Effects of Early Vocal Contact in the Neonatal Intensive Care Unit: Study Protocol for a Multi-Centre, Randomised Clinical Trial
Abstract
1. Background
1.1. Medical End Environmental Factors Lead the Preterm Infants’ Development
1.2. Atypical Auditory Environment in the NICU and Atypical Voice Treatment for Preterm Infants
1.3. Early Protective Interventions
1.4. EVC in the NICU Impacts Both the Auditory and Relational Environment
1.5. Aims
2. Hypothesis
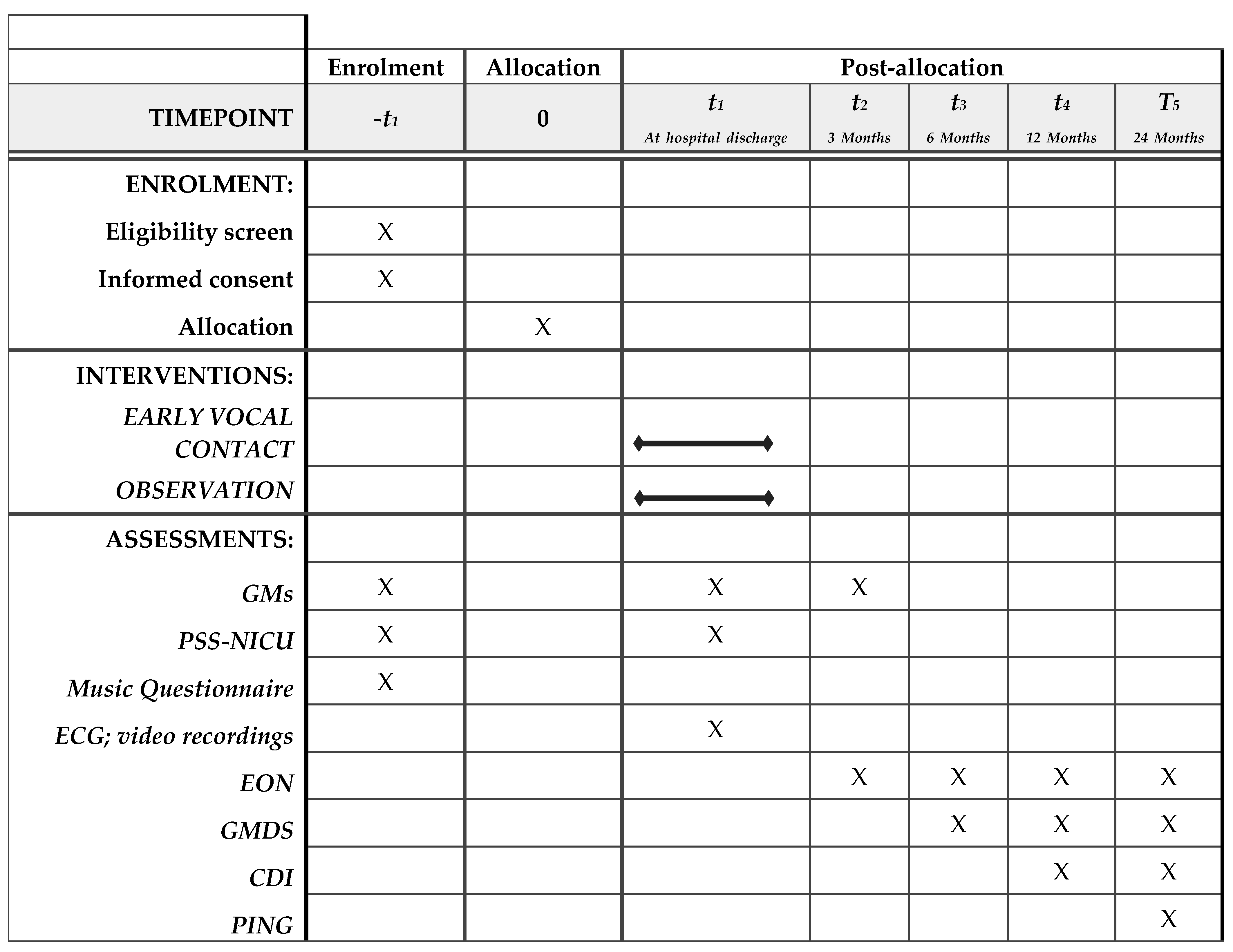
3. Methods
3.1. Design
3.2. Study Setting
3.3. Participants
3.4. Patient and Public Involvement Statement
3.5. Inclusion Criteria
3.6. Exclusion Criteria
3.7. Sample Size and Power Analysis
3.8. Randomisation
3.9. Intervention
3.10. Control Group
4. Measures
4.1. Baseline Measures
4.1.1. Infant’s Measures
4.1.2. Maternal Measures
4.1.3. Environmental Measures
5. Outcome Measures
5.1. Primary Outcome Measure
Physiological Level
5.2. Secondary Outcome Measures
5.2.1. Infant’s Measures
5.2.2. Maternal Measures
Stress Measures
Maternal Presence in the NICU
6. Blinding
7. Potential Confounds
8. Statistical Analyses
8.1. Primary Outcome
8.2. Secondary Outcomes
9. Data Management
10. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Protocol Version
List of Abbreviations
References
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Jarjour, I.T. Neurodevelopmental Outcome after Extreme Prematurity: A Review of the Literature. Pediatr. Neurol. 2015, 52, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Adams-Chapman, I.; Heyne, R.J.; DeMauro, S.B.; Duncan, A.F.; Hintz, S.R.; Pappas, A.; Vohr, B.R.; McDonald, S.A.; Das, A.; Newman, J.E.; et al. Neurodevelopmental Impairment Among Extremely Preterm Infants in the Neonatal Research Network. Pediatrics 2018, 141, e20173091. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.C.; Litt, J.S.; Smith, V.C.; Zupancic, J.A. Prematurity: An Overview and Public Health Implications. Annu. Rev. Public Health 2011, 32, 367–379. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Born Too Soon: The Global Action Report on Preterm Birth; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Anand, K.; Scalzo, F.M. Can Adverse Neonatal Experiences Alter Brain Development and Subsequent Behavior? Neonatology 2000, 77, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Grunau, R.E.; Holsti, L.; Peters, J.W. Long-term consequences of pain in human neonates. Semin. Fetal Neonatal Med. 2006, 11, 268–275. [Google Scholar] [CrossRef]
- Prechtl, H. The behavioural states of the newborn infant (A review). Brain Res. 1974, 76, 185–212. [Google Scholar] [CrossRef]
- Als, H. Newborn Individualized Developmental Care and Assessment Program (NIDCAP): New frontier for neonatal and perinatal medicine. J. Neonatal-Perinat. Med. 2009, 2, 135–147. [Google Scholar] [CrossRef]
- Roué, J.-M.; Kuhn, P.; Maestro, M.L.; Maastrup, R.A.; Mitanchez, D.; Westrup, B.; Sizun, J. Eight principles for patient-centred and family-centred care for newborns in the neonatal intensive care unit. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F364–F368. [Google Scholar] [CrossRef]
