Abstract
Fasting plasma glucose (FPG) and obesity-related indices are prognostic factors for adverse outcomes in both subjects with and without diabetes. A few studies have investigated sex differences in obesity indices related to the risk of diabetes, however no studies have compared the relationship between FPG and obesity-related indices by diabetes and sex. Therefore, in this study, we compared the curve shapes of FPG and various obesity-related indices by diabetes, and further explored sex differences in these associations. Data were derived from the Taiwan Biobank database, which included 5000 registered individuals. We used an adjusted generalized linear regression model and calculated the difference of least square means (Lsmean; standard error, SE) for males and females with and without diabetes. Associations between obesity-related indices and fasting glucose level by diabetes and sex groups were estimated, and the ORTHOREG procedure was used to construct B-splines. The post-fitting for linear models procedure was used to determine the range at which the trends separated significantly. The diabetes/sex/FPG interaction term was significant for all obesity-related indices, including body mass index, waist circumference, hip circumference, waist-to-hip ratio, waist-to-height ratio, lipid accumulation product, body roundness index, conicity index, body adiposity index and abdominal volume index. B-spline comparisons between males and females did not reach significance. However, FPG affected the trend towards obesity-related indices. As the fasting glucose level increased, the values of obesity-related indices varied more obviously in the participants without diabetes than in those with diabetes mellitus. The current study revealed that there was a different relationship between FPG and obesity-related indices by diabetes and sex. FPG affected the trend towards obesity-related indices more obviously in participants without diabetes than in those with diabetes. Further studies with a longitudinal design would provide a better understanding of the underlying mechanisms for the relationships.
1. Introduction
Diabetes is a serious health issue with an increasing prevalence worldwide. A recent study estimated a global prevalence of around about 9%, meaning that around 500 million people have diabetes worldwide. Moreover, the number is expected to increase by 25% in the next 10 years and 50% in the next 25 years []. The increase in patients with diabetes has spurred research efforts into disease control, and the incidence of acute complications such as cardiovascular disease (CVD), lower-extremity amputation, and all-cause mortality among people with diabetes has generally declined [,]. A modifiable risk factor for these complications is hyperglycemia, of which the underlying pathophysiology may be related to the induction of proinflammatory and prothrombotic pathways [,,]. The American Diabetes Association guidelines recommended a treatment goal for fasting plasma glucose (FPG) that correlates with a glycated hemoglobin (HbA1C) level of <7% []. FPG is an important determinant of morbidity and mortality in patients with diabetes, and many studies have reported that FPG level is associated with microvascular complications, CVD, and mortality in both people with prediabetes and those without diabetes [,,,,]. Furthermore, the level of FPG has been associated with the risk of future progression to diabetes in this population [,,,,].
Obesity is the major etiological cause and clinical manifestation of diabetes, and the term “diabesity” has been proposed []. Obesity is also a known risk factor for many other diseases, including metabolic syndrome, dyslipidemia, hypertension, CVD, fatty liver diseases, musculoskeletal diseases, and even cancer [,,,,]. Therefore, obesity causes a huge health economic burden []. Obesity can be classified as general and central obesity, with body mass index (BMI) commonly used to measure general obesity [] and waist circumference (WC) to measure central obesity []. Apart from a single direct WC measurement, several more complex anthropometric indices have been developed to define and quantify central obesity, including waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), lipid accumulation product (LAP), body roundness index (BRI), conicity Index (CI), body adiposity index (BAI) and abdominal volume index (AVI). Many studies have confirmed the associations among these obesity-related indices and the risk of diabetes [,,,,]. However, only a few studies have revealed obvious sex differences. Several studies have reported that some indices are adequate to discriminate diabetes from metabolically healthy individuals in both sexes, whereas other prospective studies have found a difference in some indices for the prediction of the future risk of diabetes progression [,,]. The results of these studies have been inconsistent. Moreover, no studies have compared the relationship between FPG and obesity-related indices by diabetes and sex.
Though the sex difference of metabolism was determined in diabetes mellitus (DM) or prediabetes population [,,,,], whether this difference could extend to non-DM population was uncertain, and this could affect the screening policy of related metabolic diseases in different sexes. Therefore, in this study, we enrolled 5000 individuals from the Taiwan Biobank (TWB) database and compared curve shapes of FPG and various obesity-related indices by diabetes, and further explored sex differences in these associations.
