 79, 1384–1391. [Google Scholar] [CrossRef]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Concepts, C.; Kwan, P.; Schachter, S.C.; Brodie, M.J. Drug-Resistant Epilepsy. N. Engl. J. Med. 2011, 365, 919–926. [Google Scholar]
- Kwan, P.; Brodie, M.J. Early Identification of Refractory Epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Arts, W.F.M.; Brouwer, O.F.; Peters, A.C.B.; Stroink, H.; Peeters, E.A.J.; Schmitz, P.I.M.; Van Donselaar, C.A.; Geerts, A.T. Course and prognosis of childhood epilepsy: 5-Year follow-up of the Dutch study of epilepsy in childhood. Brain 2004, 127, 1774–1784. [Google Scholar] [CrossRef]
- Kennedy, G.M.; Lhatoo, S.D. CNS adverse events associated with antiepileptic drugs. CNS Drugs 2008, 22, 739–760. [Google Scholar] [CrossRef]
- Loring, D.W.; Marino, S.; Meador, K.J. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol. Rev. 2007, 17, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.I.; Pennell, P.B. Management of epilepsy during pregnancy: An update. Ther. Adv. Neurol. Disord. 2016, 9, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef]
- Moavero, R.; Santarone, M.E.; Galasso, C.; Curatolo, P. Cognitive and behavioral effects of new antiepileptic drugs in pediatric epilepsy. Brain Dev. 2017, 39, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Arif, H.; Buchsbaum, R.; Weintraub, D.; Pierro, J.; Resor, S.R.; Hirsch, L.J. Patient-reported cognitive side effects of antiepileptic drugs: Predictors and comparison of all commonly used antiepileptic drugs. Epilepsy Behav. 2009, 14, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.W.; Besag, F.; Caplan, R.; Aldenkamp, A.; Gobbi, G.; Sillanpää, M. Psychiatric and behavioural disorders in children with epilepsy (ILAE Task Force Report): Anxiety, depression and childhood epilepsy. Epileptic Disord. 2016, 18, S24–S30. [Google Scholar] [CrossRef]
- Vermeulen, J.; Aldenkamp, A.P. Cognitive side-effects of chronic antiepileptic drug treatment: A review of 25 years of research. Epilepsy Res. 1995, 22, 65–95. [Google Scholar] [CrossRef]
- Ijff, D.M.; Aldenkamp, A.P. Cognitive side-effects of antiepileptic drugs in children. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 111, pp. 707–718. [Google Scholar] [CrossRef]
- Helal, S.I.; Megahed, H.S.; Salem, S.M.; Youness, E.R. Monotherapy versus polytherapy in epileptic adolescents. Maced. J. Med. Sci. 2013, 6, 1387–1394. [Google Scholar] [CrossRef]
- Go, T. Effect of antiepileptic drug polytherapy on urinary pH in children and young adults. Child’s Nerv. Syst. 2009, 25, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Golyala, A.; Kwan, P. Drug development for refractory epilepsy: The past 25 years and beyond. Seizure 2017, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shvo, Y.; Hashish, I. The structure of cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Lett. Nat. 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Patel, A.; Devinsky, O.; Cross, J.H.; Villanueva, V.; Wirrell, E.; VanLandingham, K.; Roberts, C.; Checketts, D. Zuberi, S.M. Cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): Results of a dose-ranging, multi-center, randomized, double-blind, placebo-controlled trial (GWPCARE3). Neurology 2017, 89, e100. [Google Scholar]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef]
- Zareie, P.; Sadegh, M.; Palizvan, M.R.; Moradi-Chameh, H. Anticonvulsive effects of endocannabinoids; an investigation to determine the role of regulatory components of endocannabinoid metabolism in the Pentylenetetrazol induced tonic- clonic seizures. Metab. Brain Dis. 2018, 33, 939–948. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behav. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Rosenberg, E.C.; Patra, P.H.; Whalley, B.J. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017, 70, 319–327. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Neuroscience: Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef]
- Núñez, E.; Benito, C.; Pazos, M.R.; Barbachano, A.; Fajardo, O.; González, S.; Tolón, R.M.; Romero, J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: An Immunohistochemical Study. Synapse 2004, 53, 208–213. [Google Scholar] [CrossRef]
