An Emerging Syndemic of Smoking and Cardiopulmonary Diseases in People Living with HIV in Africa
Abstract
1. Introduction
| Country | Official Population [6] | PWH [7] | Smoking Prevalence among Adults (Non-PWH) | Smoking Prevalence among PWH |
|---|---|---|---|---|
| Nigeria | 214 million | 1.9 million | 10.40% [8] | 22.10% [8] |
| South Africa | 56.5 million | 7.7 million | 17.60% [9] | 24.88% [10] |
| Uganda | 43.3 million | 1.4 million | 7.40% [11] | 10.00% [12] |
1.1. Smoking Prevalence Is Higher among PWH in Nigeria, South Africa, and Uganda and Is Associated with Negative Health Outcomes
1.2. Prevalence of Hypertension, COPD, and CAD Could Be Elevated among PWH Who Smoke, but Data Are Lacking
| Country | CAD Prevalence among Adults (Non-PWH) | CAD Prevalence PWH | COPD Prevalence among Adults (Non-PWH) | COPD Prevalence PWH | HTN Prevalence among Adults (Non-PWH) | HTN Prevalence PWH |
|---|---|---|---|---|---|---|
| Nigeria | 1.6% to 3.4% [57,58] | NA | 7.70% [52] | 22.19% to 15.4% [51] | 28.90% [42] | 46.0% [43] |
| South Africa | 11% [58] | NA | 16.7% to 22.20% [59] | 8.00% to 9.8% [60,61] | >40% [45] | 38.6% [44] |
| Uganda | 4.02% [62] | NA | 6.20% [63] | 3.7% to 10.4% [64] | 26.40% [54] | 10% to 11% [46,47] |
