Universal Access to Xpert MTB/RIF Testing for Diagnosis of Tuberculosis in Uzbekistan: How Well Are We Doing?
Abstract
1. Introduction
- To determine nationally in Uzbekistan, and stratified by region, for the years 2018 and 2019:
- The aggregate number of presumptive TB patients;
- The aggregate number (proportion) tested using Xpert MTB/RIF assay.
- In an individual patient-wise cohort of presumptive TB patients identified in selected health care facilities of Tashkent City and Bukhara Region during January–March 2019, to determine:
- The number (proportion) tested using Xpert MTB/RIF and/or microscopy and the number (proportion) diagnosed with TB;
- Demographic and health-facility level factors associated with not getting tested using Xpert MTB/RIF assay;
- Median duration in days between the ‘date of initial visit’ to the PHC and ‘date of PHC receiving the Xpert MTB/RIF result’.
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.2.1. Tuberculosis (TB) Control Program in Uzbekistan
2.2.2. The TB Laboratory Network
- Tier I—Sputum smear microscopy laboratories without GeneXpert, situated at district level dispensaries and PHCs;
- Tier II—Laboratories with both microscopy and GeneXpert situated at provincial, district level or PHCs;
- Tier III—Provincial level laboratories capable of performing LPA;
- Tier IV—Inter-provincial level laboratories which conduct LPA and culture and DST;
- Tier V—National reference laboratories which conduct LPA and culture and DST.
