Exposure to Particulate PAHs on Potential Genotoxicity and Cancer Risk among School Children Living Near the Petrochemical Industry
Abstract
1. Introduction
2. Methods
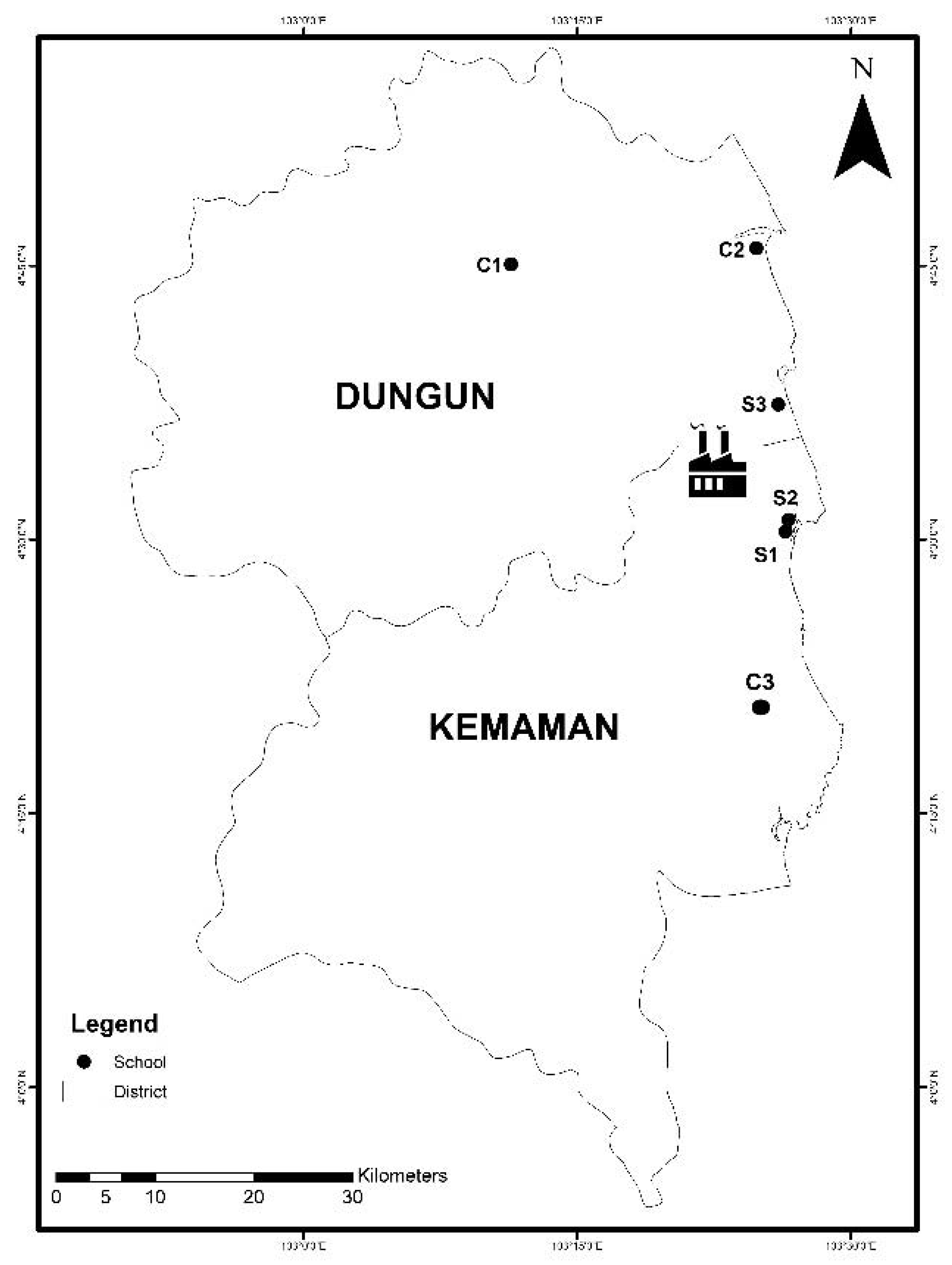
2.1. Study Location
2.2. Study Population
2.3. Measurements of Particulate Matter
2.4. PAH Analysis
2.5. Health Risk Assessment
2.6. Collection of Exfoliated Buccal Mucosa and MN Assay
2.7. Comet Assay
2.8. Statistical Analysis
2.9. Quality Control
3. Results and Discussion
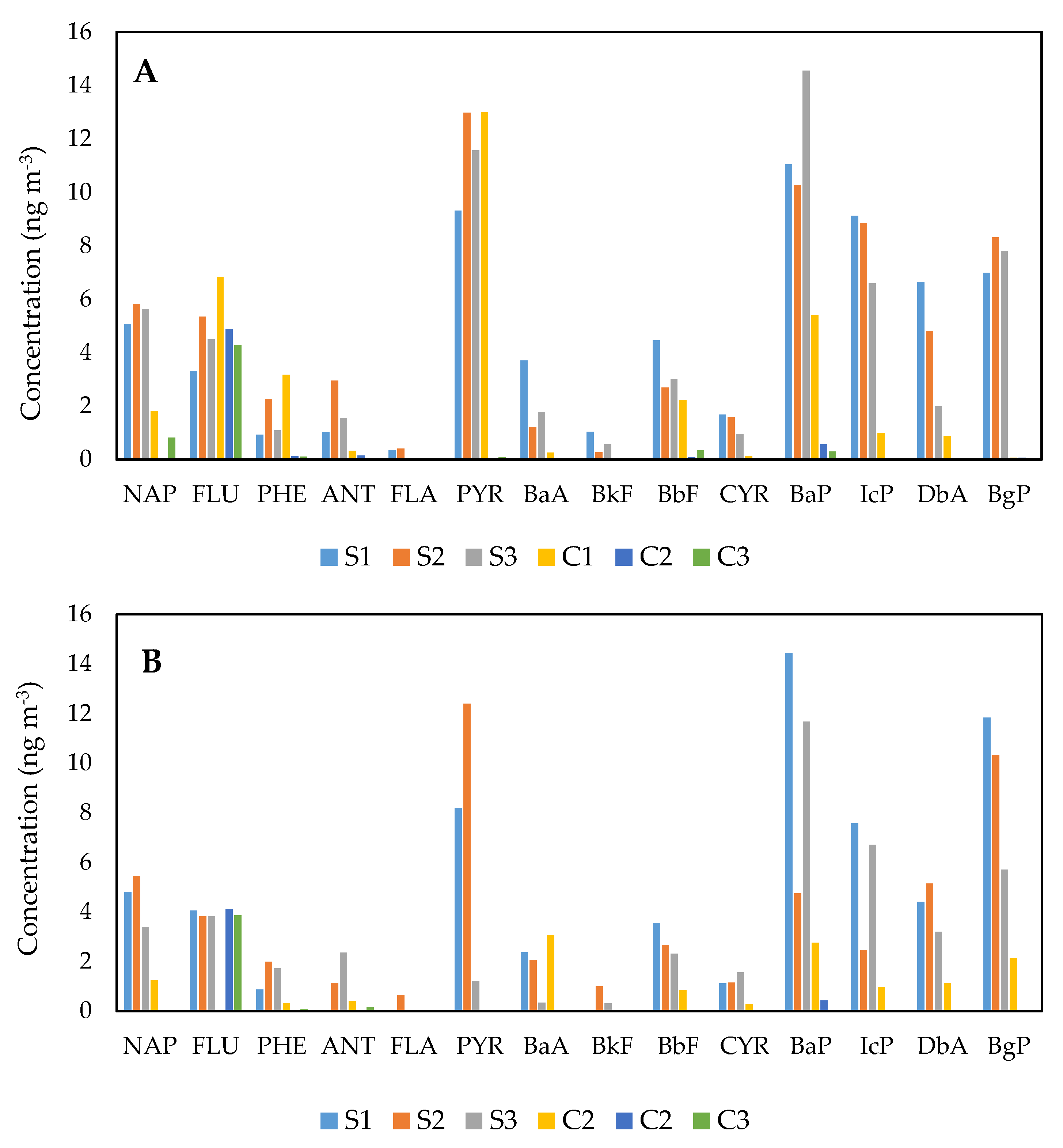
3.1. Distributions of PAH Species at the Exposed and Comparative Schools
3.2. Source Diagnostic Ratio
3.3. Principal Components Analysis (PCA)
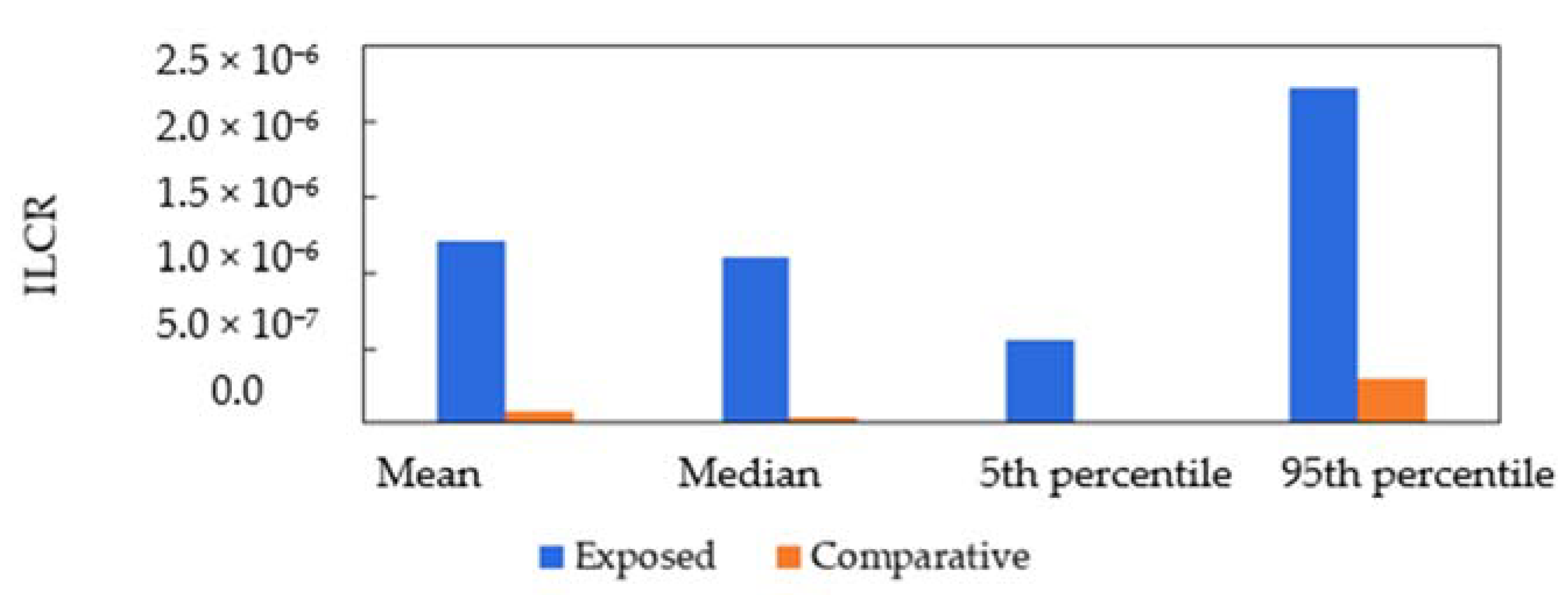
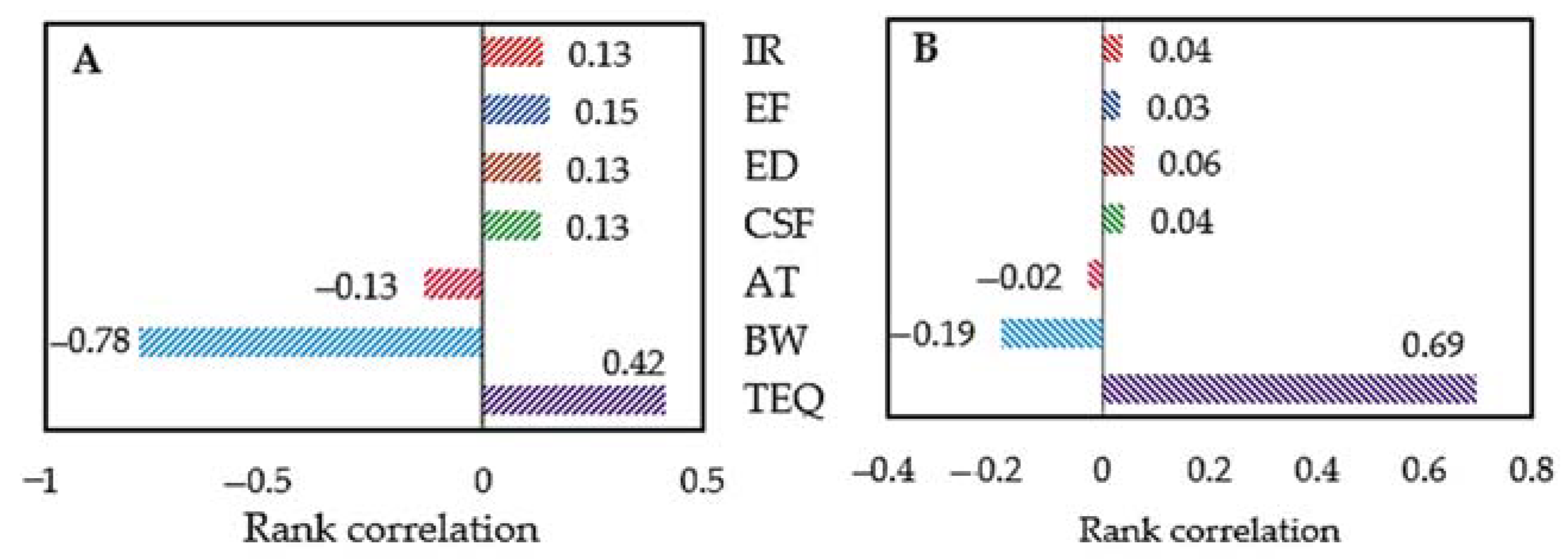
3.4. Health Risk Assessment
3.5. Individual Factors in DNA Damage
3.6. Relationship between Tail Moment with PAH Exposure and Other Risk Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, N. Polycyclic Aromatic Hydrocarbons (PAHs) in Indoor Air and Dust Samples of Different Saudi Microenvironments; Health and Carcinogenic Risk Assessment for the General Population. Sci. Total Environ. 