- Van den Hoogen, A. Putting the family at the centre of newborn health: EFCNI European Standards of Care for Newborn Health. Lancet Child Adolesc. Health 2019, 3, 1. [Google Scholar]
- Pineda, R.G.; Neil, J.; Dierker, D.; Smyser, C.D.; Wallendorf, M.; Kidokoro, H.; Reynolds, L.C.; Walker, S.; Rogers, C.; Mathur, A.M.; et al. Alterations in Brain Structure and Neurodevelopmental Outcome in Preterm Infants Hospitalized in Different Neonatal Intensive Care Unit Environments. J. Pediatr. 2014, 164, 52–60.e2. [Google Scholar] [CrossRef]
- Flacking, R.; Lehtonen, L.; Thomson, G.; Axelin, A.; Ahlqvist, S.; Moran, V.H.; Ewald, U.; Dykes, F.; SCENE Group. Closeness and separation in neonatal intensive care. Acta Paediatr. 2012, 101, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Sansavini, A.; Guarini, A.; Caselli, M.C. Preterm Birth: Neuropsychological Profiles and Atypical Developmental Pathways. Dev. Disabil. Res. Rev. 2011, 17, 102–113. [Google Scholar] [CrossRef]
- Heuvel, M.I.V.D.; Thomason, M.E. Functional Connectivity of the Human Brain in Utero. Trends Cogn. Sci. 2016, 20, 931–939. [Google Scholar] [CrossRef]
- Molliver, M.E.; Kostovic, I.; Van Der Loos, H. The development of synapses in cerebral cortex of the human fetus. Brain Res. 1973, 50, 403–407. [Google Scholar] [CrossRef]
- Lester, B.M.; Miller, R.J.; Hawes, K.; Salisbury, A.; Bigsby, R.; Sullivan, M.C.; Padbury, J.F. Infant Neurobehavioral Devel-opment. Semin. Perinatol. 2011, 35, 8–19. [Google Scholar] [CrossRef]
- Jones, T.A.; Greenough, W.T. Ultrastructural Evidence for Increased Contact between Astrocytes and Synapses in Rats Reared in a Complex Environment. Neurobiol. Learn. Mem. 1996, 65, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Greenough, W.T. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol. 2004, 1, 351–363. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Rabinowitz, N.C.; Willmore, B.D.; Schnupp, J.W.; King, A.J. Contrast Gain Control in Auditory Cortex. Neuron 2011, 70, 1178–1191. [Google Scholar] [CrossRef]
- Graven, S.N.; Browne, J.V. Auditory Development in the Fetus and Infant. Newborn Infant Nurs. Rev. 2008, 8, 187–193. [Google Scholar] [CrossRef]
- Ba, G.C.S.; Ba, J.G.; Smyser, C.; Pineda, R.; Newnham, C.; Tjoeng, T.H.; Vavasseur, C.; Wallendorf, M.; Neil, J.; Inder, T. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 2011, 70, 541–549. [Google Scholar] [CrossRef]
- Als, H.; Duffy, F.H.; McAnulty, G.B.; Rivkin, M.J.; Vajapeyam, S.; Mulkern, R.V.; Warfield, S.K.; Huppi, P.S.; Butler, S.C.; Conneman, N.; et al. Early Experience Alters Brain Function and Structure. Pediatrics 2004, 113, 846–857. [Google Scholar] [CrossRef] [PubMed]
- White, R.D.; on behalf of the Committee to Establish Recommended Standards for Newborn ICU Design; Smith, J.A.; Shepley, M.M. Recommended standards for newborn ICU design, eighth edition. J. Perinatol. 2013, 33, S2–S16. [Google Scholar] [CrossRef]
- Philbin, M.K.; Evans, J.B. Standards for the acoustic environment of the newborn ICU. J. Perinatol. 2006, 26, S27–S30. [Google Scholar] [CrossRef][Green Version]