2.2.3. Recording and Reporting
2.3. Study Population
2.4. Data Variables and Sources
2.5. Analysis and Statistics
3. Results
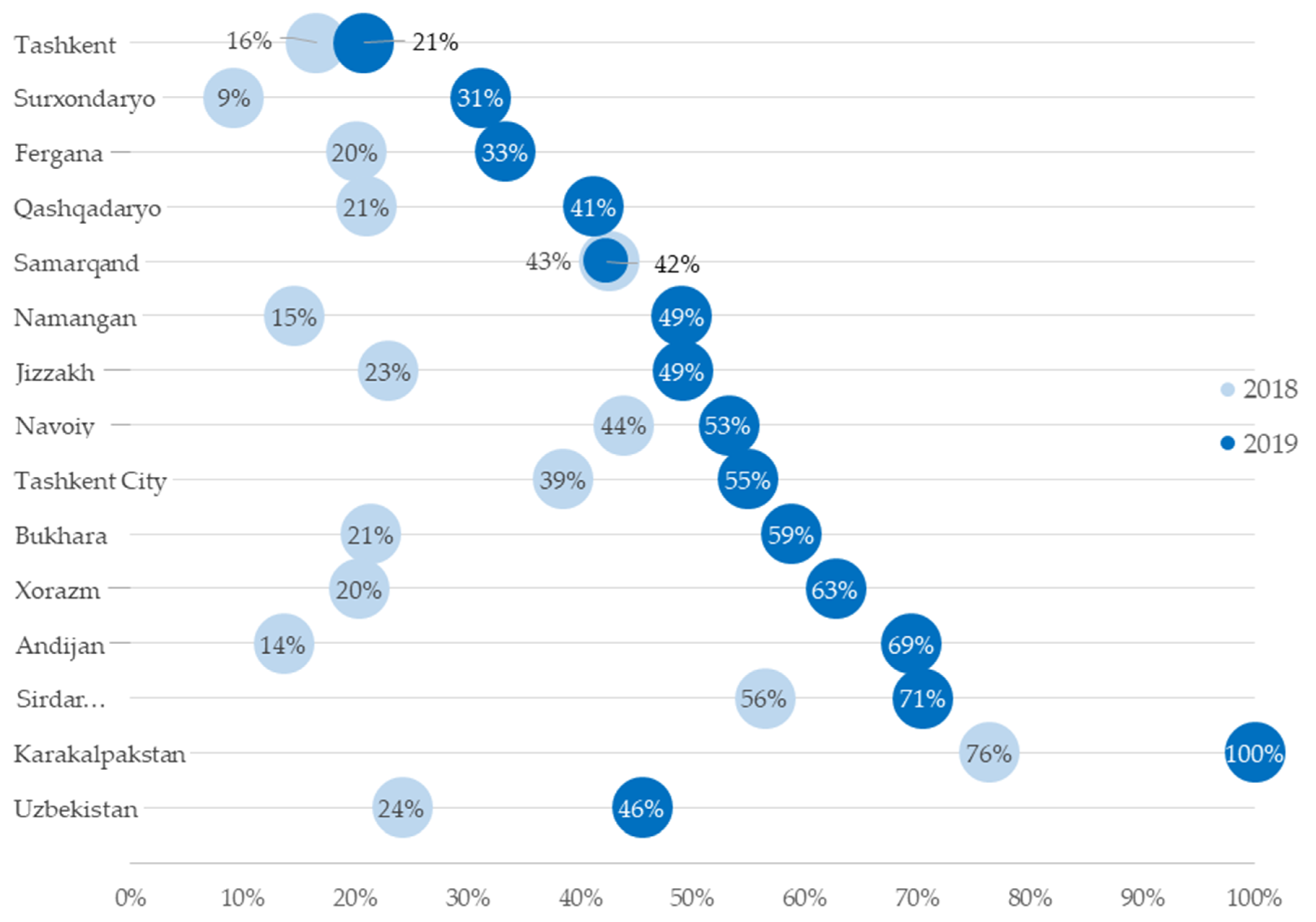
3.1. National and Regional Xpert MTB/RIF Test Coverage
3.2. Baseline Characteristics
3.3. Diagnosis of Tuberculosis by Test Used
3.4. Factors Associated with Xpert MTB/RIF Non-Testing
3.5. Turnaround Time of Laboratory Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Open Access Statement
References
- World Health Organization (WHO). Global Tuberculosis Report. 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 10 December 2020).
- Subbaraman, R.; Nathavitharana, R.R.; Mayer, K.H.; Satyanarayana, S.; Chadha, V.K.; Arinaminpathy, N.; Pai, M. Constructing care cascades for active tuberculosis: A strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 2019, 16, e1002754. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The End TB Strategy. Available online: http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1 (accessed on 9 March 2018).
- United Nations. Transforming Our World: The 2030 Agenda For Sustainable Development Transforming Our World: The 2030 Agenda For Sustainable Development; United Nations: New York, NY, USA, 2016. [Google Scholar]
- World Health Organization (WHO). Stop TB Partnership. In The Paradigm Shift 2016–2020: Global Plan to End TB; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Global Tuberculosis Report. 2018. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 12 December 2018).