- Skaper, S.D.; Buriani, A.; DAL Toso, R.; Petrelli, L.; Romanello, S.; Facci, L.; Leon, A. The ALIAmide Palmitoylethanolamide and Cannabinoids, but Not Anandamide, Are Protective in a Delayed Postglutamate Paradigm of Excitotoxic Death in Cerebellar Granule Neurons (N-methyl-D-aspartate/neurotoxicity/N-acylethanolamides/neuroprotection/receptor). Proc. Natl. Acad. Sci. USA 1996, 93, 3984–3989. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Di Marzo, V. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.A.; di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. Cannabinoid activation of peroxisome proliferator-activated receptors: An update and review of the physiological relevance. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2013, 2, 17–25. [Google Scholar] [CrossRef]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Harvey-white, J.; Huang, B.X.; Kim, H.; Luquet, S.; Palmiter, R.D.; Krystal, G.; Rai, R.; Mahadevan, A.; et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 2008, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, A.C.; Regehr, W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 2001, 29, 717–727. [Google Scholar] [CrossRef]
- Llano, I.; Leresche, N.; Marty, A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 1991, 6, 565–574. [Google Scholar] [CrossRef]
- Maejima, T.; Hashimoto, K.; Yoshida, T.; Aiba, A.; Kano, M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 2001, 31, 463–475. [Google Scholar] [CrossRef]
- Gerdeman, G.L.; Ronesi, J.; Lovinger, D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002, 5, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Chevaleyre, V.; Castillo, P.E. Heterosynaptic LTD of hippocampal GABAergic synapses: A novel role of endocannabinoids in regulating excitability. Neuron 2003, 38, 461–472. [Google Scholar] [CrossRef]
- O’Connell, B.K.; Gloss, D.; Devinsky, O. Cannabinoids in treatment-resistant epilepsy: A review. Epilepsy Behav. 2017, 70, 341–348. [Google Scholar] [CrossRef]
- Baraban, S.C. Animal Models of Epilepsy. In Neuromethods; Baraban, S.C., Ed.; Humana Press, Springer Nature: New York, NY, USA, 2009; pp. 59–74. [Google Scholar] [CrossRef]
- Löscher, W. The holy grail of epilepsy prevention: Preclinical approaches to antiepileptogenic treatments. Neuropharmacology 2020, 167, 107605. [Google Scholar] [CrossRef]
- Maljevic, S.; Reid, C.A.; Petrou, S. Models for discovery of targeted therapy in genetic epileptic encephalopathies. J. Neurochem. 2017, 143, 30–48. [Google Scholar] [CrossRef]
- Griffin, A.; Hamling, K.R.; Hong, S.; Anvar, M.; Lee, L.P.; Baraban, S.C. Preclinical Animal Models for Dravet Syndrome: Seizure Phenotypes, Comorbidities and Drug Screening. Front. Pharmacol. 2018, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Grone, B.P.; Baraban, S.C. Animal models in epilepsy research: Legacies and new directions. Nat. Neurosci. 2015, 18, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kandratavicius, L.; Balista, P.A.; Lopes-Aguiar, C.; Ruggiero, R.N.; Umeoka, E.H.; Garcia-Cairasco, N.; Bueno-Junior, L.S.; Leite, J.P. Animal models of epilepsy: Use and limitations. Neuropsychiatr. Dis. Treat. 2014, 10, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Jones, É.; Vlachou, S. A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment. Molecules 2020, 25, 4930. [Google Scholar] [CrossRef]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- During, M.J.; Spencer, D.D. Adenosine: A potential mediator of seizure arrest and postictal refractoriness. Ann. Neurol. 1992, 32, 618–624. [Google Scholar] [CrossRef]
- Brickley, S.G.; Mody, I. Extrasynaptic GABA A Receptors: Their Function in the CNS and Implications for Disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, S.; Le Foll, B. The complexity of pharmacology of cannabidiol (CBD) and its implications in the treatment of brain disorders. Neuropsychopharmacology 2020, 45, 229–230. [Google Scholar] [CrossRef]