1.3. Inflammation Underlies Cardiopulmonary Diseases in PWH
1.4. The Syndemic Burden for PWH and the Economic Impact of Not Addressing Comorbid Conditions
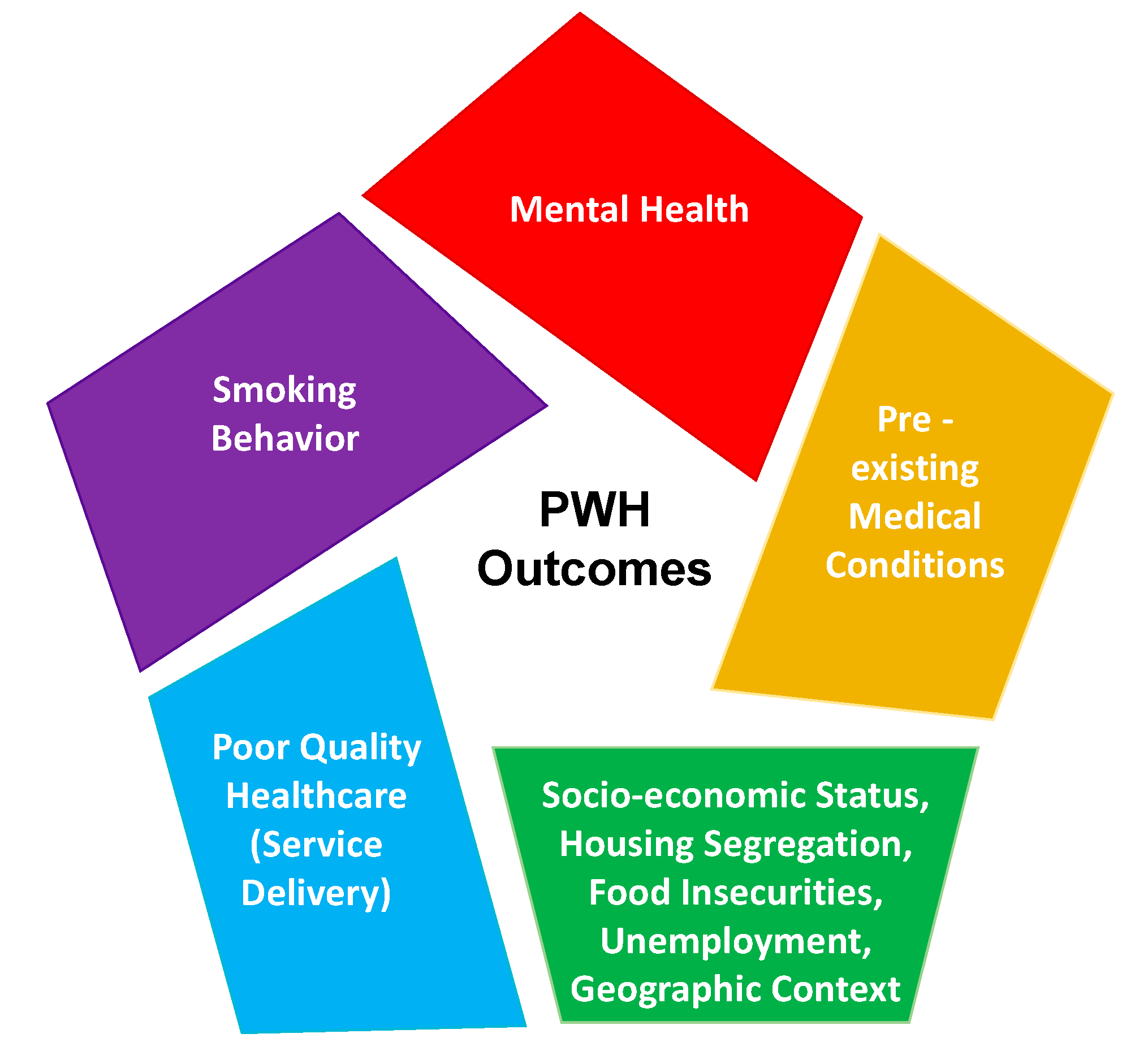
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kharsany, A.B.; Karim, Q.A. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016, 10, 34–48. [Google Scholar] [CrossRef]
- UNAIDS. Global HIV & AIDS Statistics—2020 Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 29 July 2020).
- UNAIDS. UNAIDS Country South Africa. Available online: https://www.unaids.org/en/regionscountries/countries/southafrica (accessed on 16 March 2020).
- Forum, W.E. Mapped: The Median Age of the Population on Every Continent. Available online: https://www.weforum.org/agenda/2019/02/mapped-the-median-age-of-the-population-on-every-continent/ (accessed on 25 August 2020).
- Foundation BaMG. The GoalKeepers Report. Available online: https://www.gatesfoundation.org/goalkeepers/report/2018-report/ (accessed on 25 August 2020).
- Bureau UC. Available online: https://www.census.gov (accessed on 26 August 2020).
- UNAIDS. Available online: https://www.unaids.org/en/regionscountries/countries (accessed on 26 August 2020).
