Epicardial Adipose Tissue and Cardiovascular Risk Assessment in Ultra-Marathon Runners: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
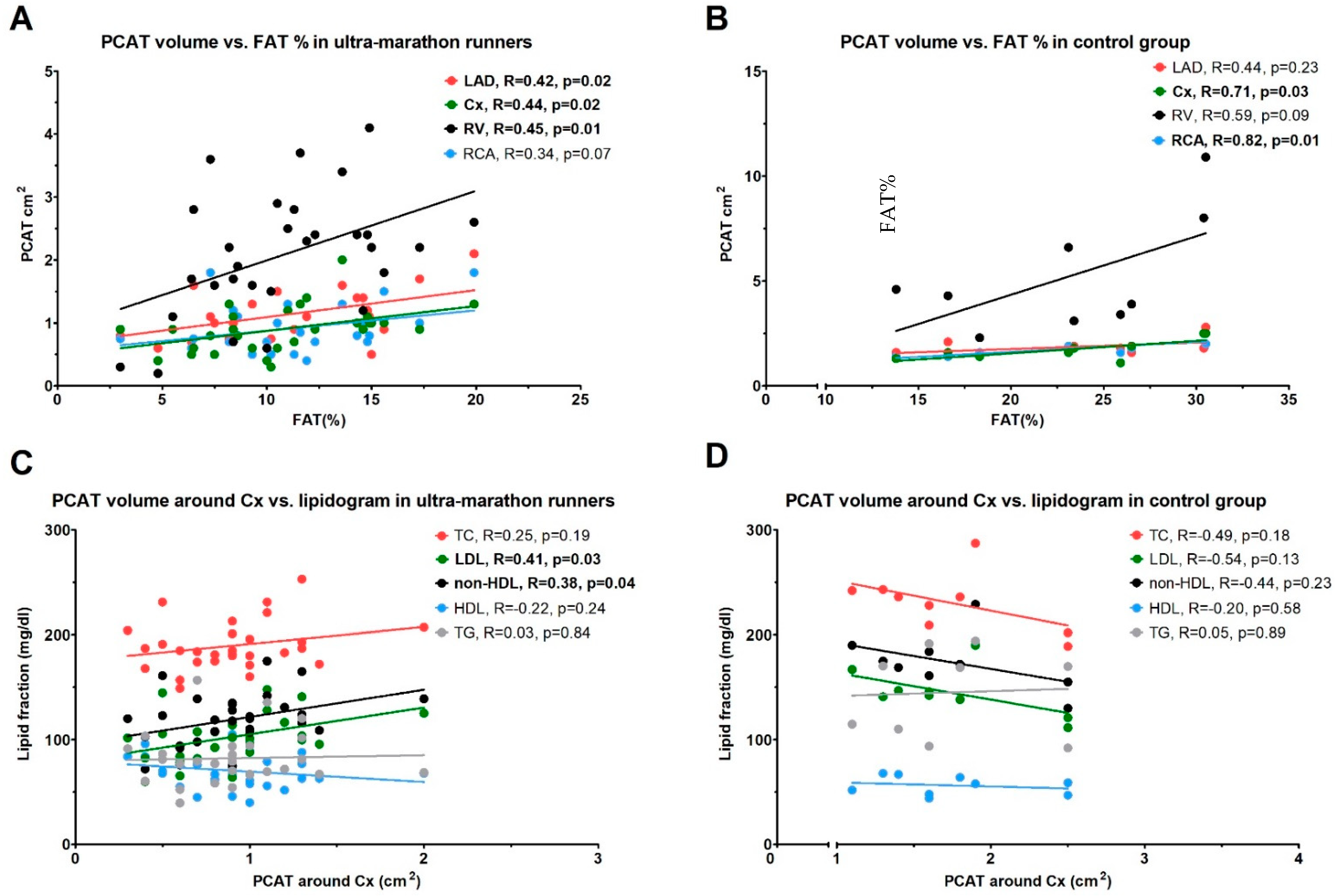
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laslett, L.J.; Alagona, P., Jr.; Clark, B.A., 3rd; Drozda, J.P., Jr.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60 (Suppl. 25), 1–49. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Roth, G.A.; Huffman, M.D.; Moran, A.E.; Feigin, V.; Mensah, G.A.; Naghavi, M.; Murray, C.J. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015, 132, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Interheart Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.; Lemieux, I.; Despres, J.P. Obesity, inflammation, and cardiovascular risk. Clin. Pharmacol. Ther. 2010, 87, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W. Epicardial fat: Properties, function and relationship to obesity. Obes. Rev. 2007, 8, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Willens, H.J.; Barbaro, G.; Sharma, A.M. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity 2008, 16, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.; Berg, M.H.; Lehmann, N.; Kälsch, H.; Bauer, M.; Kara, K.; Dragano, N.; Moebus, S.; Jockel, K.-H.; Erbel, R.; et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population. J. Am. Coll. Cardiol. 2013, 61, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Fain, J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef]
- Erdogan, T.; Durakoglukil, M.E.; Cetin, M.; Altan Kocaman, S.; Duman, H.; Çiçek, Y.; Şatıroğlu, Ö. Epicardial Adipose tissue predicts carotid intima-media thickness independently of body mass index and waist circumference. Acta Cardiol. Sin. 2019, 35, 32–41. [Google Scholar]
- Mazurek, T. Proinflammatory capacity of adipose tissue: New insights in the pathophysiology of atherosclerosis. Kardiol. Pol. 2009, 67, 1119–1124. [Google Scholar]
- Mazurek, T.; Opolski, G. Pericoronary adipose tissue: A novel therapeutic target in obesity-related coronary atherosclerosis. J. Am. Coll. Nutr. 2015, 34, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Hyde, R.T.; Wing, A.L.; Lee, I.M.; Jung, D.L.; Kampert, J.B. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N. Engl. J. Med. 1993, 328, 538–545. [Google Scholar] [CrossRef]