- Lin, C.H.; Lane, H.Y. The role of N-methyl-D-aspartate receptor neurotransmission and precision medicine in behavioral and psychological symptoms of dementia. Front. Pharmacol. 2019, 10, 540. [Google Scholar] [CrossRef]
- Pamplona, F.A.; da Silva, L.R.; Coan, A.C. Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis. Front. Neurol. 2018, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-zand, Z.; Ahmad-Molaei, L.; Motamedi, F.; Naderi, N. The role of potassium BK channels in anticonvulsant effect of cannabidiol in pentylenetetrazole and maximal electroshock models of seizure in mice. Epilepsy Behav. 2013, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Reyes, L.E.; Ladas, T.P.; Chiang, C.-C.; Durand, D.M. TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo. Exp. Neurol. 2013, 250, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Rimmerman, N.; Ben-Hail, D.; Porat, Z.; Juknat, A.; Kozela, E.; Daniels, M.P.; Connelly, P.S.; Leishman, E.; Bradshaw, H.B.; Shoshan-Barmatz, V.; et al. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: A novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013, 4, e949. [Google Scholar] [CrossRef] [PubMed]
- Consroe, P.; Wolkin, A. Cannabidiol-Antiepileptic Comparisons Experimentally and Induced Interactions Seizures Drug in rats. J. Pharmacol. Exp. Ther. 1977, 201, 26–32. [Google Scholar] [PubMed]
- Consroe, P.; Benedito, M.A.C.; Leite, J.R.; Carlini, E.A.; Mechoulam, R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur. J. Pharmacol. 1982, 83, 293–298. [Google Scholar] [CrossRef]
- Jones, N.A.; Glyn, S.E.; Akiyama, S.; Hill, T.D.M.; Hill, A.J.; Weston, S.E.; Burnett, M.D.A.; Yamasaki, Y.; Stephens, G.J.; Whalley, B.J.; et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure 2012, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; You, C.; Lei, D.; Zhang, H. High dosage of cannabidiol (CBD) alleviates pentylenetetrazole-induced epilepsy in rats by exerting an anticonvulsive effect. Int. J. Clin. Exp. Med. 2015, 8, 8820–8827. [Google Scholar] [PubMed]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.M.; Kang, H.-C. Could Cannabidiol be a Treatment Option for Intractable Childhood and Adolescent Epilepsy? J. Epilepsy Res. 2017, 7, 16–20. [Google Scholar] [CrossRef][Green Version]
- Hausman-Kedem, M.; Menascu, S.; Kramer, U. Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents—An observational, longitudinal study. Brain Dev. 2018, 40, 544–551. [Google Scholar] [CrossRef]
- Gherzi, M.; Milano, G.; Fucile, C.; Calevo, M.G.; Mancardi, M.M.; Nobili, L.; Astuni, P.; Marini, V.; Barco, S.; Cangemi, G.; et al. Safety and pharmacokinetics of medical cannabis preparation in a monocentric series of young patients with drug resistant epilepsy. Complement. Ther. Med. 2020, 51, 102402. [Google Scholar] [CrossRef]
- Mechoulam, R.; Carlini, E.A. Toward drugs derived from cannabis. Naturwissenschaften 1978, 65, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.A.; Cunha, J.M. Hypnotic and antiepileptic effects of cannabidiol. J. Clin. Pharmacol. 1981, 21, 417S–427S. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.M.; Cascio, M.G.; Romano, B.; Duncan, M.; Pertwee, R.G.; Williams, C.M.; Whalley, B.J.; Hill, A.J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 2013, 170, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Morano, A.; Fanella, M.; Albini, M.; Cifelli, P.; Palma, E.; Giallonardo, A.T.; Di Bonaventura, C. Cannabinoids in the treatment of epilepsy: Current status and future prospects. Neuropsychiatr. Dis. Treat. 2020, 16, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Weston, S.E.; Jones, N.A.; Smith, I.; Bevan, S.A.; Williamson, E.M.; Stephens, G.J.; Williams, C.M.; Whalley, B.J. 9-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia 2010, 51, 1522–1532. [Google Scholar] [CrossRef]
- Hill, A.J.; Jones, N.A.; Smith, I.; Hill, C.L.; Williams, C.M.; Stephens, G.J.; Whalley, B.J. Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci. Lett. 2014, 566, 269–274. [Google Scholar] [CrossRef]