2. Materials and Methods
2.1. The Taiwan Biobank
The TWB is the largest government-supported biobank in Taiwan [,]. It includes genomic and lifestyle data of community-based individuals aged 30–70 years with no history of cancer, with all registered individuals providing informed consent, blood samples, and information on personal and lifestyle factors through questionnaires administered by TWB researchers. All of the individuals also underwent physical examinations. In this study, we included 5000 individuals registered in the TWB from December 2008 to April 2014.
2.2. Collection of Demographic, Medical and Laboratory Data
The TWB includes data on body weight, height, WC, hip circumference (HC), WHR, WHtR and BMI. The following baseline variables were recorded: demographic features (age and sex), medical history (DM), history of smoking and drinking alcohol, examination findings (systolic blood pressure (SBP) and diastolic blood pressure (DBP)) and laboratory data (estimated glomerular filtration rate (eGFR) and uric acid, fasting glucose, HbA1c, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides (TG)). After ten minutes rest, the blood pressures were averaged from three times measurement. Fasting blood samples were obtained, and laboratory data were measured using an autoanalyzer (Roche Diagnostics GmbH, D-68298 Mannheim COBAS Integra 400). Serum creatinine was measured according to the compensated Jaffé (kinetic alkaline picrate) method using the same autoanalyzer (Roche/Integra 400, Roche Diagnostics) and a calibrator that could be used in isotope-dilution mass spectrometry. The eGFR was calculated using the Modification of Diet in Renal Disease 4-variable equation [].
2.3. Definitions of Diabetes and Non-Diabetes
Participates who had a past history of diabetes, used hypoglycemic agents, and had a fasting glucose level ≥126 mg/dL or HbA1c ≥ 6.5% were considered to have diabetes (diabetes group) []. Participates who had no past history of diabetes and whose fasting glucose level was <126 mg/dL and HbA1c was <6.5% were considered to not have diabetes (non-diabetes group).
2.4. Obesity-Related Indices
For males, LAP was calculated as: LAP = ; and for females:
LAP = [].
BRI was calculated as: BRI = [].
CI was calculated using the Valdez equation based on the values for body weight, height and WC: CI = [].
BAI was calculated according to the method of Bergman and colleagues as:
BAI = [].
AVI was calculated as: AVI = [].
2.5. Ethics Statement
Ethical approval was granted by the Ethics and Governance Council of the TWB and the Institutional Review Board on Biomedical Science Research/IRB-BM, Academia Sinica, Taiwan. Each participant provided written informed consent, and the study was conducted in accordance with the principles of the Declaration of Helsinki. In addition, the Institutional Review Board of Kaohsiung Medical University Hospital approved this study (KMUHIRB-E(I)-20180242).
2.6. Statistical Analysis
Continuous variables are presented as mean (standard deviation, SD). Continuous outcome variables exhibiting a skewed distribution were transformed using the natural logarithms. Categorical variables are expressed as number of subjects (%). Data of continuous and categorical variables were analyzed using the t test and chi-squared test to compare the diabetes group and with that of the comparison group according to sex.
Next, we conducted the adjusted generalized linear regression model and calculated the difference of least square means (Lsmean; standard error, SE) for males and females with and without DM. Multiple comparison analysis testing was by using Bonferroni method. The interaction between DM and sex was tested after adjusted covariates were included such as DBP, total cholesterol, Ln (TG), HDL-cholesterol, eGFR and uric acid.
Outcome variables included were BMI, WC, HC, WHR, WHtR, LAP, BRI, CI, BAI and AVI. Outcome variables in fasting glucose level according to DM by sex groups were estimated and the ORTHOREG procedure constructs B-splines. To perform multiple comparisons among predicted values in a model with group-specific trends (DM and sex) that are modeled through regression splines. In order to determine the range on which the trends separate significantly, the post-fitting for linear models (PLM) procedure is executed.
All data was analyzed using Statistical Analysis Software, version 9.4 (SAS Institute, Cary, NC, USA) with a statistically significant level of two tailed p-value < 0.05.