- Graven, S.N. Sound and the Developing Infant in the NICU: Conclusions and Recommendations for Care. J. Perinatol. 2000, 20, S88–S93. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Zores, C.; Pebayle, T.; Hoeft, A.; Langlet, C.; Escande, B.; Astruc, D.; Dufour, A. Infants born very preterm react to variations of the acoustic environment in their incubator from a minimum signal-to-noise ratio threshold of 5 to 10 dBA. Pediatr. Res. 2012, 71, 386–392. [Google Scholar] [CrossRef]
- Gray, L.; Philbin, M. Effects of the neonatal intensive care unit on auditory attention and distraction. Clin. Perinatol. 2004, 31, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Bartha-Doering, L.; Alexopoulos, J.; Giordano, V.; Stelzer, L.; Kainz, T.; Benavides-Varela, S.; Wartenburger, I.; Klebermass-Schrehof, K.; Olischar, M.; Seidl, R.; et al. Absence of neural speech discrimination in preterm infants at term-equivalent age. Dev. Cogn. Neurosci. 2019, 39, 100679. [Google Scholar] [CrossRef] [PubMed]
- Adam-Darque, A.; Pittet, M.P.; Grouiller, F.; A Rihs, T.; Leuchter, R.H.-V.; Lazeyras, F.; Michel, C.M.; Hüppi, P.S. Neural Correlates of Voice Perception in Newborns and the Influence of Preterm Birth. Cereb. Cortex 2020, 30, 5717–5730. [Google Scholar] [CrossRef]
- Philbin, M.K. The sound environments and auditory perceptions of the fetus and preterm newborn. In Early Vocal Contact and Preterm Infant Brain Development; Springer: Berlin/Heridelberg, Germany, 2017; pp. 91–111. [Google Scholar]
- Browne, J.V. Recorded maternal voice, recorded music, or live intervention: A bioecological perspective. In Early Vocal Contact and Preterm Infant Brain Development; Springer: Berlin/Heidelberg, Germany, 2017; pp. 183–201. [Google Scholar]
- Ramey, C.T.; Ramey, S.L. Early intervention and early experience. Am. Psychol. 1998, 53, 109. [Google Scholar] [CrossRef]
- Vanderveen, J.A.; Bassler, D.; Robertson, C.M.T.; Kirpalani, H. Early interventions involving parents to improve neurodevelopmental outcomes of premature infants: A meta-analysis. J. Perinatol. 2009, 29, 343–351. [Google Scholar] [CrossRef]
- Ionio, C.; Lista, G.; Mascheroni, E.; Olivari, M.G.; Confalonieri, E.; Mastrangelo, M.; Brazzoduro, V.; Balestriero, M.A.; Banfi, A.; Bonanomi, A.; et al. Premature birth: Complexities and difficulties in building the mother–child relationship. J. Reprod. Infant Psychol. 2017, 35, 509–523. [Google Scholar] [CrossRef]
- Schore, A.N. Back to Basics: Attachment, Affect Regulation, and the Developing Right Brain: Linking Developmental Neuroscience to Pediatrics. Pediatr. Rev. 2005, 26, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Fernald, A. Intonation and communicative intent in mothers’ speech to infants: Is the melody the message? Child Dev. 1989, 60, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Schore, A.N. The effects of early relational trauma on right brain development, affect regulation, and infant mental health. Infant Ment. Health J. Off. Publ. World Assoc. Infant Ment. Health 2001, 22, 201–269. [Google Scholar] [CrossRef]
- Haslbeck, F.B. Music therapy for premature infants and their parents: An integrative review. Nord. J. Music Ther. 2012, 21, 203–226. [Google Scholar] [CrossRef]
