Microbiological Surveillance of Endoscopes in a Southern Italian Transplantation Hospital: A Retrospective Study from 2016 to 2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Endoscopes
2.2. Reprocessing
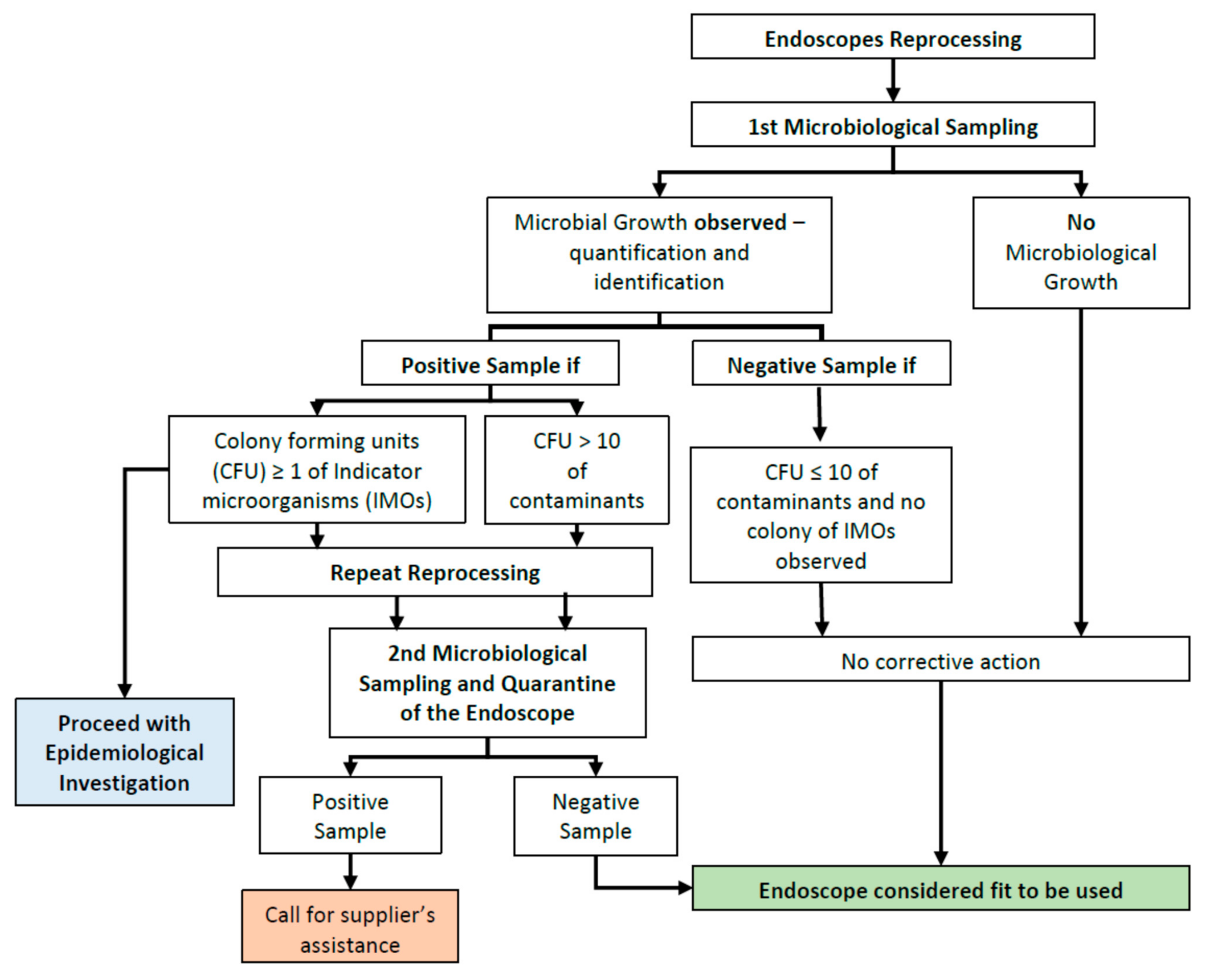
2.3. Microbiological Sampling
2.4. Report and Follow-Up
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Spaulding, E.H. Chemical disinfection of medical and surgical materials. In Disinfection, Sterilization, and Preservation; Lawrence, C.A., Block, S.S., Eds.; Lea and Febiger: Philadelphia, PA, USA, 1968; p. 517. [Google Scholar]
- Beilenhoff, U.; Neumann, C.S.; Rey, J.F.; Biering, H.; Blum, R.; Cimbro, M.; Kampf, B.; Rogers, M.; Schmidt, V. ESGE-ESGENA Guideline: Cleaning and disinfection in gastrointestinal endoscopy. Endoscopy 2008, 40, 939–957. [Google Scholar] [CrossRef]
- Gastroenterological Society of Australia. Available online: https://www.gesa.org.au/public/13/files/ClinicalUpdatesandGuidelines/Endoscopy_Microbiological_Testing.pdf (accessed on 17 July 2020).