3. Results
The mean age of the 5000 participants (2335 males and 2665 females) was 49.6 ± 10.7 years. The overall prevalence rate of type 2 DM was 10.3%. The participants were stratified into four groups according to DM and sex as follows: DM males (n = 295), non-DM males (n = 2040), DM females (n = 220) and non-DM females (n = 2445).
3.1. Comparison of Clinical Characteristics of the Study Population between Males and Females with and without DM
A comparison of the clinical characteristics among the participants with and without DM indifferent sex is shown in Table 1. In males, compared to the participants without DM, those with DM were older, had higher weight, lower height, higher BMI, higher WC, higher HC, higher WHR, higher WHtR, higher SBP, higher DBP, higher fasting glucose, higher HbA1c, higher TG, lower total cholesterol, lower HDL-cholesterol, lower LDL-cholesterol, higher eGFR and lower uric acid. Regarding obesity-related indices, in males, compared to the participants without DM, those with DM had higher LAP, higher BRI, higher CI, higher VAI, higher BAI and higher AVI. In females, compared to the participants without DM, those with DM were older, had higher weight, lower height, higher BMI, higher WC, higher HC, higher WHR, higher WHtR, higher SBP, higher DBP, higher fasting glucose, higher HbA1c, higher TG, higher total cholesterol, lower HDL-cholesterol, higher LDL-cholesterol, higher uric acid, higher LAP, higher BRI, higher CI, higher VAI, higher BAI and higher AVI.

Table 1.
Characteristics of the study population between males and females with and without diabetes mellitus (DM).
3.2. B-Spline Comparisons for Fasting Glucose with Obesity-Related Indices
Multiple comparison analysis testing of the interaction between DM and sex after adjustments for age, DBP, total cholesterol, Ln (TG), HDL-cholesterol, eGFR and uric acid is shown in Table 2. Whether in non-DM or DM participants, B-spline comparisons between males and females are not achieving significance. In males, comparing DM and non-DM participants, B-spline comparisons achieved significance in WHR, LAP and CI. In females, comparing DM and non-DM participants, B-spline comparisons achieved significance in LAP and BAI. However, the DM–Sex–fasting glucose interaction term was calculated significantly for all obesity-related indices, including BMI, WC, HC, WHR, WHtR, LAP, BRI, CI, BAI and AVI.

Table 2.
B-spline comparisons for DM by sex or sex by DM in AC sugar levels with obesity-related indices.
3.3. The Relationship between Fasting Glucose and Various Obesity-Related Indices by DM and Sex
Figure 1 illustrates the relationship between fasting glucose and various obesity-related indices by DM and sex: BMI (A), WC (B), HC(C), WHR (D), WHtR (E), LAP (F), BRI (G), CI (H), BAI (I) and AVI (J). The figure presents the sex difference of obesity-related indices in association with continuous FPG change more directly in non-diabetic participants.
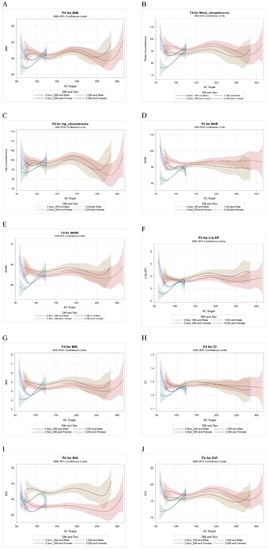
Figure 1.
The relationship between fasting glucose (AC sugar) and various obesity-related indices by DM and sex: BMI (A), WC (B), HC (C), WHR (D), WHtR (E), LAP (F), BRI (G), CI (H), BAI (I) and AVI (J). Abbreviations. DM, diabetes mellitus; BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio; LAP, lipid accumulation product; BRI, body roundness index; CI, conicity index; BAI, body adiposity index; AVI, abdominal volume index.
3.4. Separate Trends between Obesity-Related Indices and Fasting Glucose between Males and Females with and without DM
In order to determine the range on which the trends separate significantly, the PLM procedure was executed (Table 3). In Table 3, we grabbed part of the range of fasting glucose: 70–110 mg/dL in non-DM and 120–160 mg/dL in DM. Although B-spline comparisons between males and females did not achieve significance, whether DM or non-DM in Table 2, however, fasting glucose affected the trend towards obesity-related indices. While fasting glucose increased, the values of obesity-related indices varied more obvious in non-DM than in DM participants.