- Ulmasova, D.J.; Uzakova, G.; Tillyashayhov, M.N.; Turaev, L.; van Gemert, W.; Hoffmann, H.; Zignol, M.; Kremer, K.; Gombogaram, T.; Gadoev, J.; et al. Multidrug-resistant tuberculosis in Uzbekistan: Results of a nationwide survey, 2010 to 2011. Eurosurveillance 2013, 18, 20609. [Google Scholar] [CrossRef] [PubMed]
- Shewade, D.; Kokane, A.M.; Singh, A.R.; Verma, M.; Parmar, M.; Chauhan, A.; Chahar, S.S.; Tiwari, M.; Khan, S.N.; Gupta, V.; et al. High pre-diagnosis attrition among patients with presumptive MDR-TB: An operational research from Bhopal district, India. BMC Health Serv. Res. 2017, 17, 249. [Google Scholar] [CrossRef]
- Hasker, E.; Khodjikhanov, M.; Sayfiddinova, S.; Rasulova, G.; Yuldashova, U.; Uzakova, G.; Butabekov, I.; Veen, J.; van der Werf, M.J.; Lefevre, P. Why do tuberculosis patients default in Tashkent City, Uzbekistan? A qualitative study. Int. J. Tuberc. Lung Dis. 2010, 14, 1132–1139. [Google Scholar] [PubMed]
- Oo, T.; Kyaw, K.W.Y.; Soe, K.T.; Saw, S.; Satyanarayana, S.; Aung, S.T. Magnitude and reasons for pre-diagnosis attrition among presumptive multi-drug resistant tuberculosis patients in Bago Region, Myanmar: A mixed methods study. Sci. Rep. 2019, 9, 7189. [Google Scholar] [CrossRef] [PubMed]
- Murongazvombo, A.S.; Dlodlo, R.A.; Shewade, H.D.; Robertson, V.; Hirao, S.; Pikira, E.; Zhanero, C.; Taruvinga, R.K.; Andifasi, P.; Tshuma, C. Where, when, and how many tuberculosis patients are lost from presumption until treatment initiation? A step by step assessment in a rural district in Zimbabwe. Int. J. Infect. Dis. 2019, 78, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Botha, E.; Den Boon, S.; Lawrence, K.-A.; Reuter, H.; Verver, S.; Lombard, C.J.; Dye, C.; Enarson, D.A.; Beyers, N. From Suspect to Patient: Tuberculosis Diagnosis and Treatment Initiation in Health Facilities in South Africa. Int. J. Tuberc. Lung Dis. 2008, 12, 936–941. [Google Scholar] [PubMed]
- Shewade, H.D.; Nair, D.; Klinton, J.S.; Parmar, M.; Lavanya, J.; Murali, L.; Gupta, V.; Tripathy, J.P.; Swaminathan, S.; Kumar, A.M.V. Low pre-diagnosis attrition but high pre-treatment attrition among patients with MDR-TB: An operational research from Chennai, India. J. Epidemiol. Glob. Health 2017, 7, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Shewade, H.D.; Govindarajan, S.; Sharath, B.N.; Tripathy, J.P.; Chinnakali, P.; Kumar, A.M.V.; Muthaiah, M.; Vivekananda, K.; Paulraj, A.K.; Roy, G. MDR-TB screening in a setting with molecular diagnostic techniques: Who got tested, who didn’t and why? Public Health Action 2015, 5, 132–139. [Google Scholar] [CrossRef] [PubMed]
- The World Bank Data. Available online: https://data.worldbank.org/?locations=UZ-XN (accessed on 8 March 2021).
- McNutt, L.-A.; Wu, C.; Xue, X.; Hafner, J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003, 157, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qian, L.; Shi, J.; Franklin, M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med. Res. Methodol. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- MSF. How We’re Helping in Uzbekistan: Treating Patients Living with HIV and Tuberculosis. Available online: https://www.doctorswithoutborders.org/what-we-do/countries/uzbekistan (accessed on 16 December 2020).
- Ardizzoni, E.; Fajardo, E.; Saranchuk, P.; Casenghi, M.; Page, A.-L.; Varaine, F.; Kosack, C.S.; Hepple, P. Implementing the Xpert® MTB/RIF Diagnostic Test for Tuberculosis and Rifampicin Resistance: Outcomes and Lessons Learned in 18 Countries. PLoS ONE 2015, 10, e0144656. [Google Scholar] [CrossRef] [PubMed]
- Trébucq, A.; Enarson, D.A.; Chiang, C.Y.; Van Deun, A.; Harries, A.D.; Boillot, F.; Detjen, A.; Fujiwara, P.I.; Graham, S.M.; Monedero, I.; et al. Xpert® MTB/RIF for national tuberculosis programmes in low-income countries: When, where and how? Int. J. Tuberc. Lung Dis. 2011, 15, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Jokwiro, A.; Timire, C.; Harries, A.D.; Gwinji, P.T.; Mulema, A.; Takarinda, K.C.; Mafaune, P.T.; Sandy, C. Has the utilisation of Xpert ® MTB/RIF in Manicaland Province, Zimbabwe, improved with new guidance on whom to test? Public Health Action 2018, 8, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.; Michel, C.; Manhiça, I.; Mutaquiha, C.; Monivo, C.; Saize, D.; Beste, J.; Creswell, J.; Codlin, A.J.; Gloyd, S. Remote monitoring of Xpert® MTB/RIF testing in Mozambique: Results of programmatic implementation of GxAlert. Int. J. Tuberc. Lung Dis. 2016, 20, 335–341. [Google Scholar] [CrossRef] [PubMed]
- MSF Uzbekistan: Fighting Tuberculosis in Karakalpakstan. Available online: https://www.doctorswithoutborders.org/what-we-do/news-stories/story/uzbekistan-fighting-tuberculosis-karakalpakstan#:~:text=InUzbekistan’sAutonomousRepublic,supporttoimprovetreatmentadherence (accessed on 16 December 2020).