- Elsaid, S.; Kloiber, S.; Le Foll, B. Effects of Cannabidiol (CBD) in Neuropsychiatric Disorders: A Review of Pre-Clinical and Clinical Findings. Prog. Mol. Biol. Transl. Sci. 2019, 167, 25–75. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R. The path to the first FDA-approved cannabis-derived treatment and what comes next. JAMA J. Am. Med. Assoc. 2018, 320, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Metternich, B.; Wagner, K.; Geiger, M.J.; Hirsch, M.; Schulze-Bonhage, A.; Klotz, K.A. Cognitive and behavioral effects of cannabidiol in patients with treatment-resistant epilepsy. Epilepsy Behav. 2021, 114, 107558. [Google Scholar] [CrossRef] [PubMed]
- Amada, N.; Yamasaki, Y.; Williams, C.M.; Whalley, B.J. Cannabidivarin (CBDV) suppresses pentylenetetrazole (PTZ)-induced increases in epilepsy-related gene expression. PeerJ 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Nicolussi, S.; Gertsch, J. Endocannabinoid transport revisited. Vitam. Horm. 2015, 98, 441–485. [Google Scholar] [CrossRef] [PubMed]
- Chicca, A.; Nicolussi, S.; Bartholomäus, R.; Blunder, M.; Rey, A.A.; Petrucci, V.; Reynoso-Moreno, I.D.C.; Viveros-Paredes, J.M.; Gens, M.D.; Lutz, B.; et al. Chemical probes to potently and selectively inhibit endocannabinoid cellular reuptake. Proc. Natl. Acad. Sci. USA 2017, 114, E5006–E5015. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic plant cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), activate and desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) channels in vitro: Potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef]
- Sawamura, S.; Shirakawa, H.; Nakagawa, T.; Mori, Y.; Kaneko, S. TRP Channels in the Brain. In Neurobiology of TRP Channels; CRC Press/Taylor & Francis: Oxford, UK, 2017; pp. 295–322. [Google Scholar] [CrossRef]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef]
- Vennekens, R.; Menigoz, A.; Nilius, B. TRPs in the Brain. Rev. Physiol. Biochem. Pharmacol. 2012, 49, 27–64. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Nabissi, M. TRP Channels: New Potential Therapeutic Approaches in CNS Neuropathies. CNS Neurol. Disord. Drug Targets 2013, 12, 274–293. [Google Scholar] [CrossRef]
- Bialer, M.; Johannessen, S.I.; Levy, R.H.; Perucca, E.; Tomson, T.; White, H.S. Progress report on new antiepileptic drugs: A summary of the Thirteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIII). Epilepsia 2017, 58, 181–221. [Google Scholar] [CrossRef] [PubMed]
- Bialer, M.; Johannessen, S.I.; Koepp, M.J.; Levy, R.H.; Perucca, E.; Tomson, T.; White, H.S. A summary of data presented at the XIV conference on new antiepileptic drug and devices (EILAT XIV). Epilepsy Res. 2019, 153, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Whalley, B.; Williams, C.; Stephens, G.; Futamura, O. Use of the Phytocannabinoid Cannabidivarin (CBDV) in the Treatment of Epilepsy. CA 2794620, Issued: 13-3-2018; Canadian Intellectual Property Office: Gatineau, QC, Canada, 2018. [Google Scholar]
- Gill, E.W.; Paton, W.D.M.; Pertwee, R.G. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature 1970, 228, 134–136. [Google Scholar] [CrossRef]
- Harrison, C.; Traynor, J.R. The [35S]GTPγS binding assay: Approaches and applications in pharmacology. Life Sci. 2003, 74, 489–508. [Google Scholar] [CrossRef]
- Thomas, A.; Stevenson, L.A.; Wease, K.N.; Price, M.R.; Baillie, G.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid Δ 9- tetrahydrocannabivarin is a cannabinoid CB 1 and CB 2 receptor antagonist. Br. J. Pharmacol. 2005, 146, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Dennis, I.; Whalley, B.J.; Stephens, G.J. Effects of Δ9-tetrahydrocannabivarin on [35S]GTPγS binding in mouse brain cerebellum and piriform cortex membranes. Br. J. Pharmacol. 2008, 154, 1349–1358. [Google Scholar] [CrossRef]
- Ma, Y.L.; Weston, S.E.; Whalley, B.J.; Stephens, G.J. The phytocannabinoid Δ9-tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. Br. J. Pharmacol. 2008, 154, 204–215. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef]