Table 3.
Separate trends between obesity-related indices and fasting glucose between males and females with and without DM.
4. Discussion
In this study, the different correspondences between obesity-related indices and DM status in different sexes were unmasked if we took FPG into consideration. Furthermore, the differences were more obvious in the non-DM group than in the DM group, but gradually declined as the FPG increased in the general Taiwanese population.
The first important finding of this study is that there were differences in the relationships between FPG and obesity-related indices by DM and sex. Sex differences in obesity indices related to the risk of diabetes have been reported in previous studies [,,]. The underlying mechanism could be complex. In addition to the persistent biological effects of different sex hormones and sex-specific gene expressions, lifelong psychosocial factors such as gender-sensitive economic, behavioral, cultural, and environmental factors may also aggravate the difference between males and females, and the overall reason may be explained as evolutionary maladaptation to relative food security in the modern age [,]. Previous studies have reported that males have larger increases in WC with weight gain than women, the so called “apple shape” in males and “pear shape” in females [,], and this may partially explain why the prevalence of diabetes or FPG is mildly higher in males than in females [,,].
The second important finding of this study is that B-spline comparisons between males and females were not significant in the non-DM group. However, we found more obvious sex differences in the association between obesity-related indices and continuous changes in FPG in the non-diabetes group. Previous studies have reported inconclusive results of gender differences in indices related to the risk of diabetes. Although some studies have concluded that central obesity based on WC or WHR may be a more specific risk factor in males than in females [,], other studies have reported opposite conclusions, in that the general obesity index BMI is more specific in males and that central obesity indices are more specific in female [,,]. In our study, the females in the non-DM group were more compatible with background knowledge, because a higher FPG level related to increasing obesity indices and could be regarded as a risk for diabetes [,]. In contrast, the obesity indices in males decreased to a nadir before FPG fell to the lowest point and then rebounded, resulting in a U-shaped curve, which has never been reported before. This implies that some overweight or obese males had very low FPG levels far from a diagnosis of diabetes, similar to the concept of metabolically healthy obesity, which likely represents a transient phenotype between lean men without diabetes and obese men with impaired fasting glucose. Therefore, at least avoiding further weight gain would be recommended in these individuals [,].
The other important finding of this study is that FPG affected the trend towards obesity-related indices more obviously in the non-DM group than in the DM group. Managing obesity is recommended in all obese or overweight diabetic patients as it can improve further glycemic control by improving insulin resistance [,]. In the current study, the obesity indices were basically stable and accompanied increasing FPG in the DM group, which is in contrast to some previous studies [,,,]. There are several reasons that could explain this discrepancy. First, due to the study design, we could not investigate the duration of diabetes as in previous studies, and this may have affected the results of glycemic burden independently of obesity status. Second, we did not have records of anti-hyperglycemic medication use in our participants, and this may also have impacted glycemic control. Third, rather than using HbA1C to evaluate glycemic burden as in the prior studies, we used FPG. FPG shows greater variability than HbA1C in patients with diabetes, and this could have amplified the influence of various factors, such as the aforementioned anti-hyperglycemic medication history.
Furthermore, we observed that differences in obesity-related indices corresponding to the same FPG level between sexes gradually decreased; that is, the two curves gradually converged, along with increasing FPG from the non-diabetic to diabetic participants. The reason why the associations among FPG, obesity-related indices, and sex did not extend from the non-diabetic to diabetic participants may be due to the aforementioned unknown duration of diabetes and anti-hyperglycemic medication use, as both can attenuate the impact of sex- and obesity-related indices. In addition, the nadir of the FPG level corresponded to a BMI of 22.5 kg/m2 and WC of 77 cm in the female participants, and a BMI of 24.5 kg/m2 and WC of 87 cm in the male participants. This observation generally responds to the different criteria for WC in men and women to diagnose metabolic syndrome, however previous same BMI definitions of overweight or obesity for males and females do not reflect different thresholds for metabolic abnormalities [,,,].