- Petersen, B.T.; Cohen, J.; Hambrick, R.D., 3rd; Buttar, N.; Greenwald, D.A.; Buscaglia, J.M.; Collins, J.; Eisen, G. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest. Endosc. 2017, 85, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Available online: https://www.cdc.gov/hicpac/recommendations/flexible-endoscope-reprocessing.html (accessed on 17 July 2020).
- Calderwood, A.H.; Day, L.W.; Muthusamy, V.R.; Collins, J.; Hambrick, R.D.; Brock, A.S.; Guda, N.M.; Buscaglia, J.M.; Petersen, B.T.; Buttar, N.S.; et al. ASGE guideline for infection control during GI endoscopy. Gastrointest. Endosc. 2018, 87, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B. Infectious disease complications of GI endoscopy: Part II, exogenous infections. Gastrointest. Endosc. 2003, 57, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Muscarella, L.F. Current issues in endoscope reprocessing and infection control during gastrointestinal endoscopy. World J. Gastroenterol. 2006, 12, 3953–3964. [Google Scholar] [CrossRef]
- Kovaleva, J.; Peters, F.T.M.; van der Mei, H.C.; Degener, J.E. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin. Microbiol. Rev. 2013, 26, 231–254. [Google Scholar] [CrossRef]
- Brandabur, J.J.; Leggett, J.E.; Wang, L.; Bartles, R.L.; Baxter, L.; Diaz, G.A.; Grunkemeier, G.L.; Hove, S.; Oethinger, M. Surveillance of guideline practices for duodenoscope and linear echoendoscope reprocessing in a large healthcare system. Gastrointest. Endosc. 2016, 84, 392–399.e3. [Google Scholar] [CrossRef] [PubMed]
- Aumeran, C.; Poincloux, L.; Souweine, B.; Robin, F.; Laurichesse, H.; Baud, O.; Bommelaer, G.; Traoré, O. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy 2010, 42, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Carbonne, A.; Thiolet, J.M.; Fournier, S.; Fortineau, N.; Kassis-Chikhani, N.; Boytchev, I.; Aggoune, M.; Séguier, J.C.; Sénéchal, H.; Tavolacci, M.P.; et al. Control of a multi-hospital outbreak of KPC-producing Klebsiella pneumoniae type 2 in France, September to October 2009. Eurosurveillance 2010, 15. [Google Scholar] [CrossRef]
- Epstein, L.; Hunter, J.C.; Arwady, M.A.; Tsai, V.; Stein, L.; Gribogiannis, M.; Frias, M.; Guh, A.Y.; Laufer, A.S.; Black, S.; et al. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 2014, 312, 1447–1455. [Google Scholar] [CrossRef]
- Wendorf, K.A.; Kay, M.; Baliga, C.; Weissman, S.J.; Gluck, M.; Verma, P.; D’Angeli, M.; Swoveland, J.; Kang, M.-G.; Eckmann, K.; et al. Endoscopic retrograde cholangiopancreatography-associated AmpC Escherichia coli outbreak. Infect. Control. Hosp. Epidemiol. 2015, 36, 634–642. [Google Scholar] [CrossRef]
- Moses, F.M.; Lee, J. Surveillance cultures to monitor quality of gastrointestinal endoscope reprocessing. Am. J. Gastroenterol. 2003, 98, 77–81. [Google Scholar] [CrossRef]
- Alrabaa, S.F.; Nguyen, P.; Sanderson, R.; Baluch, A.; Sandin, R.L.; Kelker, D.; Karlapalem, C.; Thompson, P.; Sams, K.; Martin, S.; et al. Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am. J. Infect. Control 2013, 41, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Murray, P. Preventable Tragedies: Superbugs and How Ineffective Monitoring of Medical Device Safety Fails Patients; Agency for Healthcare Research and Quality: Washington, DC, USA, 2016.