- Leon, A.S.; Connett, J.; Jacobs, D.R., Jr.; Rauramaa, R. Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. JAMA 1987, 258, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Sui, X.; Artero, E.G.; Lee, I.M.; Church, T.S.; McAuley, P.A.; Stanford, F.C.; Kohl, H.W., 3rd; Blair, S.N. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: The Aerobics Center Longitudinal Study. Circulation 2011, 124, 2483–2490. [Google Scholar] [CrossRef]
- Kubota, Y.; Evenson, K.R.; Maclehose, R.F.; Roetker, N.S.; Joshu, C.E.; Folsom, A.R. Physical Activity and Lifetime Risk of Cardiovascular Disease and Cancer. Med. Sci. Sports Exerc. 2017, 49, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, K.; Żmijewski, P.; Kozdroń, E.; Fojt, A.; Czajkowska, A.; Szczypiorski, P.; Mazurek, T. Cardiovascular risk reduction in sedentary postmenopausal women during organized physical activity. Kardiol. Pol. 2017, 75, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Małek, Ł.A.; Barczuk-Falęcka, M.; Werys, K.; Czajkowska, A.; Mróz, A.; Witek, K.; Burrage, M.; Bakalarski, W.; Nowicki, D.; Roik, D.; et al. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur. J. Radiol. 2019, 117, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Nieves, D.J.; Cnop, M.; Retzlaff, B.; Walden, C.E.; Brunzell, J.D.; Knopp, R.H.; Kahn, S.E. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 2003, 52, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, M.; Wojciechowska, M.; Zarębiński, M.; Cudnoch-Jędrzejewska, A.; Mazurek, T. Adiponectin promotes ischemic heart preconditioning–PRO and CON. Cytokine 2020, 127, 154981. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, M.; Penttila, A.; Kovanen, P.T. Mast cells accompany microvessels in human coronary atheromas: Implications for intimal neovascularisation and hemorrhage. Atherosclerosis 1996, 123, 123–131. [Google Scholar] [CrossRef]