The strengths of the current study include detailed data collection from 5000 individuals, and the new finding of different curve shapes of FPG and various obesity-related indices by diabetes and sex, which has not previously been reported. There are two major limitations to this study. First, this was a cross-sectional study, so we could not define causal relationships between the obesity indices and FPG. Second, due to the study design, we did not have data on some important determinants of diabetes, including anti-hyperglycemic medications and the duration of diabetes.
5. Conclusions
In conclusion, the current study revealed a different trend of obesity-related indices in different sexes if we compared not only DM status, but also included the FPG. FPG affected the trend towards obesity-related indices more obviously in the non-DM group than in the DM group. Further studies with longitudinal designs would provide a better understanding of the underlying mechanisms for these relationships.
Author Contributions
Conceptualization, W.-L.W., P.-Y.W., J.-C.H., H.-P.T. and S.-C.C.; methodology, W.-L.W., H.-P.T. and S.-C.C.; software, H.-P.T. and S.-C.C.; validation, W.-L.W., H.-P.T. and S.-C.C.; formal analysis, H.-P.T. and S.-C.C.; investigation, W.-L.W., P.-Y.W., J.-C.H., H.-P.T. and S.-C.C.; writing—original draft preparation, W.-L.W. and S.-C.C.; writing—review and editing, H.-P.T. and S.-C.C.; supervision, H.-P.T. and S.-C.C.; project administration, H.-P.T. and S.-C.C.; funding acquisition, H.-P.T. and S.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported partially by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and by Kaohsiung Medical University Research Center Grant (KMU-TC109A01-1), the Ministry of Science and Technology (grant number: MOST 109-2314-B-037-071) and Kaohsiung Municipal Siaogang Hospital (kmhk-109-032).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUHIRB-E(I)-20180242 and 2018/8/3 approval.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this study is from the Taiwan Biobank. Due to restrictions placed on the data by the Personal Information Protection Act of Taiwan, the minimal data set cannot be made publicly available. Data may be available upon request to interested researchers. Please send data requests to: Szu-Chia Chen, PhD, MD. Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University.
Conflicts of Interest
We have no financial interest in the information contained in the manuscript.
References
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Gregg, E.W.; Sattar, N.; Ali, M.K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016, 4, 537–547. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Park, K.; Li, Q. Selective Insulin Resistance and the Development of Cardiovascular Diseases in Diabetes: The 2015 Edwin Bierman Award Lecture. Diabetes 2016, 65, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S66–S76. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Kowall, B.; Rathmann, W.; Heier, M.; Giani, G.; Peters, A.; Thorand, B.; Huth, C.; Icks, A.; Meisinger, C. Categories of glucose tolerance and continuous glycemic measures and mortality. Eur. J. Epidemiol. 2011, 26, 637–645. [Google Scholar] [CrossRef]
- Wong, T.Y.; Liew, G.; Tapp, R.J.; Schmidt, M.I.; Wang, J.J.; Mitchell, P.; Klein, R.; Klein, B.E.K.; Zimmet, P.; Shaw, J. Relation between fasting glucose and retinopathy for diagnosis of diabetes: Three population-based cross-sectional studies. Lancet 2008, 371, 736–743. [Google Scholar] [CrossRef]
- Palladino, R.; Tabak, A.G.; Khunti, K.; Valabhji, J.; Majeed, A.; Millett, C.; Vamos, E.P. Association between pre-diabetes and microvascular and macrovascular disease in newly diagnosed type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001061. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Papanas, N.; Vinik, A.I.; Shaw, J.E. Epidemiology of polyneuropathy in diabetes and prediabetes. Diabetes Nerv. Syst. 2014, 126, 3–22. [Google Scholar] [CrossRef]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Hillier, T.A.; Brown, J.B. Normal Fasting Plasma Glucose and Risk of Type 2 Diabetes Diagnosis. Am. J. Med. 2008, 121, 519–524. [Google Scholar] [CrossRef]