- Cottarelli, A.; De Giusti, M.; Solimini, A.G.; Venuto, G.; Palazzo, C.; Del Cimmuto, A.; Osborn, J.; Marinelli, L. Microbiological surveillance of endoscopes and implications for current reprocessing procedures adopted by an Italian teaching hospital. Ann. Ig 2020, 32, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Ofstead, C.L.; Wetzler, H.P.; Doyle, E.M.; Rocco, C.K.; Visrodia, K.H.; Baron, T.H.; Tosh, P.K. Persistent contamination on colonoscopes and gastroscopes detected by biologic cultures and rapid indicators despite reprocessing performed in accordance with guidelines. Am. J. Infect. Control. 2015, 43, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Paula, H.; Tribl, B.; Presterl, E.; Diab-El Schahawi, M. Prospective microbiologic evaluation of the forceps elevator in closed-channel duodenoscopes after reprocessing. Am. J. Infect. Control 2017, 45, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, E.E.; Kotsanas, D.; Stuart, R.L. Microbiological monitoring of endoscopes: 5-year review. J. Gastroenterol. Hepatol. 2008, 23, 1069–1074. [Google Scholar] [CrossRef]
- Battaglia, G. AIGO-SIED-SIGE per la Sicurezza in Endoscopia Digestiva Commissione “reprocessing”- Pulizia e Disinfezione in Endoscopia Digestiva. Giorn. Ital. End. Dig. 2007, 30, 25–33. [Google Scholar]
- Cirigliano, V.; Cordioli, D.; Gabrielli, L.; Mattiola, R.; Nembrini, L.; Rivara, C.; Salardi, I. Linee Guida Pulizia E Disinfezione in Endoscopia—Update 2011. Available online: https://www.anoteanigea.it/linee-guida-public/linee-guida-pulizia-e-disinfezione-in-endoscopia-update-2011 (accessed on 22 July 2020).
- Sage Design of Endoscopic Retrograde Cholangiopancreatography (ERCP) Duodenoscopes May Impede Effective Cleaning: FDA Safety Communication. Available online: https://www.sages.org/design-of-endoscopic-retrograde-cholangiopancreatography-ercp-duodenoscopes-may-impede-effective-cleaning-fda-safety-communication (accessed on 22 July 2020).
- Alvarado, C.J.; Reichelderfer, M. APIC guideline for infection prevention and control in flexible endoscopy. Association for Professionals in Infection Control. Am. J. Infect. Control 2000, 28, 138–155. [Google Scholar] [CrossRef]
- Speer, T.; Alfa, M.; Cowen, A.; Jones, D.; Vickery, K.; Griffiths, H.; Nelson, D.; Saenz, R.; LeMair, A. Endoscope Disinfection Update: A Guide to Resource-Sensitive Reprocessing; World Gastroenterology Organisation: Melbourne, Australia, 2019. [Google Scholar]
- Di Mento, G.; Cuscino, N.; Carcione, C.; Cardinale, F.; Conaldi, P.G.; Douradinha, B. Emergence of a Klebsiella pneumoniae ST392 clone harbouring KPC-3 in an Italian transplantation hospital. J. Hosp. Infect. 2017, 10–11. [Google Scholar] [CrossRef]
- Monaco, F.; Mento, G.D.; Cuscino, N.; Conaldi, P.G.; Douradinha, B. Infant colonisation with Escherichia coli and Klebsiella pneumoniae strains co-harbouring blaOXA-48and blaNDM-1carbapenemases genes: A case report. Int. J. Antimicrob. Agents 2018, 52, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Di Mento, G.; Carreca, A.P.; Monaco, F.; Cuscino, N.; Cardinale, F.; Conaldi, P.G.; Douradinha, B. Mycobacterium saskatchewanense strain associated with a chronic kidney disease patient in an Italian transplantation hospital and almost misdiagnosed as Mycobacterium tuberculosis. Infect. Control. Hosp. Epidemiol. 2019, 40, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Martiny, H.; Floss, H.; Zühlsdorf, B. The importance of cleaning for the overall results of processing endoscopes. J. Hosp. Infect. 2004, 56 (Suppl. 2), S16–S22. [Google Scholar] [CrossRef]