- Yamashita, K.; Yamamoto, M.H.; Igawa, W.; Ono, M.; Kido, T.; Ebara, S.; Okabe, T.; Saito, S.; Amemiya, K.; Isomura, N.; et al. Association of Epicardial Adipose Tissue Volume and Total Coronary Plaque Burden in Patients with Coronary Artery Disease. Int. Heart J. 2018, 59, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Kochman, J.; Kobylecka, M.; Wilimski, R.; Filipiak, K.; Krolicki, L.; Opolski, G. Inflammatory activity of pericoronary adipose tissue may affect plaque composition in patients with acute coronary syndrome without persistent ST-segment elevation: Preliminary results. Kardiol. Pol. 2013, 72, 410–416. [Google Scholar] [CrossRef]
- Thanassoulis, G.; Massaro, J.M.; O’Donnell, C.J.; Hoffmann, U.; Levy, D.; Ellinor, P.T.; Wang, T.J.; Schnabel, R.B.; Vasan, R.S.; Fox, C.S.; et al. Pericardial fat is associated with prevalent atrial fibrillation: The Framingham Heart Study. Circ. Arrhythmia Electrophysiol. 2010, 3, 345–350. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Tamarappoo, B.K.; Hachamovitch, R.; Kwon, D.H.; Alraies, M.C.; Halliburton, S.; Schoenhagen, P.; Dey, D.; Berman, D.S.; Marwick, T.H. Association of epicardial fat, hypertension, subclinical coronary artery disease, and metabolic syndrome with left ventricular diastolic dysfunction. Am. J. Cardiol. 2012, 110, 1793–1798. [Google Scholar] [CrossRef]
- Rado, S.D.; Lorbeer, R.; Gatidis, S.; Machann, J.; Storz, C.; Nikolaou, K.; Rathmann, W.; Hoffmann, U.; Peters, A.; Bamberg, F.; et al. MRI-based assessment and characterization of epicardial and paracardial fat depots in the context of impaired glucose metabolism and subclinical left-ventricular alterations. Br. J. Radiol. 2019, 92, 20180562. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, Y.; Bulut, A. Epicardial fat thickness is significantly increased and related to LDL cholesterol level in patients with familial hypercholesterolemia. J. Ultrasound 2019, 22, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Natale, F.; Tedesco, M.A.; Mocerino, R.; de Simone, V.; Di Marco, G.M.; Aronne, L.; Credendino, M.; Siniscalchi, C.; Calabrò, P.; Cotrufo, M.; et al. Visceral adiposity and arterial stiffness: Echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur. J. Echocardiogr. 2009, 10, 549–555. [Google Scholar] [CrossRef]