- Abioye, A.; Ayodele, O.; Marinkovic, A.; Patidar, R.; Akinwekomi, A.; Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A commentary on potential therapeutic benefit for the management of obesity and diabetes. J. Cannabis Res. 2020, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, D.; Costa, B.; Maione, S.; Comelli, F.; Marini, P.; Di Marzo, V.; Parolaro, D.; Ross, R.A.; Gauson, L.A.; Cascio, M.G.; et al. The plant cannabinoid Δ 9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br. J. Pharmacol. 2010, 160, 677–687. [Google Scholar] [CrossRef]
- Wargent, E.T.; Zaibi, M.S.; Silvestri, C.; Hislop, D.C.; Stocker, C.J.; Stott, C.G.; Guy, G.W.; Duncan, M.; Di Marzo, V.; Cawthorne, M.A. The cannabinoid Δ9-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutr. Diabetes 2013, 3, e68-10. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Grunfeld, Y.; Edery, H. Psychopharmacological activity of the active constituents of hashish and some related cannabinoids. Psychopharmacologia 1969, 14, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Goodwin, J.M.; Limebeer, C.L.; Breuer, A.; Pertwee, R.G.; Mechoulam, R.; Parker, L.A. Interaction between non-psychotropic cannabinoids in marihuana: Effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology 2011, 215, 505–512. [Google Scholar] [CrossRef]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef]
- Nadal, X.; del Río, C.; Casano, S.; Palomares, B.; Ferreiro-Vera, C.; Navarrete, C.; Sánchez-Carnerero, C.; Cantarero, I.; Bellido, M.L.; Meyer, S.; et al. Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br. J. Pharmacol. 2017, 174, 4263–4276. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; de Medina, V.S.; Rivas-Santisteban, R.; Callado, C.S.C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1-CB2 heteroreceptor complexes. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Ferré, S.; Ciruela, F.; Woods, A.S.; Lluis, C.; Franco, R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007, 30, 440–446. [Google Scholar] [CrossRef]
- Guiard, B.P.; Di Giovanni, G. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: The missing link? Front. Pharmacol. 2015, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nabbout, R.; Andrade, D.M.; Bahi-Buisson, N.; Cross, H.; Desquerre, I.; Dulac, O.; Granata, T.; Hirsch, E.; Navarro, V.; Ouss, L.; et al. Outcome of childhood-onset epilepsy from adolescence to adulthood: Transition issues. Epilepsy Behav. 2017, 69, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Wyska, E.; Szafarz, M.; Nieoczym, D.; Wlaź, P. Acute effect of cannabidiol on the activity of various novel antiepileptic drugs in the maximal electroshock- and 6 Hz-induced seizures in mice: Pharmacodynamic and pharmacokinetic studies. Neuropharmacology 2019, 158, 107733. [Google Scholar] [CrossRef]
- MARIN, S. The Impact of Epilepsy on the Adolescent. MCN Am. J. Matern. Nurs. 2005, 30, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2019, 18, 87–96. [Google Scholar] [CrossRef]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Ortega-Gutiérrez, S.; Van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef]
- Aguado, T. The Endocannabinoid System Promotes Astroglial Differentiation by Acting on Neural Progenitor Cells. J. Neurosci. 2006, 26, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Lim, D.A. For the Long Run. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Chiurchiù, V.; Díaz-Alonso, J.; Bari, M.; Guzmán, M.; Maccarrone, M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013, 52, 633–650. [Google Scholar] [CrossRef]
- Prenderville, J.A.; Kelly, Á.M.; Downer, E.J. The role of cannabinoids in adult neurogenesis. Br. J. Pharmacol. 2015, 172, 3950–3963. [Google Scholar] [CrossRef]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaça, M.V.; Aguiar, D.C.; Díaz-Alonso, J.; Ortega-Gutiérrez, S.; Vázquez-Villa, H.; Moreira, F.A.; Guzmán, M.; et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Marchalant, Y.; Rosi, S.; Wenk, G.L. Anti-inflammatory property of the cannabinoid agonist WIN-55212-2 in a rodent model of chronic brain inflammation. Neuroscience 2007, 144, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Marchalant, Y.; Brothers, H.M.; Wenk, G.L. Cannabinoid agonist WIN-55,212-2 partially restores neurogenesis in the aged rat brain. Mol. Psychiatry 2009, 14, 1068–1069. [Google Scholar] [CrossRef]