| Province or City | Number of GeneXpert Machines in 2018 | Number of GeneXpert Machines in 2019 | Number of Districts or Cities | Districts with GeneXpert (%) |
|---|---|---|---|---|
| Andijan | 3 | 4 | 17 | 24% |
| Bukhara | 1 | 4 | 13 | 31% |
| Fergana | 2 | 4 | 19 | 21% |
| Jizzakh | 1 | 4 | 13 | 31% |
| Karakalpakstan | 6 | 8 | 16 | 50% |
| Namangan | 3 | 5 | 12 | 42% |
| Navoiy | 1 | 3 | 10 | 30% |
| Qashqadaryo | 1 | 4 | 15 | 27% |
| Samarqand | 2 | 4 | 16 | 25% |
| Sirdaryo | 1 | 3 | 11 | 27% |
| Surxondaryo | 2 | 3 | 14 | 21% |
| Tashkent | 2 | 4 | 19 | 21% |
| Tashkent City | 2 | 4 | 11 | 36% |
| Xorazm | 1 | 3 | 13 | 23% |
| National | 28 * | 57 ** | 199 | 29% |
| Province/Region | 2018 | 2019 | ||||
|---|---|---|---|---|---|---|
| Number of Presumptive TB Patients * | Xpert MTB/RIF Testing | Number of Presumptive TB Patients * | Xpert MTB/RIF Testing | |||
| N | n | (%) | N | n | (%) | |
| Andijan | 19,419 | 2641 | (14) | 9973 | 6929 | (69) |
| Bukhara | 22,109 | 4731 | (21) | 20,897 | 12,290 | (59) |
| Fergana | 25,540 | 5114 | (20) | 25,162 | 8391 | (33) |
| Jizzakh | 11,685 | 2678 | (23) | 11,499 | 5646 | (49) |
| Karakalpakstan | 15,614 | 11,926 | (76) | 11,462 | 11,462 | (100) |
| Namangan | 26,280 | 3835 | (15) | 13,819 | 6774 | (49) |
| Navoiy | 5670 | 2490 | (44) | 9656 | 5138 | (53) |
| Qashqadaryo | 15,371 | 3230 | (21) | 17,115 | 7049 | (41) |
| Samarqand | 9737 | 4151 | (43) | 16,813 | 7124 | (42) |
| Sirdaryo | 5206 | 2937 | (56) | 6406 | 4517 | (71) |
| Surxondaryo | 32,637 | 3000 | (9) | 15,164 | 4726 | (31) |
| Tashkent | 25,592 | 4217 | (16) | 40,979 | 8512 | (21) |
| Tashkent City | 9054 | 3487 | (39) | 8722 | 4791 | (55) |
| Xorazm | 10,835 | 2202 | (20) | 6667 | 4181 | (63) |
| National | 23,4749 | 56,639 | (24) | 214,334 | 97,530 | (46) |
| Characteristics | Tashkent | Bukhara | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 1282 | (100) | 658 | (100) | 1940 | (100) |
| Age (Years) | ||||||
| Less than 15 | 17 | (1.3) | 3 | (0.5) | 20 | (1.0) |
| 15–34 | 293 | (22.9) | 66 | (10.0) | 359 | (18.5) |
| 35–54 | 444 | (34.6) | 173 | (26.3) | 617 | (31.8) |
| 55–74 | 461 | (36.0) | 308 | (46.8) | 769 | (39.6) |
| 75 and above | 67 | (5.2) | 108 | (16.4) | 175 | (9.0) |
| Sex | ||||||
| Male | 738 | (57.6) | 284 | (43.2) | 1022 | (52.7) |
| Female | 544 | (42.4) | 374 | (56.8) | 918 | (47.3) |
| Site of Health Facility | ||||||