- Sengul, C.; Cevik, C.; Ozveren, O.; Oduncu, V.; Sunbul, A.; Akgun, T.; Can, M.M.; Semiz, E.; Dindar, I. Echocardiographic epicardial fat thickness is associated with carotid intima-media thickness in patients with metabolic syndrome. Echocardiography 2011, 28, 853–858. [Google Scholar] [CrossRef]
- Barros, E.S.; Nascimento, D.C.; Prestes, J.; Nóbrega, O.T.; Córdova, C.; Sousa, F.; Boullosa, D.A. Acute and Chronic Effects of Endurance Running on Inflammatory Markers: A Systematic Review. Front. Physiol. 2017, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Schmidt-Trucksäss, A.; Brugger, N.; Schäfer, D.; Saner, H.; Wilhelm, M. Ultra-endurance sports have no negative impact on indices of arterial stiffness. Eur. J. Appl. Physiol. 2014, 114, 49–57. [Google Scholar] [CrossRef]
- Nickel, T.; Emslander, I.; Sisic, Z.; David, R.; Schmaderer, C.; Marx, N.; Schmidt-Trucksäss, A.; Hoster, E.; Halle, M.; Weis, M.; et al. Modulation of dendritic cells and toll-like receptors by marathon running. Eur. J. Appl. Physiol. 2012, 112, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
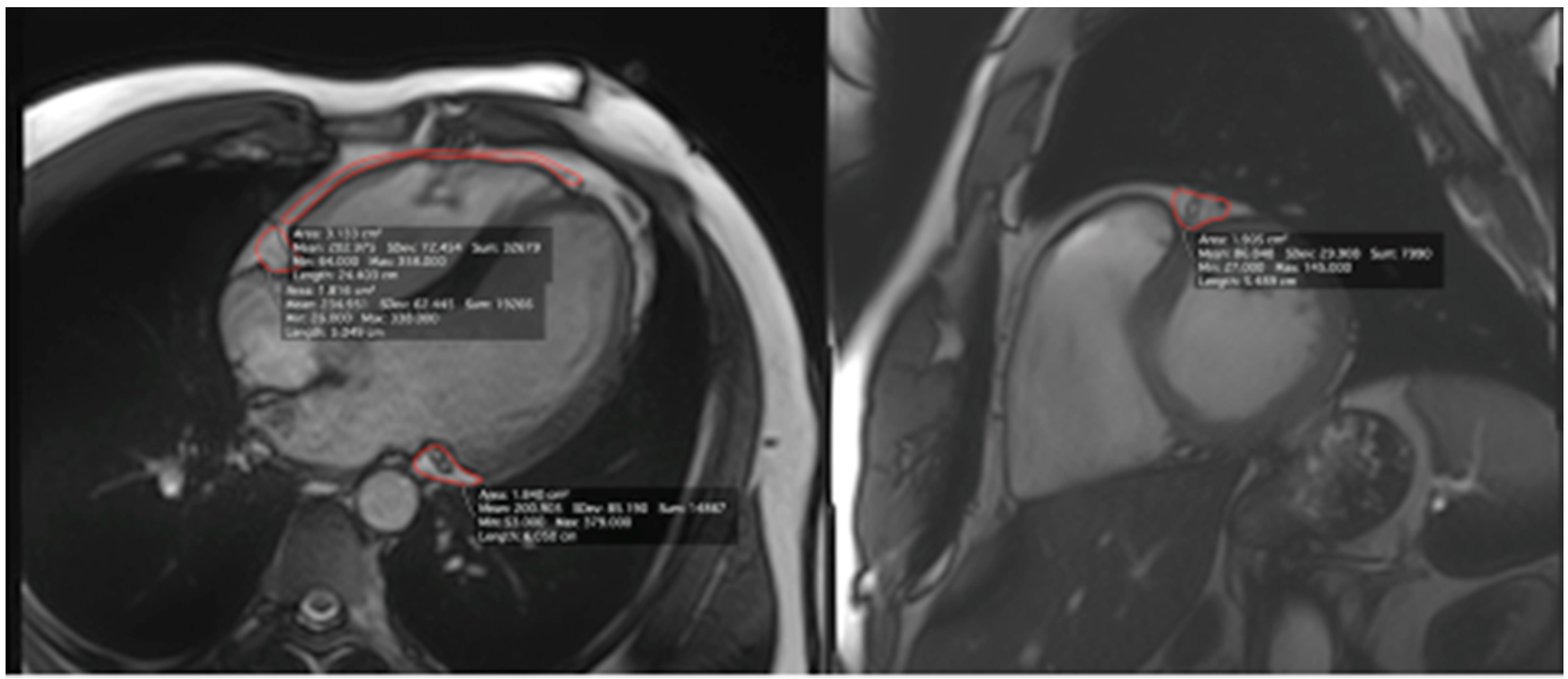
| Parameter | Ultra-Marathon Runners n = 30, Median (IQR) |
|---|---|
| Years of running | 9 (7–15) |
| Age at start of ultra-running | 34 (29–39) |
| Total covered distance (km) | 25,000 (20,000-40,000) |
| Weekly running distance (km) | 80 (70–90) |
| Number of ultra-races completed | 15 (10–27.5) |
| Number of ultra-races during last 2 years | 5.5 (4–9) |
| Number of completed ultra-races >100 km | 3.5 (2–7) |
| Best place achieved in an ultra-race | 5 (1–13) |
| Baseline Characteristics | |||
| Parameter | Ultra-marathon Runners (n = 30) | Control Group (n = 9) | p |
| Age (years) | 40.93 ± 6.57 | 40.78 ± 8.32 | 0.95 |
| Heigth (cm) | 172.02 ± 32.49 | 179.89 ± 6.43 | 0.48 |
| Weight (kg) | 72.73 ± 5.19 | 96.10 ± 19.39 | <0.001 |
| BMI (cm/m2) | 23.09 ± 1.54 | 30.00 ± 5.41 | <0.001 |
| FAT % (%) | 10.78 ± 4.01 | 23.17 ± 5.91 | <0.001 |
| FAT mass (kg) | 8.02 ± 3.32 | 23.16 ± 10.16 | <0.001 |
| FFM (kg) | 65.30 ± 3.44 | 74.34 ± 14.86 | 0.004 |
| TBW (kg) | 47.81 ± 2.52 | 53.40 ± 7.17 | <0.001 |
| Blood Test Results | |||
| Parameter | Ultra-marathon Runners (n = 30) | Control Group (n = 9) | p |
| TC (mg/dl) | 189.66 ± 23.38 | 230.22 ± 28.60 | <0.001 |
| HDL (mg/dl) | 70.52 ± 15.96 | 56.33 ± 8.99 | 0.016 |