- Brambilla, P.; La Valle, E.; Falbo, R.; Limonta, G.; Signorini, S.; Cappellini, F.; Mocarelli, P. Normal Fasting Plasma Glucose and Risk of Type 2 Diabetes. Diabetes Care 2011, 34, 1372–1374. [Google Scholar] [CrossRef][Green Version]
- Hayashino, Y.; Fukuhara, S.; Suzukamo, Y.; Okamura, T.; Tanaka, T.; Ueshima, H. Normal fasting plasma glucose levels and type 2 diabetes: The high-risk and population strategy for occupational health promotion (HIPOP-OHP) [corrected] study. Acta Diabetol. 2007, 44, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Shai, I.; Tekes-Manova, D.; Pereg, D.; Shochat, T.; Kochba, I.; Rudich, A.; Eran Israeli Diabetes Research Group. Normal Fasting Plasma Glucose Levels and Type 2 Diabetes in Young Men. N. Engl. J. Med. 2005, 353, 1454–1462. [Google Scholar] [CrossRef]
- Astrup, A.; Finer, N. Redefining Type 2 diabetes: ‘Diabesity’ or ‘Obesity Dependent Diabetes Mellitus’? Obes. Rev. 2000, 1, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obes. 2007, 32, 211–222. [Google Scholar] [CrossRef]
- Tremmel, M.; Gerdtham, U.-G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.-H.; Flegal, K.M.; Chang, H.-Y.; Yeh, W.-T.; Yeh, C.-J.; Lee, W.-C. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: Implications for definitions of overweight and obesity for Asians. Am. J. Clin. Nutr. 2004, 79, 31–39. [Google Scholar] [CrossRef]
- Chang, Y.; Guo, X.; Chen, Y.; Guo, L.; Li, Z.; Yu, S.; Yang, H.; Sun, Y. A body shape index and body roundness index: Two new body indices to identify diabetes mellitus among rural populations in northeast China. BMC Public Health 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K.; Heidari, B. Is Waist Circumference A Better Predictor of Diabetes Than Body Mass Index or Waist-To-Height Ratio in Iranian Adults? Int. J. Prev. Med. 2015, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Ku, B.; Nam, J.; Pham, D.D.; Kim, J.Y. Prediction of Fasting Plasma Glucose Status Using Anthropometric Measures for Diagnosing Type 2 Diabetes. IEEE J. Biomed. Health Inform. 2013, 18, 555–561. [Google Scholar] [CrossRef]
- Snijder, M.B.; Dekker, J.M.; Visser, M.; Bouter, L.M.; Stehouwer, C.D.; Kostense, P.J.; Yudkin, J.S.; Heine, R.J.; Nijpels, G.; Seidell, J.C. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: The Hoorn Study. Am. J. Clin. Nutr. 2003, 77, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, G.; Duval, S.; Jacobs, J.D.R.; Silventoinen, K. Comparison of Body Mass Index, Waist Circumference, and Waist/Hip Ratio in Predicting Incident Diabetes: A Meta-Analysis. Epidemiol. Rev. 2007, 29, 115–128. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, X.; Zhang, H.; Zhao, W.; Li, J.; Shu, Y.; Li, S.; Yang, M.; Cai, L.; Zhou, J.; et al. Prevalence of diabetes and predictions of its risks using anthropometric measures in southwest rural areas of China. BMC Public Health 2012, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, Y.; Li, L.; Liu, X.; Zhang, H.; Zhang, X.; Qian, X.; Zhou, W.; Jiang, J.; Zhao, J.; et al. Gender-specific associations of body mass index and waist circumference with type 2 diabetes mellitus in Chinese rural adults: The Henan Rural Cohort Study. J. Diabetes Its Complicat. 2018, 32, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Lasky, D.; Becerra, E.; Boto, W.; Otim, M.; Ntambi, J. Obesity and gender differences in the risk of type 2 diabetes mellitus in Uganda. Nutrition 2002, 18, 417–421. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yang, J.-H.; Chiang, C.W.; Hsiung, C.-N.; Wu, P.-E.; Chang, L.-C.; Chu, H.-W.; Chang, J.; Yuan-Tsong, C.; Yang, S.-L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-T.; Hung, T.-H.; Yeh, C.-K. Taiwan Regulation of Biobanks. J. Law Med. Ethic 2015, 43, 816–826. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D.R. A More Accurate Method to Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S15. [Google Scholar]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Valdez, R. A simple model-based index of abdominal adiposity. J. Clin. Epidemiol. 1991, 44, 955–956. [Google Scholar] [CrossRef]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A Better Index of Body Adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Abdominal volume index. an anthropometry-based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch. Med. Res. 2003, 34, 428–432. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef]