| Tashkent peripheral | 159 | (12.4) | 0 | (0.0) | 159 | (8.2) |
| Tashkent central | 1123 | (87.6) | 0 | (0.0) | 1123 | (57.9) |
| Bukhara peripheral | 0 | (0.0) | 524 | (79.6) | 524 | (27.0) |
| Bukhara central | 0 | (0.0) | 134 | (20.4) | 134 | (6.9) |
| Diagnostic Capacity | ||||||
| Microscopy and GeneXpert | 452 | (35.3) | 103 | (15.7) | 555 | (28.6) |
| Microscopy only | 720 | (56.2) | 223 | (33.9) | 943 | (48.6) |
| No microscopy no GeneXpert | 110 | (8.6) | 332 | (50.5) | 442 | (22.8) |
| Distance (km) * | ||||||
| 0–9 | 652 | (50.9) | 240 | (36.5) | 892 | (46.0) |
| 10–19 | 553 | (43.1) | 167 | (25.4) | 720 | (37.1) |
| 20 and above | 77 | (6.0) | 251 | (38.1) | 328 | (16.9) |
| Total | Not Tested Using Xpert | RR | (95% CI) | aRR | (95% CI) | ||
|---|---|---|---|---|---|---|---|
| N | (%) | ||||||
| Total | 1940 | 832 | (43) | ||||
| Sex | |||||||
| Male | 1022 | 402 | (39) | Ref. | Ref. | ||
| Female | 918 | 430 | (47) | 1.19 | (1.07,1.32) | 1.04 | (0.96,1.12) |
| Age (Years) | |||||||
| Less than 15 | 20 | 8 | (40) | 1.71 | (0.97,3.02) | 1.74 | (1.09,2.77) |
| 15–34 | 359 | 84 | (23) | Ref. | Ref. | ||
| 35–54 | 617 | 241 | (39) | 1.67 | (1.35,2.06) | 1.35 | (1.15,1.59) |
| 55–74 | 769 | 390 | (51) | 2.17 | (1.77,2.65) | 1.46 | (1.25,1.71) |
| 75 and above | 175 | 109 | (62) | 2.66 | (2.14,3.32) | 1.45 | (1.22,1.73) |
| Distance (km) * | |||||||
| 0–9 | 892 | 232 | (26) | Ref. | Ref. | ||
| 10–19 | 720 | 359 | (50) | 1.92 | (1.68,2.19) | 1.66 | (1.45,1.88) |
| 20 and above | 328 | 241 | (73) | 2.83 | (2.48,3.21) | 1.77 | (1.52,2.05) |
| Site of Health Facility | |||||||
| Tashkent central | 1123 | 203 | (18) | Ref. | Ref. | ||
| Tashkent peripheral | 159 | 152 | (96) | 5.29 | (4.65,6.02) | 4.69 | (4.03,5.47) |
| Bukhara central | 134 | 96 | (72) | 3.96 | (3.36,4.67) | 6.26 | (5.12,7.66) |
| Bukhara peripheral | 524 | 381 | (73) | 4.02 | (3.51,4.60) | 2.79 | (2.43,3.19) |
| Diagnostic Capacity | |||||||
| Microscopy and GeneXpert | 555 | 22 | (4) | Ref. | Ref. | ||
| Microscopy only | 943 | 454 | (48) | 12.15 | (8.02,18.39) | 7.75 | (5.22,11.50) |
| No Microscopy no GeneXpert | 442 | 356 | (81) | 20.32 | (13.46,30.68) | 6.31 | (4.17,9.55) |
| Duration (Days) | Number Eligible | Number Assessed | (%) | Median Days (IQR) | Max (Days) |
|---|---|---|---|---|---|
| Total (Both the Regions) | |||||
| Initial visit to PHC and sputum collection | 1385 | 1368 | (99) | 0 (0,0) | 8 |