- Leung, J.W. Reprocessing of flexible endoscopes. J. Gastroenterol. Hepatol. 2000, 15, G73–G77. [Google Scholar] [CrossRef]
- Heeg, P. Reprocessing endoscopes: National recommendations with a special emphasis on cleaning--the German perspective. J. Hosp Infect. 2004, 56 (Suppl. 2), S23–S26. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Reprocessing endoscopes: United States perspective. J. Hosp. Infect. 2004, 56 (Suppl. 2), S27–S39. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.P.; Kim, W.H. Recent Update on Microbiological Monitoring of Gastrointestinal Endoscopes after High-Level Disinfection. Clin. Endosc. 2015, 48, 369–373. [Google Scholar] [CrossRef]
- Saliou, P.; Cholet, F.; Jézéquel, J.; Robaszkiewicz, M.; Le Bars, H.; Baron, R. The Use of Channel-Purge Storage for Gastrointestinal Endoscopes Reduces Microbial Contamination. Infect. Control. Hosp. Epidemiol. 2015, 36, 1100–1102. [Google Scholar] [CrossRef]
- Saviuc, P.; Picot-Guéraud, R.; Shum Cheong Sing, J.; Batailler, P.; Pelloux, I.; Brenier-Pinchart, M.-P.; Dobremez, V.; Mallaret, M.-R. Evaluation of the Quality of Reprocessing of Gastrointestinal Endoscopes. Infect. Control. Hosp. Epidemiol. 2015, 36, 1017–1023. [Google Scholar] [CrossRef]
- McCafferty, C.E.; Aghajani, M.J.; Abi-Hanna, D.; Gosbell, I.B.; Jensen, S.O. An update on gastrointestinal endoscopy-associated infections and their contributing factors. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 36. [Google Scholar] [CrossRef]
- Bisset, L.; Cossart, Y.E.; Selby, W.; West, R.; Catterson, D.; O’hara, K.; Vickery, K. A prospective study of the efficacy of routine decontamination for gastrointestinal endoscopes and the risk factors for failure. Am. J. Infect. Control 2006, 34, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Balan, G.G.; Sfarti, C.V.; Chiriac, S.A.; Stanciu, C.; Trifan, A. Duodenoscope-associated infections: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Rauwers, A.W.; Voor In ’t Holt, A.F.; Buijs, J.G.; de Groot, W.; Erler, N.S.; Bruno, M.J.; Vos, M.C. Nationwide risk analysis of duodenoscope and linear echoendoscope contamination. Gastrointest. Endosc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Barbero, L.; Borio, N.; Franco, C.B.; Cerina, E.; Demaria, F.; Grivetto, M.; Lunetta, S.; Pozzebon, M.; Rivara, C. LINEE GUIDA REPROCESSING IN BRONCOSCOPIA Esperienza di un Gruppo di Lavoro Infermieristico Piemontese. Available online: https://www.anoteanigea.it/download-allegato/MxdCskgA-udpuEoklht5onCBWcSOPXj-mkbFOwVS7-k, (accessed on 23 December 2020).
- Mongardi, M.; Bedosti, C.; Moro, M.L. Reprocessing Degli Endoscopi Indicazioni Operative. Available online: http://asr.regione.emilia-romagna.it/wcm/asr/collana_dossier/doss133.htm (accessed on 23 December 2020).