- Shoemark, H. Time Together: A Feasible Program to Promote parent-infant Interaction in the NICU. Music Ther. Perspect. 2017, 36, 6–16. [Google Scholar] [CrossRef]
- Haslbeck, F.B. The interactive potential of creative music therapy with premature infants and their parents: A qualitative analysis. Nord. J. Music Ther. 2013, 23, 36–70. [Google Scholar] [CrossRef]
- Shoemark, H. Infant-Directed Singing as a Vehicle for Regulation Rehearsal in the Medically Fragile Full-Term Infant. Voices World Forum Music Ther. 2008, 8, 54. [Google Scholar] [CrossRef]
- Loewy, J.; Stewart, K.; Dassler, A.-M.; Telsey, A.; Homel, P. The Effects of Music Therapy on Vital Signs, Feeding, and Sleep in Premature Infants. Pediatrics 2013, 131, 902–918. [Google Scholar] [CrossRef]
- Shoemark, H. Translating ‘infant-directed singing’into a strategy for the hospitalized family. Music Ther. Parent-Infant Bond. 2011, 161–178. [Google Scholar]
- Haslbeck, F.; Hugoson, P. Sounding Together: Family-Centered Music Therapy as Facilitator for Parental Singing during Skin-to-Skin Contact. In Early Vocal Contact and Preterm Infant Brain Development; Springer: Berlin/Heidelberg, Germany, 2017; pp. 217–238. [Google Scholar]
- Loewy, J. NICU music therapy: Song of kin as critical lullaby in research and practice. Ann. N. Y. Acad. Sci. 2015, 1337, 178–185. [Google Scholar] [CrossRef]
- Feldman, R.; Rosenthal, Z.; Eidelman, A.I. Maternal-Preterm Skin-to-Skin Contact Enhances Child Physiologic Organization and Cognitive Control across the First 10 Years of Life. Biol. Psychiatry 2014, 75, 56–64. [Google Scholar] [CrossRef]
- Filippa, M.; Kuhn, P.; Westrup, B. Early Vocal Contact and Preterm Infant Brain Development; Springer International Publish-ing: Cham, Switzerland, 2017. [Google Scholar]
- Feldman, R.; Singer, M.; Zagoory, O. Touch attenuates infants’ physiological reactivity to stress. Dev. Sci. 2010, 13, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Filippa, M.; Devouche, E.; Arioni, C.; Imberty, M.; Gratier, M. Live maternal speech and singing have beneficial effects on hospitalized preterm infants. Acta Paediatr. 2013, 102, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M.; Stephens, B.; Tucker, R.; Vohr, B. Adult Talk in the NICU with Preterm Infants and Developmental Outcomes. Pediatrics 2014, 133, e578–e584. [Google Scholar] [CrossRef]
- Filippa, M.; Menin, D.; Panebianco, R.; Monaci, M.G.; Dondi, M.; Grandjean, D. Live Maternal Speech and Singing Increase Self-Touch and Eye-Opening in Preterm Newborns: A Preliminary Study. J. Nonverbal Behav. 2020, 44, 453–473. [Google Scholar] [CrossRef]
- Filippa, M.; Gratier, M.; Devouche, E.; Grandjean, D. Changes in infant-directed speech and song are related to preterm infant facial expression in the neonatal intensive care unit. Interact. Stud. 2018, 19, 427–444. [Google Scholar] [CrossRef]
- Saliba, S.; Gratier, M.; Filippa, M.; Devouche, E.; Esseily, R. Fathers’ and Mothers’ Infant Directed Speech Influences Preterm Infant Behavioral State in the NICU. J. Nonverbal Behav. 2020, 44, 1–15. [Google Scholar] [CrossRef]