| Sample collection at PHC to receipt at GX laboratory | 1368 | 576 | (42) | 1 (1,1) | 13 |
| Sample receipt at GX laboratory to result receipt at PHC | 576 | 575 | (99) | 0 (0,0) | 10 |
| Total (Initial visit to PHC to receipt of result at PHC) | 1385 | 575 | (42) | 1 (1,2) | 18 |
| Tashkent City | |||||
| Initial visit to PHC and sputum collection | 830 | 813 | (98) | 0 (0,1) | 8 |
| Sample collection at PHC to receipt at GX laboratory | 813 | 476 | (59) | 1 (1,1) | 8 |
| Sample receipt at GX laboratory to result receipt at PHC | 476 | 475 | (99) | 0 (0,0) | 1 |
| Total (Initial visit to PHC to receipt of result at PHC) | 830 | 475 | (57) | 1 (1,2) | 9 |
| Bukhara Region | |||||
| Initial visit to PHC and sputum collection | 555 | 555 | (100) | 0 (0,0) | 0 |
| Sample collection at PHC to receipt at GX laboratory | 555 | 100 | (18) | 2 (1,4) | 13 |
| Sample receipt at GX laboratory to result receipt at PHC | 100 | 100 | (100) | 0 (0,4) | 10 |
| Total (Initial visit to PHC to receipt of result at PHC) | 555 | 100 | (18) | 3 (1,6) | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turaev, L.; Kumar, A.; Nabirova, D.; Alaverdyan, S.; Parpieva, N.; Abdusamatova, B. Universal Access to Xpert MTB/RIF Testing for Diagnosis of Tuberculosis in Uzbekistan: How Well Are We Doing? Int. J. Environ. Res. Public Health 2021, 18, 2915. https://doi.org/10.3390/ijerph18062915
Turaev L, Kumar A, Nabirova D, Alaverdyan S, Parpieva N, Abdusamatova B. Universal Access to Xpert MTB/RIF Testing for Diagnosis of Tuberculosis in Uzbekistan: How Well Are We Doing? International Journal of Environmental Research and Public Health. 2021; 18(6):2915. https://doi.org/10.3390/ijerph18062915
Chicago/Turabian StyleTuraev, Laziz, Ajay Kumar, Dilyara Nabirova, Sevak Alaverdyan, Nargiza Parpieva, and Barno Abdusamatova. 2021. "Universal Access to Xpert MTB/RIF Testing for Diagnosis of Tuberculosis in Uzbekistan: How Well Are We Doing?" International Journal of Environmental Research and Public Health 18, no. 6: 2915. https://doi.org/10.3390/ijerph18062915
APA StyleTuraev, L., Kumar, A., Nabirova, D., Alaverdyan, S., Parpieva, N., & Abdusamatova, B. (2021). Universal Access to Xpert MTB/RIF Testing for Diagnosis of Tuberculosis in Uzbekistan: How Well Are We Doing? International Journal of Environmental Research and Public Health, 18(6), 2915. https://doi.org/10.3390/ijerph18062915