- Saliou, P.; Le Bars, H.; Payan, C.; Narbonne, V.; Cholet, F.; Jézéquel, J.; Scotet, V.; Robaszkiewicz, M.; Cornec, D.; Héry-Arnaud, G.; et al. Measures to improve microbial quality surveillance of gastrointestinal endoscopes. Endoscopy 2016, 48, 704–710. [Google Scholar] [CrossRef]
- Rauwers, A.W.; Voor In ’t Holt, A.F.; Buijs, J.G.; de Groot, W.; Hansen, B.E.; Bruno, M.J.; Vos, M.C. High prevalence rate of digestive tract bacteria in duodenoscopes: A nationwide study. Gut 2018, 67, 1637–1645. [Google Scholar] [CrossRef]
- Cattoir, L.; Vanzieleghem, T.; Florin, L.; Helleputte, T.; De Vos, M.; Verhasselt, B.; Boelens, J.; Leroux-Roels, I. Surveillance of Endoscopes: Comparison of Different Sampling Techniques. Infect. Control. Hosp. Epidemiol. 2017, 38, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Obee, P.C.; Griffith, C.J.; Cooper, R.A.; Cooke, R.P.; Bennion, N.E.; Lewis, M. Real-time monitoring in managing the decontamination of flexible gastrointestinal endoscopes. Am. J. Infect. Control 2005, 33, 202–206. [Google Scholar] [CrossRef]
- Fernando, G.; Collignon, P.; Beckingham, W. ATP bioluminescence to validate the decontamination process of gastrointestinal endoscopes. Healthc. Infect. 2014, 19, 59–64. [Google Scholar] [CrossRef]
| Type of Endoscope | Instruments (n) | Samplings (n) | Indicator Microorganisms (IMOs) ≥ 1 (%) | Contaminants ≥ 10 (%) | Contaminants < 10 (%) | No Growth (%) |
|---|---|---|---|---|---|---|
| Duodenoscopes | 3 | 114 | 1 (0.9%) | 0 (0.0%) | 3 (2.6%) | 110 (96.5%) |
| Echoendoscopes | 3 | 117 | 0 (0.0%) | 0 (0.0%) | 2 (1.7%) | 115 (98.3%) |
| Bronchoscopes | 12 | 370 | 3 (1.0%) | 1 (0.3%) | 22 (5.9%) | 344 (93.0%) |
| Colonoscopes | 5 | 67 | 0 (0.0%) | 0 (0.0%) | 1 (1.5%) | 66 (98.5%) |
| Gastroscopes | 12 | 143 | 5 (3.5%) | 2 (1.4%) | 7 (4.9%) | 129 (90.2%) |
| Total | 35 | 811 | 9 (1.1%) | 3 (0.4%) | 35 (4.3%) | 764 (94.2%) |
| Type of Endoscope | 2016 | 2017 | 2018 | 2019 | Total |
|---|---|---|---|---|---|
| Duodenoscopes | 29 (0.0%) | 17 (5.9%) | 26 (0.0%) | 42 (0.0%) | 114 (0.9%) |
| Echoendoscopes | 28 (0.0%) | 26 (0.0%) | 29 (0.0%) | 34 (0.0%) | 117 (0.0%) |
| Bronchoscopes | 72 (1.4%) | 78 (3.8%) | 102 (0.0%) | 118 (0.8%) | 370 (1.4%) † |
| Colonoscopes | 14 (0.0%) | 13 (0.0%) | 18 (0.0%) | 22 (0.0%) | 67 (0.0%) |
| Gastroscopes | 29 (6.9%) | 35 (5.7%) | 41 (4.9%) | 38 (2.6%) | 143 (4.9%) † |