- Adeloye, D.; Auta, A.; Fawibe, A. Current prevalence pattern of tobacco smoking in Nigeria: A systematic review and meta-analysis. BMC Public Health 2019, 19, 1719. [Google Scholar] [CrossRef]
- Reddy, P.; Zuma, K.; Shisana, O.; Kim, J.; Sewpaul, R. Prevalence of tobacco use among adults in South Africa: Results from the first South African National Health and Nutrition Examination Survey. S. Afr. Med. J. 2015, 105, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Elf, J.L.; Variava, E.; Chon, S. Prevalence and Correlates of Smoking Among People Living with HIV in South Africa. Nicotine Tob. Res. 2017, 20, 1124–1131. [Google Scholar] [CrossRef]
- Kabwama, S.N.; Ndyanabangi, S.; Mutungi, G.; Wesonga, R.; Bahendeka, S.K.; Guwatudde, D. Tobacco use and associated factors among Adults in Uganda: Findings from a nationwide survey. Tob. Induc. Dis. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Kruse, G.R.; Bangsberg, D.R.; Hahn, J.A. Tobacco use among adults initiating treatment for HIV infection in rural Uganda. AIDS Behav. 2014, 18, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.H.; Sande, E.; Ogwal, M. Progress toward UNAIDS 90-90-90 targets: A respondent-driven survey among female sex workers in Kampala, Uganda. PLoS ONE 2018, 13, e0201352. [Google Scholar] [CrossRef] [PubMed]
- Nachega, J.B.; Sam-Agudu, N.A.; Mofenson, L.M.; Schechter, M.; Mellors, J.W. Achieving Viral Suppression in 90% of People Living with Human Immunodeficiency Virus on Antiretroviral Therapy in Low- and Middle-Income Countries: Progress, Challenges, and Opportunities. Clin. Infect. Dis. 2018, 66, 1487–1491. [Google Scholar] [CrossRef]
- Green, D.; Tordoff, D.M.; Kharono, B. Evidence of sociodemographic heterogeneity across the HIV treatment cascade and progress towards 90-90-90 in sub-Saharan Africa—A systematic review and meta-analysis. J. Int. AIDS Soc. 2020, 23, e25470. [Google Scholar] [CrossRef]
- UNAIDS. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Available online: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf (accessed on 25 August 2020).
- Justman, J.E.; Mugurungi, O.; El-Sadr, W.M. HIV Population Surveys—Bringing Precision to the Global Response. N. Engl. J. Med. 2018, 378, 1859–1861. [Google Scholar] [CrossRef]
- Brathwaite, R.; Addo, J.; Smeeth, L.; Lock, K. A Systematic Review of Tobacco Smoking Prevalence and Description of Tobacco Control Strategies in Sub-Saharan African Countries; 2007 to 2014. PLoS ONE 2015, 10, e0132401. [Google Scholar] [CrossRef]
- GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- Pacek, L.R.; Cioe, P.A. Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living with HIV. Curr. HIV/AIDS Rep. 2015, 12, 413–420. [Google Scholar] [CrossRef]
- Desalu, O.O.; Oluboyo, P.O.; Olokoba, A.B. Prevalence and determinants of tobacco smoking among HIV patients in North Eastern Nigeria. Afr. J. Med. Med Sci. 2009, 38, 103–108. [Google Scholar]
- Murphy, J.D.; Liu, B.; Parascandola, M. Smoking and HIV in Sub-Saharan Africa: A 25-Country Analysis of the Demographic Health Surveys. Nicotine Tob. Res. 2019, 21, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Page-Shafer, K.; Chin, D.P. Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care STDs 2001, 15, 615–624. [Google Scholar] [CrossRef]
- Reynolds, N.R. Cigarette smoking and HIV: More evidence for action. AIDS Educ. Prev. 2009, 21 (Suppl. 3), 106–121. [Google Scholar] [CrossRef]