2019, 696, 133995. [Google Scholar] [CrossRef]
- Dai, Y.; Huo, X.; Cheng, Z.; Wang, Q.; Zhang, Y.; Xu, X. Alterations in Platelet Indices Link Polycyclic Aromatic Hydrocarbons Toxicity to Low-Grade Inflammation in Preschool Children. Environ. Int. 2019, 131, 105043. [Google Scholar] [CrossRef]
- Huang, X.; Deng, X.; Li, W.; Liu, S.; Chen, Y.; Yang, B.; Liu, Q. Internal Exposure Levels of Polycyclic Aromatic Hydrocarbons in Children and Adolescents: A Systematic Review and Meta-Analysis. Environ. Health Prev. Med. 2019, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.A.; Obi, G.; Uzoekwe, S.A.; Egobueze, F.E.; Odali, E.W.; Tesi, G.O.; Nwajei, G.E.; Martincigh, B.S. Distribution, sources and risk of exposure to polycyclic aromatic hydrocarbons in indoor dusts from electronic repair workshops in southern Nigeria. Emerg. Contam. 2019, 5, 23–30. [Google Scholar] [CrossRef]
- Sulong, N.A.; Latif, M.T.; Sahani, M.; Khan, M.F.; Fadzil, M.F.; Mohd Tahir, N.; Mohamad, N.; Sakai, N.; Fujii, Y.; Othman, M.; et al. Distribution, sources and potential health risks of polycyclic aromatic hydrocarbons (PAHs) in PM2.5 collected during different monsoon seasons and haze episode in Kuala Lumpur. Chemosphere 2019, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Delerue-matos, C.; Carmo, M.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxid; WHO: Geneva, Switzerland, 2006; Available online: http://apps.who.int/iris/bitstream/handle/10665/69477/WHO_SDE_PHE_OEH_06.02_eng.pdf,sequence=1 (accessed on 14 June 2018).
- Agency for Toxic Substances and Disease Registry (ATSDR). ATSDR’s Substance Priority List. 2020. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 20 July 2020).
- Abdel-shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Mu, X.; Zhao, L.; Gu, C.; Gao, H.; Ma, J.; Mao, X.; Huang, T. A WRF-CMAQ modeling of atmospheric