- Saliba, S.; Esseily, R.; Filippa, M.; Gratier, M.; Grandjean, D. Changes in the vocal qualities of mothers and fathers are related to preterm infant’s behavioural states. Acta Paediatr. 2020, 109, 2271–2277. [Google Scholar] [CrossRef]
- Fyfe, K.L.; Yiallourou, S.R.; Wong, F.Y.; Odoi, A.; Walker, A.M.; Horne, R.S. The Effect of Gestational Age at Birth on Post-Term Maturation of Heart Rate Variability. Sleep 2015, 38, 1635–1644. [Google Scholar] [CrossRef]
- Arnon, S.; Diamant, C.; Bauer, S.; Regev, R.; Sirota, G.; Litmanovitz, I. Maternal singing during kangaroo care led to autonomic stability in preterm infants and reduced maternal anxiety. Acta Paediatr. 2014, 103, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W.; Davila, M.I.; Lewis, G.F.; Kolacz, J.; Okonmah-Obazee, S.; Hane, A.A.; Kwon, K.Y.; Ludwig, R.J.; Myers, M.M.; Welch, M.G. Autonomic regulation of preterm infants is enhanced by Family Nurture Intervention. Dev. Psychobiol. 2019, 61, 942–952. [Google Scholar] [CrossRef]
- Als, H.; Duffy, F.H.; McAnulty, G.; Butler, S.C.; Lightbody, L.; Kosta, S.; I Weisenfeld, N.; Robertson, R.; Parad, R.B.; A Ringer, S.; et al. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J. Perinatol. 2012, 32, 797–803. [Google Scholar] [CrossRef]
- Feldman, R.; Eidelman, A.I.; Sirota, L.; Weller, A. Comparison of Skin-to-Skin (Kangaroo) and Traditional Care: Parenting Outcomes and Preterm Infant Development. Pediatrics 2002, 110, 16–26. [Google Scholar] [CrossRef]
- Field, T.; Diego, M.; Hernandez-Reif, M. Preterm infant massage therapy research: A review. Infant Behav. Dev. 2010, 33, 115–124. [Google Scholar] [CrossRef]
- Benzies, K.M.; E Magill-Evans, J.; Hayden, K.A.; Ballantyne, M. Key components of early intervention programs for preterm infants and their parents: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2013, 13, S10. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.R.; Bergman, N.; Anderson, G.C.; Medley, N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst. Rev. 2016, 11, CD003519. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Jacobs, S.E. NIDCAP: A Systematic Review and Meta-analyses of Randomized Controlled Trials. Pediatrics 2013, 131, e881–e893. [Google Scholar] [CrossRef] [PubMed]
- Sansavini, A.; Guarini, A.; Savini, S.; Broccoli, S.; Justice, L.; Alessandroni, R.; Faldella, G. Longitudinal trajectories of gestural and linguistic abilities in very preterm infants in the second year of life. Neuropsychologia 2011, 49, 3677–3688. [Google Scholar] [CrossRef] [PubMed]
- Guarini, A.; Marini, A.; Savini, S.; Alessandroni, R.; Faldella, G.; Sansavini, A. Linguistic features in children born very preterm at preschool age. Dev. Med. Child Neurol. 2016, 58, 949–956. [Google Scholar] [CrossRef]
- Hofer, M.A. On the Nature and Consequences of Early Loss. Psychosom. Med. 1996, 58, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.A. The emerging neurobiology of attachment and separation: How parents shape their infant’s brain and behav-iour. In Relational Perspectives Book Series; Coates, S.W., Rosenthal, J.L., Schechter, D.S., Eds.; The Analytic Press/Taylor & Francis Group: Abigdon, UK, 2003; pp. 191–209. [Google Scholar]