- Jha, P. The hazards of smoking and the benefits of cessation: A critical summation of the epidemiological evidence in high-income countries. eLife 2020, 9, 9. [Google Scholar] [CrossRef]
- Ogbo, F.A.; Ogeleka, P.; Okoro, A. Tuberculosis disease burden and attributable risk factors in Nigeria, 1990–2016. Trop. Med. Health 2018, 46, 34. [Google Scholar] [CrossRef]
- Gunasekera, K.; Cohen, T.; Gao, W.; Ayles, H.; Godfrey-Faussett, P.; Claassens, M. Smoking and HIV associated with subclinical tuberculosis: Analysis of a population-based prevalence survey. Int. J. Tuberc. Lung Dis. 2020, 24, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Muttamba, W.; Kirenga, B.; Ssengooba, W.; Sekibira, R.; Katamba, A.; Joloba, M.L. Prevalence of Tuberculosis Risk Factors among Bacteriologically Negative and Bacteriologically Confirmed Tuberculosis Patients from Five Regional Referral Hospitals in Uganda. Am. J. Trop. Med. Hyg. 2019, 100, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Bronner Murrison, L.; Martinson, N.; Moloney, R.M. Tobacco Smoking and Tuberculosis among Men Living with HIV in Johannesburg, South Africa: A Case-Control Study. PLoS ONE 2016, 11, e0167133. [Google Scholar] [CrossRef] [PubMed]
- Steel, H.C.; Venter, W.D.F.; Theron, A.J. Effects of Tobacco Usage and Antiretroviral Therapy on Biomarkers of Systemic Immune Activation in HIV-Infected Participants. Mediat. Inflamm. 2018, 2018, 8357109. [Google Scholar] [CrossRef] [PubMed]
- Pollack, T.M.; Duong, H.T.; Pham, T.T.; Do, C.D.; Colby, D. Cigarette smoking is associated with high HIV viral load among adults presenting for antiretroviral therapy in Vietnam. PLoS ONE 2017, 12, e0173534. [Google Scholar] [CrossRef]
- Lagathu, C.; Cossarizza, A.; Bereziat, V.; Nasi, M.; Capeau, J.; Pinti, M. Basic science and pathogenesis of ageing with HIV: Potential mechanisms and biomarkers. Aids 2017, 31 (Suppl. 2), S105–S119. [Google Scholar] [CrossRef] [PubMed]
- Hyle, E.P.; Mayosi, B.M.; Middelkoop, K. The association between HIV and atherosclerotic cardiovascular disease in sub-Saharan Africa: A systematic review. BMC Public Health 2017, 17, 954. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Stelzle, D.; Lee, K.K. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation 2018, 138, 1100–1112. [Google Scholar] [CrossRef]
- Kibirige, D.; Sanya, R.E.; Nantanda, R.; Worodria, W.; Kirenga, B. Availability and affordability of medicines and diagnostic tests recommended for management of asthma and chronic obstructive pulmonary disease in sub-Saharan Africa: A systematic review. Allergy Asthma Clin. Immunol. 2019, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Finney, L.J.; Feary, J.R.; Leonardi-Bee, J.; Gordon, S.B.; Mortimer, K. Chronic obstructive pulmonary disease in sub-Saharan Africa: A systematic review. Int. J. Tuberc. Lung Dis. 2013, 17, 583–589. [Google Scholar] [CrossRef]
- Ortblad, K.F.; Baeten, J.M.; Cherutich, P.; Wamicwe, J.N.; Wasserheit, J.N. The arc of HIV epidemics in sub-Saharan Africa: New challenges with concentrating epidemics in the era of 90-90-90. Curr. Opin. HIV AIDS 2019, 14, 354–365. [Google Scholar] [CrossRef]
- WHO. A Global Brief on Hypertension Silent Killer, Global Public Health Crisis. Available online: https://apps.who.int/iris/bitstream/handle/10665/79059/WHO_DCO_WHD_2013.2_eng.pdf;jsessionid=96EAD2203EDE2EA7E6CFA57014B13B53?sequence=1 (accessed on 20 August 2020).