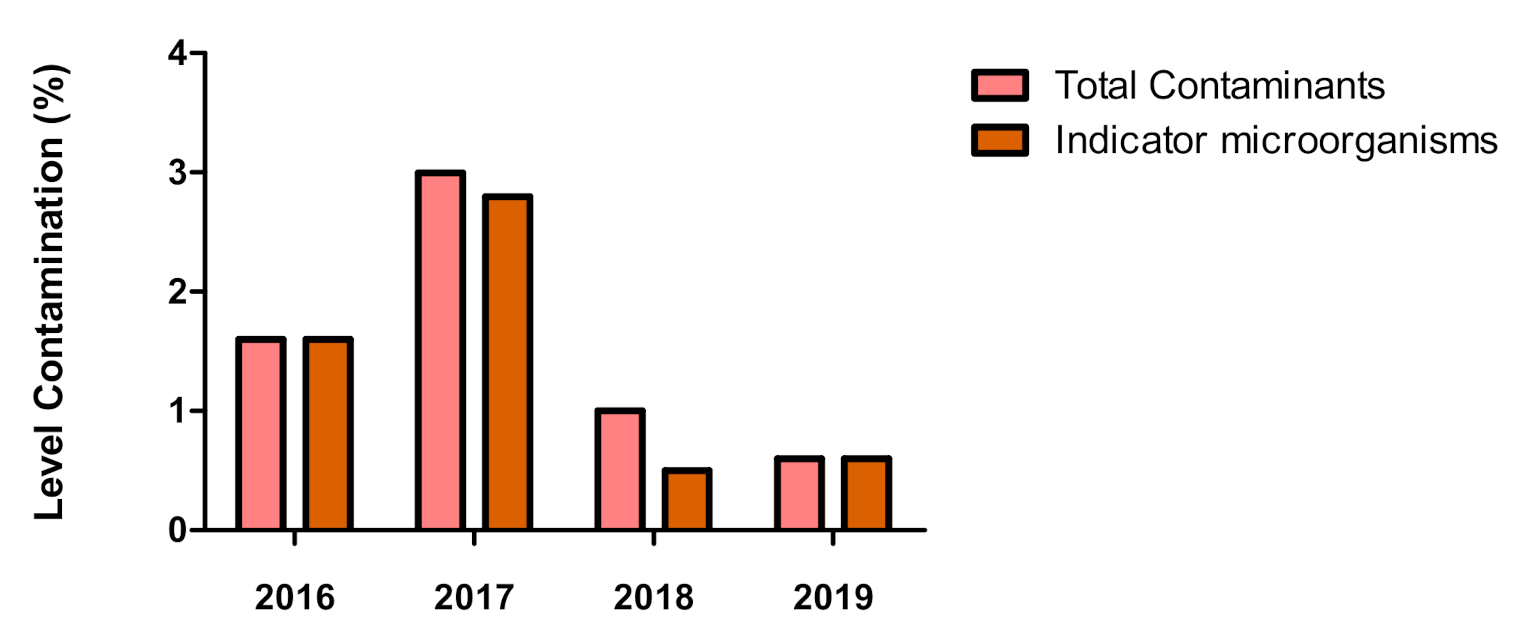
| Total | 172 (1.6%) | 169 (3.0%) | 216 (1.0%) | 254 (0.6%) | 811 (1.5%) |
| Type of Endoscope | 2016 | 2017 | 2018 | 2019 | Total |
|---|---|---|---|---|---|
| Duodenoscopes | 0 | 1 | 0 | 0 | 1 |
| Echoendoscopes | 0 | 0 | 0 | 0 | 0 |
| Bronchoscopes | 0 | 3 | 0 | 1 | 5 |
| Colonoscopes | 0 | 0 | 0 | 0 | 0 |
| Gastroscopes | 2 | 2 | 2 | 1 | 7 |
| Total | 2 | 6 | 2 | 2 | 12 |
| Indicator Microorganisms (IMOs) | Contaminants |
|---|---|
| Pseudomonas aeruginosa Klebsiella pneumoniae Candida parapsilosis Candida glabrata Aspergillus niger Enterococcus faecalis Candida tropicalis Enterobacter cloacae Pseudomonas putida | Coagulase-negative Staphylococci Moraxella osloensis Rothia mucillaginosa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchese, V.; Di Carlo, D.; Fazio, G.; Gioè, S.M.; Luca, A.; Alduino, R.; Rizzo, M.; Tuzzolino, F.; Monaco, F.; Conaldi, P.G.; et al. Microbiological Surveillance of Endoscopes in a Southern Italian Transplantation Hospital: A Retrospective Study from 2016 to 2019. Int. J. Environ. Res. Public Health 2021, 18, 3057. https://doi.org/10.3390/ijerph18063057
Marchese V, Di Carlo D, Fazio G, Gioè SM, Luca A, Alduino R, Rizzo M, Tuzzolino F, Monaco F, Conaldi PG, et al. Microbiological Surveillance of Endoscopes in a Southern Italian Transplantation Hospital: A Retrospective Study from 2016 to 2019. International Journal of Environmental Research and Public Health. 2021; 18(6):3057. https://doi.org/10.3390/ijerph18063057
Chicago/Turabian StyleMarchese, Valentina, Daniele Di Carlo, Gaetano Fazio, Santi Mauro Gioè, Angelo Luca, Rossella Alduino, Monica Rizzo, Fabio Tuzzolino, Francesco Monaco, Pier Giulio Conaldi, and et al. 2021. "Microbiological Surveillance of Endoscopes in a Southern Italian Transplantation Hospital: A Retrospective Study from 2016 to 2019" International Journal of Environmental Research and Public Health 18, no. 6: 3057. https://doi.org/10.3390/ijerph18063057
APA StyleMarchese, V., Di Carlo, D., Fazio, G., Gioè, S. M., Luca, A., Alduino, R., Rizzo, M., Tuzzolino, F., Monaco, F., Conaldi, P. G., Douradinha, B., & Di Martino, G. (2021). Microbiological Surveillance of Endoscopes in a Southern Italian Transplantation Hospital: A Retrospective Study from 2016 to 2019. International Journal of Environmental Research and Public Health, 18(6), 3057. https://doi.org/10.3390/ijerph18063057