- Westrup, B. Newborn Individualized Developmental Care and Assessment Program (NIDCAP)—Family-centered developmentally supportive care. Early Hum. Dev. 2007, 83, 443–449. [Google Scholar] [CrossRef]
- Miles, M.S.; Funk, S.G.; Carlson, J. Parental Stressor Scale: Neonatal intensive care unit. Nurs. Res. 1993, 42, 148–152. [Google Scholar] [CrossRef]
- Montirosso, R.; Provenzi, L.; Calciolari, G.; Borgatti, R.; NEO-ACQUA Study Group. Measuring maternal stress and perceived support in 25 Italian NICUs. Acta Paediatr. 2011, 101, 136–142. [Google Scholar] [CrossRef]
- Coutinho, E.; Scherer, K. Geneva Music Background Questionnaire (GEMUBAQ); Unpublished Report; Geneva, Switzerland, 2014. [Google Scholar]
- Ferrari, F.; Cioni, G.; Prechtl, H. Qualitative changes of general movements in preterm infants with brain lesions. Early Hum. Dev. 1990, 23, 193–231. [Google Scholar] [CrossRef]
- Griffiths, R. The Griffiths Mental Development Scales from Birth to 2 Years; Manual: The 1996 Revision; Association for Research in Infant and Child Development: London, UK, 1996. [Google Scholar]
- Fenson, L. MacArthur-Bates Communicative Development Inventories; Paul, H., Ed.; Brookes Publishing Company: Bal-timore, MD, USA, 2007. [Google Scholar]
- Caselli, M.C.; Bello, A.; Rinaldi, P.; Stefanini, S.; Pasqualetti, P. Il Primo Vocabolario del Bambino: Gesti, Parole e Frasi. Valori di Riferimento fra 8 e 36 mesi delle Forme Complete e delle Forme Brevi del Questionario; Angeli, F., Ed.; MacArthur-Bates CDI: Milan, Italy, 2015. [Google Scholar]
- Bello, A.; Caselli, M.C.; Pettenati, P.; Stefanini, S. PinG Parole in Gioco. Una Prova di Comprensione e Produzione Lessicale per la Prima Infanzia; Giunti, O.S., Ed.; Organizzazioni Speciali: Florence, Italy, 2010. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippa, M.; Della Casa, E.; D’amico, R.; Picciolini, O.; Lunardi, C.; Sansavini, A.; Ferrari, F. Effects of Early Vocal Contact in the Neonatal Intensive Care Unit: Study Protocol for a Multi-Centre, Randomised Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 3915. https://doi.org/10.3390/ijerph18083915
Filippa M, Della Casa E, D’amico R, Picciolini O, Lunardi C, Sansavini A, Ferrari F. Effects of Early Vocal Contact in the Neonatal Intensive Care Unit: Study Protocol for a Multi-Centre, Randomised Clinical Trial. International Journal of Environmental Research and Public Health. 2021; 18(8):3915. https://doi.org/10.3390/ijerph18083915
Chicago/Turabian StyleFilippa, Manuela, Elisa Della Casa, Roberto D’amico, Odoardo Picciolini, Clara Lunardi, Alessandra Sansavini, and Fabrizio Ferrari. 2021. "Effects of Early Vocal Contact in the Neonatal Intensive Care Unit: Study Protocol for a Multi-Centre, Randomised Clinical Trial" International Journal of Environmental Research and Public Health 18, no. 8: 3915. https://doi.org/10.3390/ijerph18083915
APA StyleFilippa, M., Della Casa, E., D’amico, R., Picciolini, O., Lunardi, C., Sansavini, A., & Ferrari, F. (2021). Effects of Early Vocal Contact in the Neonatal Intensive Care Unit: Study Protocol for a Multi-Centre, Randomised Clinical Trial. International Journal of Environmental Research and Public Health, 18(8), 3915. https://doi.org/10.3390/ijerph18083915