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Seedat, Y.; Ali, A.; Ferdinand, K.C. Hypertension and cardiovascular disease in the sub-Saharan African context. Ann. Transl. Med. 2018, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Amegah, A.K. Tackling the Growing Burden of Cardiovascular Diseases in Sub-Saharan Africa. Circulation 2018, 138, 2449–2451. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Basquill, C.; Aderemi, A.V.; Thompson, J.Y.; Obi, F.A. An estimate of the prevalence of hypertension in Nigeria: A systematic review and meta-analysis. J. Hypertens. 2015, 33, 230–242. [Google Scholar] [CrossRef]
- Amusa, G.; Awokola, B.; Isiguzo, G. OS 37-02 Hypertension in HIV-Infected Adults in North-Central Nigeria: Prevalence, Associated Risk Factors and Assessment of Risk Using the Framingham Risk Score. J. Hypertens. 2016, 34, e405. [Google Scholar] [CrossRef]
- Mutemwa, M.; Peer, N.; de Villiers, A. Prevalence, detection, treatment, and control of hypertension in human immunodeficiency virus (HIV)-infected patients attending HIV clinics in the Western Cape Province, South Africa. Medicine 2018, 97, 35. [Google Scholar] [CrossRef]
- Gómez-Olivé, F.X.; Ali, S.A.; Made, F. Regional and Sex Differences in the Prevalence and Awareness of Hypertension: An H3Africa AWI-Gen Study Across 6 Sites in Sub-Saharan Africa. Glob. Heart 2017, 12, 81–90. [Google Scholar] [CrossRef]
- Kwarisiima, D.; Atukunda, M.; Owaraganise, A. Hypertension control in integrated HIV and chronic disease clinics in Uganda in the SEARCH study. BMC Public Health 2019, 19, 511. [Google Scholar] [CrossRef]
- Kwarisiima, D.; Balzer, L.; Heller, D. Population-Based Assessment of Hypertension Epidemiology and Risk Factors among HIV-Positive and General Populations in Rural Uganda. PLoS ONE 2016, 11, e0156309. [Google Scholar] [CrossRef]
- Hyle, E.P.; Bekker, L.-G.; Martey, E.B. Cardiovascular risk factors among ART-experienced people with HIV in South Africa. J. Int. AIDS Soc. 2019, 22, e25274. [Google Scholar] [CrossRef]
- (CDC) CfDCaP. Health Effects of Cigarette Smoking. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm (accessed on 13 March 2020).
- WHO. Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 13 March 2020).
- Akanbi, M.O.; Taiwo, B.O.; Achenbach, C.J. HIV Associated Chronic Obstructive Pulmonary Disease in Nigeria. J. AIDS Clin. Res. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Obaseki, D.O.; Erhabor, G.E.; Gnatiuc, L.; Adewole, O.O.; Buist, S.A.; Burney, P.G. Chronic Airflow Obstruction in a Black African Population: Results of BOLD Study, Ile-Ife, Nigeria. COPD 2016, 13, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Siddharthan, T.; Grigsby, M.; Morgan, B. Prevalence of chronic respiratory disease in urban and rural Uganda. Bull. World Health Organ. 2019, 97, 318–327. [Google Scholar] [CrossRef]
- Guwatudde, D.; Mutungi, G.; Wesonga, R. The Epidemiology of Hypertension in Uganda: Findings from the National Non-Communicable Diseases Risk Factor Survey. PLoS ONE 2015, 10, e0138991. [Google Scholar]
- Muddu, M.; Tusubira, A.K.; Sharma, S.K.; Akiteng, A.R.; Ssinabulya, I.; Schwartz, J.I. Integrated Hypertension and HIV Care Cascades in an HIV Treatment Program in Eastern Uganda: A Retrospective Cohort Study. J. Acquir Immune Defic. Syndr. 2019, 81, 552–561. [Google Scholar] [CrossRef] [PubMed]
- North, C.M.; Allen, J.G.; Okello, S. HIV Infection, Pulmonary Tuberculosis, and COPD in Rural Uganda: A Cross-Sectional Study. Lung 2018, 196, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Essien, O.E.; Andy, J.; Ansa, V.; Out, A.A.; Udoh, A. Coronary artery disease and the profile of cardiovascular risk factors in South South Nigeria: A clinical and autopsy study. Cardiol. Res. Pract. 2014, 2014, 804751. [Google Scholar] [CrossRef]
- Keates, A.K.; Mocumbi, A.O.; Ntsekhe, M.; Sliwa, K.; Stewart, S. Cardiovascular disease in Africa: Epidemiological profile and challenges. Nat. Rev. Cardiol. 2017, 14, 273–293. [Google Scholar] [CrossRef]
- Buist, A.S.; McBurnie, M.A.; Vollmer, W.M. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet 2007, 370, 741–750. [Google Scholar] [CrossRef]
- Gupte, A.N.; Wong, M.L.; Msandiwa, R. Factors associated with pulmonary impairment in HIV-infected South African adults. PLoS ONE 2017, 12, e0184530. [Google Scholar] [CrossRef]
- Varkila, M.R.J.; Vos, A.G.; Barth, R.E. The association between HIV infection and pulmonary function in a rural African population. PLoS ONE 2019, 14, e0210573. [Google Scholar] [CrossRef]
- Uganda: Coronary Heart DiseaseWorld Health Rankings—Live Longer, Live Better. Available online: https://www.worldlifeexpectancy.com/uganda-coronary-heart-disease (accessed on 19 August 2020).