- Curia, G.; Lucchi, C.; Vinet, J.; Gualtieri, F.; Marinelli, C.; Torsello, A.; Costantino, L.; Biagini, G. Pathophysiogenesis of Mesial Temporal Lobe Epilepsy: Is Prevention of Damage Antiepileptogenic? Curr. Med. Chem. 2014, 21, 663–688. [Google Scholar] [CrossRef]
- Aguareles, J.; Paraíso-Luna, J.; Palomares, B.; Bajo-Grañeras, R.; Navarrete, C.; Ruiz-Calvo, A.; García-Rincón, D.; García-Taboada, E.; Guzmán, M.; Muñoz, E.; et al. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration. Transl. Neurodegener. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Pazos, M.R.; Tolón, R.M.; Moro, M.A.; Romero, J.; Lizasoain, I.; Martínez-Orgado, J. The Cannabinoid Agonist Win55212 Reduces Brain Damage in an In Vivo Model of Hypoxic-Ischemic Encephalopathy in Newborn Rats. Pediatr. Res. 2007, 62, 255–260. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Lafuente, H.; Carmen Rey-Santano, M.; Mielgo, V.E.; Gastiasoro, E.; Rueda, M.; Pertwee, R.G.; Castillo, A.I.; Romero, J.; Martínez-Orgado, J. Neuroprotective Effects of the Nonpsychoactive Cannabinoid Cannabidiol in Hypoxic-Ischemic Newborn Piglets. Pediatr. Res. 2008, 64, 653–658. [Google Scholar] [CrossRef]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Hallak, J.E.C.; Crippa, J.A.S. Neuropharmacological Effects of the Main Phytocannabinoids: A Narrative Review. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2021; Volume 1264, pp. 29–45. [Google Scholar] [CrossRef]
- Greco, M.; Varriale, G.; Coppola, G.; Operto, F.; Verrotti, A.; Iezzi, M.L. Investigational small molecules in phase II clinical trials for the treatment of epilepsy. Expert Opin. Investig. Drugs 2018, 27, 971–979. [Google Scholar] [CrossRef] [PubMed]

| Phytocannabinoid | Dose | Species | Effect | Traditional AED in Tandem | Reference |
|---|---|---|---|---|---|
Cannabidiol (CBD)
| 12 & 17 mg/kg | Rats | ↑ | ✓ | [81] |
| 60 mg/kg | Albino mice (m) | ↑ | ✗ | [82] | |
| 1, 10 & 100 mg/kg | Wistar rats (m) | ↑ | ✗ | [83] | |
| 2 μL (1 μL per side) | NMRI mice (m) | ↑ | ✗ | [78] | |
| 10, 20 & 50 mg/kg | Sprague-dawley rats (m) | ↑ | ✗ | [84] | |
| 200 mg/kg | Epilepsy Patient Volunteers | ↑ | ✓ | [89] |
| 200–300 mg/kg | Epilepsy Patients (Ages 14–49 years) | ↑ | ✗ | [90] | |
| 2–50 mg/kg | Epilepsy Patients (Ages 1–30 years) | ↑ | ✓ | [15] | |
| 20 mg/kg | Children and Young adults | ↑ | ✓ | [37] | |
| 10 & 20 mg/kg | Epilepsy Patients (Ages 2–55 years) | ↑ | ✓ | [38] | |
Cannabidivarin (CBDV)
| 50–200 mg/kg | DBA/2 mice, CD-1 mice & Wistar rats (m) | ↑ | ✓ | [91] |
| 50–422 mg/kg | Wistar rats, MF1 mice & DBA/2 mice (m) | ↑ | ✗ | [92] | |
| 400 mg/kg | Adult Epilepsy Patients | — | ✓ | [93] |
Delta-9-tetrahydrocannabivarin (
Δ9-THCV)
| 10–50 μM & 0.025–2.5 mg/kg | Wistar rats (m) | ↑ | ✗ | [94] |
Cannabigerol (CBG)
| 50–200 mg/kg | Wistar rats (m) | — | ✗ | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrelly, A.M.; Vlachou, S.; Grintzalis, K. Efficacy of Phytocannabinoids in Epilepsy Treatment: Novel Approaches and Recent Advances. Int. J. Environ. Res. Public Health 2021, 18, 3993. https://doi.org/10.3390/ijerph18083993
Farrelly AM, Vlachou S, Grintzalis K. Efficacy of Phytocannabinoids in Epilepsy Treatment: Novel Approaches and Recent Advances. International Journal of Environmental Research and Public Health. 2021; 18(8):3993. https://doi.org/10.3390/ijerph18083993
Chicago/Turabian StyleFarrelly, Aaron M., Styliani Vlachou, and Konstantinos Grintzalis. 2021. "Efficacy of Phytocannabinoids in Epilepsy Treatment: Novel Approaches and Recent Advances" International Journal of Environmental Research and Public Health 18, no. 8: 3993. https://doi.org/10.3390/ijerph18083993
APA StyleFarrelly, A. M., Vlachou, S., & Grintzalis, K. (2021). Efficacy of Phytocannabinoids in Epilepsy Treatment: Novel Approaches and Recent Advances. International Journal of Environmental Research and Public Health, 18(8), 3993. https://doi.org/10.3390/ijerph18083993