- Stevens, J.; Katz, E.G.; Huxley, R.R. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 2010, 64, 6–15. [Google Scholar] [CrossRef]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Nordström, A.; Hadrévi, J.; Olsson, T.; Franks, P.W.; Nordström, P. Higher Prevalence of Type 2 Diabetes in Men Than in Women Is Associated With Differences in Visceral Fat Mass. J. Clin. Endocrinol. Metab. 2016, 101, 3740–3746. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine (Baltimore) 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Færch, K.; Borch-Johnsen, K.; Vaag, A.; Jørgensen, T.; Witte, D.R. Sex differences in glucose levels: A consequence of physiology or methodological convenience? The Inter99 study. Diabetologia 2010, 53, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Zainal, H.; Harun, S.N.; Ghadzi, S.M.S.; Ghafoor, S. Gender differences in the modifiable risk factors associated with the presence of prediabetes: A systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Faerch, K.; Vaag, A.; Witte, D.R.; Jørgensen, T.; Pedersen, O.; Borch-Johnsen, K. Predictors of future fasting and 2-h post-OGTT plasma glucose levels in middle-aged men and women-the Inter99 study. Diabet. Med. 2009, 26, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Papacosta, O.; Whincup, P.H.; Carson, C.; Thomas, M.C.; Lawlor, D.A.; Ebrahim, S.; Sattar, N. Assessing prediction of diabetes in older adults using different adiposity measures: A 7 year prospective study in 6,923 older men and women. Diabetol. 2010, 53, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Satman, I.; Omer, B.; Tutuncu, Y.; Kalaca, S.; Gedik, S.; Dinccag, N.; Karsidag, K.; Genc, S.; Telci, A.; Canbaz, B.; et al. Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur. J. Epidemiol. 2013, 28, 169–180. [Google Scholar] [CrossRef]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Guan, S.; Liu, H.; Wu, X.; Zhang, Z.; Gu, X.; Zhang, Y.; Zhao, Y.; Tse, L.A.; et al. The Prevalence of Metabolically Healthy and Unhealthy Obesity according to Different Criteria. Obes. Facts 2019, 12, 78–90. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S89–S97. [Google Scholar] [CrossRef] [PubMed]
- Grams, J.; Garvey, W.T. Weight Loss and the Prevention and Treatment of Type 2 Diabetes Using Lifestyle Therapy, Pharmacotherapy, and Bariatric Surgery: Mechanisms of Action. Curr. Obes. Rep. 2015, 4, 287–302. [Google Scholar] [CrossRef]
- Alzaheb, R.; Altemani, A.H. The prevalence and determinants of poor glycemic control among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Lage, M.; Mo, D.; Nelson, D.; Hoogwerf, B. Obesity and glycemic control in patients with diabetes mellitus: Analysis of physician electronic health records in the US from 2009–2011. J. Diabetes its Complicat. 2016, 30, 212–220. [Google Scholar] [CrossRef]
- Zhu, H.-T.; Yu, M.; Hu, H.; He, Q.-F.; Pan, J.; Hu, R.-Y. Factors associated with glycemic control in community-dwelling elderly individuals with type 2 diabetes mellitus in Zhejiang, China: A cross-sectional study. BMC Endocr. Disord. 2019, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Hameed, E.K.; AbdulQahar, Z.H. Visceral adiposity index in female with type 2 diabetic mellitus and its association with the glycemic control. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Consultation, W.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar]
- Chiu, M.; Austin, P.C.; Manuel, D.G.; Shah, B.R.; Tu, J.V. Deriving Ethnic-Specific BMI Cutoff Points for Assessing Diabetes Risk. Diabetes Care 2011, 34, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.C.; Araneta, M.R.G.; Kanaya, A.M.; Chiang, J.L.; Fujimoto, W. BMI Cut Points to Identify At-Risk Asian Americans for Type 2 Diabetes Screening: Table 1. Diabetes Care 2015, 38, 150–158. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).