- van Gemert, F.; Kirenga, B.; Chavannes, N. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): A prospective cross-sectional observational study. Lancet Glob. Health 2015, 3, e44–e51. [Google Scholar] [CrossRef]
- Mugisha, J.O.; Schatz, E.J.; Randell, M. Chronic disease, risk factors and disability in adults aged 50 and above living with and without HIV: Findings from the Wellbeing of Older People Study in Uganda. Glob. Health Action 2016, 9, 31098. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Helleberg, M.; May, M.T. Myocardial infarction among Danish HIV-infected individuals: Population-attributable fractions associated with smoking. Clin. Infect. Dis. 2015, 60, 1415–1423. [Google Scholar] [CrossRef]
- Triant, V.A. Epidemiology of coronary heart disease in patients with human immunodeficiency virus. Rev. Cardiovasc. Med. 2014, 15 (Suppl. 1), S1–S8. [Google Scholar] [PubMed]
- Hsue, P.Y.; Waters, D.D. HIV infection and coronary heart disease: Mechanisms and management. Nat. Rev. Cardiol. 2019, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, M.F.; Sliwa, K.; Kengne, A.P.; Mocumbi, A.O.; Bukhman, G. Cardiovascular Diseases in Sub-Saharan Africa Compared to High-Income Countries: An Epidemiological Perspective. Glob. Heart 2020, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Nkoke, C.; Luchuo, E.B. Coronary heart disease in sub-Saharan Africa: Still rare, misdiagnosed or underdiagnosed? Cardiovasc. Diagn. Ther. 2016, 6, 64–66. [Google Scholar] [PubMed]
- Almahmeed, W.; Arnaout, M.S.; Chettaoui, R. Coronary artery disease in Africa and the Middle East. Ther. Clin. Risk Manag. 2012, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, O.; Awolola, N.A.; Ajuluchukwu, J.N. Prevalence and pattern of cardiovascular-related causes of out-of- hospital deaths in Lagos, Nigeria. Afr. Health Sci. 2018, 18, 942–949. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Crothers, K.; Goulet, J.L.; Rodriguez-Barradas, M.C. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ. Prev. 2009, 21 (Suppl. 3), 40–53. [Google Scholar] [CrossRef]
- Helleberg, M.; May, M.T.; Ingle, S.M. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS 2015, 29, 221–229. [Google Scholar] [CrossRef]
- Monnig, M.A.; Kahler, C.W.; Cioe, P.A. Alcohol use predicts elevation in inflammatory marker soluble CD14 in men living with HIV. AIDS Care 2016, 28, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Tracy, R.; Belloso, W. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008, 5, e203. [Google Scholar] [CrossRef]
- Duprez, D.A.; Neuhaus, J.; Kuller, L.H. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE 2012, 7, e44454. [Google Scholar] [CrossRef]
- Wada, N.I.; Bream, J.H.; Martínez-Maza, O. Inflammatory Biomarkers and Mortality Risk Among HIV-Suppressed Men: A Multisite Prospective Cohort Study. Clin. Infect. Dis. 2016, 63, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.V.; Sharma, S.; Grund, B. Systemic Inflammation, Coagulation, and Clinical Risk in the START Trial. Open Forum Infect. Dis. 2017, 4, ofx262. [Google Scholar] [CrossRef]
- Zicari, S.; Sessa, L.; Cotugno, N. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Eskan, M.A.; Rose, B.G.; Benakanakere, M.R. TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells. Eur. J. Immunol. 2008, 38, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef]