| LDL (mg/dl) | 102.68 ± 22.45 | 144.86 ± 23.12 | <0.001 |
| non-HDL (mg/dl) | 119.14 ± 25.07 | 173.89 ± 27.10 | <0.001 |
| TG (mg/dl) | 82.27 ± 24.73 | 145.07 ± 41.82 | <0.001 |
| IL-6 (pg/mL) | 1.29 ± 0.75 | 1.70 ± 0.72 | 0.156 |
| Results of Imaging Tests | |||
| Cardiac Magnetic Resonance | |||
| Parameter | Ultra-marathon Runners (n = 30) | Control Group (n = 9) | p |
| LAD (cm2) | 1.12 ± 0.4 | 1.86 ± 0.41 | <0.001 |
| RCA (cm2) | 0.88 ± 0.39 | 1.78 ± 0.34 | <0.001 |
| Cx (cm2) | 0.90 ± 0.36 | 1.74 ± 0.49 | <0.001 |
| RV (cm2) | 2.07 ± 0.97 | 5.23 ± 2.77 | <0.001 |
| Ultrasonography | |||
| Left IMT (cm) | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.99 |
| Right IMT (cm) | 0.08 ± 0.03 | 0.08 ± 0.01 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konwerski, M.; Postuła, M.; Barczuk-Falęcka, M.; Czajkowska, A.; Mróz, A.; Witek, K.; Bakalarski, W.; Gąsecka, A.; Małek, Ł.A.; Mazurek, T. Epicardial Adipose Tissue and Cardiovascular Risk Assessment in Ultra-Marathon Runners: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3136. https://doi.org/10.3390/ijerph18063136
Konwerski M, Postuła M, Barczuk-Falęcka M, Czajkowska A, Mróz A, Witek K, Bakalarski W, Gąsecka A, Małek ŁA, Mazurek T. Epicardial Adipose Tissue and Cardiovascular Risk Assessment in Ultra-Marathon Runners: A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(6):3136. https://doi.org/10.3390/ijerph18063136
Chicago/Turabian StyleKonwerski, Michał, Marek Postuła, Marzena Barczuk-Falęcka, Anna Czajkowska, Anna Mróz, Katarzyna Witek, Wawrzyniec Bakalarski, Aleksandra Gąsecka, Łukasz A. Małek, and Tomasz Mazurek. 2021. "Epicardial Adipose Tissue and Cardiovascular Risk Assessment in Ultra-Marathon Runners: A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 6: 3136. https://doi.org/10.3390/ijerph18063136
APA StyleKonwerski, M., Postuła, M., Barczuk-Falęcka, M., Czajkowska, A., Mróz, A., Witek, K., Bakalarski, W., Gąsecka, A., Małek, Ł. A., & Mazurek, T. (2021). Epicardial Adipose Tissue and Cardiovascular Risk Assessment in Ultra-Marathon Runners: A Pilot Study. International Journal of Environmental Research and Public Health, 18(6), 3136. https://doi.org/10.3390/ijerph18063136