- Yoshida, T.; Tuder, R.M. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol. Rev. 2007, 87, 1047–1082. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Lorré, K.; Fransen, L.; Ceuppens, J.L. Interleukin-2 induces tumor necrosis factor-alpha production by activated human T cells via a cyclosporin-sensitive pathway. Eur. Cytokine Netw. 1992, 3, 321–330. [Google Scholar] [PubMed]
- Crotty Alexander, L.E.; Shin, S.; Hwang, J.H. Inflammatory Diseases of the Lung Induced by Conventional Cigarette Smoke: A Review. Chest 2015, 148, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhihong, H.; Wenlong, L. The Nucleotide-Binding Oligomerization Domain-Like Receptor Family Pyrin Domain-Containing 3 Inflammasome Regulates Bronchial Epithelial Cell Injury and Proapoptosis after Exposure to Biomass Fuel Smoke. Am. J. Respir. Cell Mol. Biol. 2016, 55, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Yoon, C.M.; Kim, B.H. Suppression of NLRX1 in chronic obstructive pulmonary disease. J. Clin. Investig. 2015, 125, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Liang, M.; Li, M. A large lung gene expression study identifying IL1B as a novel player in airway inflammation in COPD airway epithelial cells. Inflamm. Res. 2018, 67, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Buscetta, M.; Di Vincenzo, S.; Miele, M.; Badami, E.; Pace, E.; Cipollina, C. Cigarette smoke inhibits the NLRP3 inflammasome and leads to caspase-1 activation via the TLR4-TRIF-caspase-8 axis in human macrophages. FASEB J. 2020, 34, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Adipogen.com. Inflammasomes. Available online: https://adipogen.com/inflammasomes (accessed on 19 March 2020).
- Helleberg, M.; Afzal, S.; Kronborg, G. Mortality attributable to smoking among HIV-1-infected individuals: A nationwide, population-based cohort study. Clin. Infect. Dis. 2013, 56, 727–734. [Google Scholar] [CrossRef]
- Nyunoya, T.; Monick, M.M.; Klingelhutz, A.; Yarovinsky, T.O.; Cagley, J.R.; Hunninghake, G.W. Cigarette smoke induces cellular senescence. Am. J. Respir. Cell Mol. Biol. 2006, 35, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Scherzer, R.; Shah, S.J.; Secemsky, E. Association of Biomarker Clusters with Cardiac Phenotypes and Mortality in Patients with HIV Infection. Circ. Heart Fail. 2018, 11, e004312. [Google Scholar] [CrossRef]
- York, A. Undetectable equals untransmittable. Nat. Rev. Microbiol. 2019, 17, 399. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Dieffenbach, C.W.; Fauci, A.S. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA 2019, 321, 451–452. [Google Scholar] [CrossRef]
- Peprah, E.; Caler, E.; Snyder, A.; Ketema, F. Deconstructing Syndemics: The Many Layers of Clustering Multi-Comorbidities in People Living with HIV. Int. J. Environ. Res. Public Health 2020, 17, 4704. [Google Scholar] [CrossRef]
- Singer, M. AIDS and the health crisis of the U.S. urban poor; the perspective of critical medical anthropology. Soc. Sci. Med. 1994, 39, 931–948. [Google Scholar] [CrossRef]
- Tsai, A.C.; Mendenhall, E.; Trostle, J.A.; Kawachi, I. Co-occurring epidemics, syndemics, and population health. Lancet 2017, 389, 978–982. [Google Scholar] [CrossRef]
- Singer, M.; Bulled, N.; Ostrach, B.; Mendenhall, E. Syndemics and the biosocial conception of health. Lancet 2017, 389, 941–950. [Google Scholar] [CrossRef]
- Sampson, U.K.A.; Kaplan, R.M.; Cooper, R.S. Reducing Health Inequities in the U.S.: Recommendations from the NHLBI’s Health Inequities Think Tank Meeting. J. Am. Coll. Cardiol. 2016, 68, 517–524. [Google Scholar] [CrossRef]
- Remien, R.H.; Stirratt, M.J.; Nguyen, N.; Robbins, R.N.; Pala, A.N.; Mellins, C.A. Mental health and HIV/AIDS: The need for an integrated response. AIDS 2019, 33, 1411–1420. [Google Scholar] [CrossRef]
- Schumacher, J.E.; McCullumsmith, C.; Mugavero, M.J. Routine depression screening in an HIV clinic cohort identifies patients with complex psychiatric co-morbidities who show significant response to treatment. AIDS Behav. 2013, 17, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, Z.; Mekuriaw, B.; Mehare, T.; Shumye, S.; Tsehay, M. Magnitude and predictors of common mental disorder among people with HIV/AIDS in Ethiopia: A systematic review and meta-analysis. BMC Public Health 2020, 20, 689. [Google Scholar]
- Yousuf, A.; Mohd Arifin, S.R.; Musa, R.; Md Isa, M.L. Depression and HIV Disease Progression: A Mini-Review. Clin. Pr. Epidemiol. Ment. Health 2019, 15, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.X.; Nguyen, L.H.; Turner, H.C. Economic evaluation studies in the field of HIV/AIDS: Bibliometric analysis on research development and scopes (GAP(RESEARCH)). BMC Health Serv. Res. 2019, 19, 834. [Google Scholar] [CrossRef]
- Peprah, E.; Xu, H.; Tekola-Ayele, F.; Royal, C.D. Genome-wide association studies in Africans and African Americans: Expanding the framework of the genomics of human traits and disease. Public Health Genom. 2015, 18, 40–51. [Google Scholar] [CrossRef]
- Tekola-Ayele, F.; Peprah, E. Examining How Our Shared Evolutionary History Shapes Future Disease Outcomes. Glob. Heart 2017, 12, 169–171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peprah, E.; Armstrong-Hough, M.; Cook, S.H.; Mukasa, B.; Taylor, J.Y.; Xu, H.; Chang, L.; Gyamfi, J.; Ryan, N.; Ojo, T.; et al. An Emerging Syndemic of Smoking and Cardiopulmonary Diseases in People Living with HIV in Africa. Int. J. Environ. Res. Public Health 2021, 18, 3111. https://doi.org/10.3390/ijerph18063111
Peprah E, Armstrong-Hough M, Cook SH, Mukasa B, Taylor JY, Xu H, Chang L, Gyamfi J, Ryan N, Ojo T, et al. An Emerging Syndemic of Smoking and Cardiopulmonary Diseases in People Living with HIV in Africa. International Journal of Environmental Research and Public Health. 2021; 18(6):3111. https://doi.org/10.3390/ijerph18063111
Chicago/Turabian StylePeprah, Emmanuel, Mari Armstrong-Hough, Stephanie H. Cook, Barbara Mukasa, Jacquelyn Y. Taylor, Huichun Xu, Linda Chang, Joyce Gyamfi, Nessa Ryan, Temitope Ojo, and et al. 2021. "An Emerging Syndemic of Smoking and Cardiopulmonary Diseases in People Living with HIV in Africa" International Journal of Environmental Research and Public Health 18, no. 6: 3111. https://doi.org/10.3390/ijerph18063111
APA StylePeprah, E., Armstrong-Hough, M., Cook, S. H., Mukasa, B., Taylor, J. Y., Xu, H., Chang, L., Gyamfi, J., Ryan, N., Ojo, T., Snyder, A., Iwelunmor, J., Ezechi, O., Iyegbe, C., O’Reilly, P., & Pascal Kengne, A. (2021). An Emerging Syndemic of Smoking and Cardiopulmonary Diseases in People Living with HIV in Africa. International Journal of Environmental Research and Public Health, 18(6), 3111. https://doi.org/10.3390/ijerph18063111