PAH cycling and health risks in the heavy petrochemical industrialised Lanzhou valley, Northwest China. J. Clean Prod. 2021, 291, 125989. [Google Scholar] [CrossRef]
- Yuan, T.; Shie, R.; Chin, Y.; Chan, C. Assessment of the levels of urinary 1-hydroxypyrene and air polycyclic aromatic hydrocarbon in PM2.5 for adult exposure to the petrochemical complex emissions. Environ. Res. 2015, 136, 219–226. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, S.; Zhou, Y.; He, D.; Li, Y.; Ren, M.; Xu, Z.; Fang, J. Characterisation and quantification of PAH atmospheric pollution from a large petrochemical complex in Guangzhou: GC–MS/MS analysis. Microchem. J. 2015, 119, 140–144. [Google Scholar] [CrossRef]
- Dong, T.T.T.; Lee, B.K. Characteristics, toxicity, and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in road dust of Ulsan, Korea. Chemosphere 2009, 74, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Bozlaker, A.; Muezzinoglu, A.; Odabasi, M. Atmospheric concentrations, dry deposition and air-soil exchange of polycyclic aromatic hydrocarbons (PAHs) in an industrial region in Turkey. J. Hazard. Mater. 2008, 153, 1093–1102. [Google Scholar] [CrossRef]
- WHO. Air Pollution and Child Health: Prescribing Clean Air. Summary; World Health Organization: Geneva, Switzerland, 2018; pp. 1–18. [Google Scholar]
- Fan, R.; Wang, D.; Mao, C.; Ou, S.; Lian, Z.; Huang, S.; Lin, Q.; Ding, R.; She, J. Preliminary study of children’s exposure to PAHs and its association with 8-hydroxy-2′-deoxyguanosine in Guangzhou, China. Environ. Int. 2012, 42, 53–58. [Google Scholar] [CrossRef]
- Murawski, A.; Roth, A.; Schwedler, G.; Schmied-tobies, M.I.H.; Rucic, E.; Pluym, N.; Scherer, M.; Scherer, G.; Conrad, A.; Kolossa-Gehring, M. Polycyclic Aromatic Hydrocarbons (PAH) in Urine of Children and Adolescents in Germany—Human Biomonitoring Results of the German Environmental Survey 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 2020, 226, 113491. [Google Scholar] [CrossRef]
- Tang, C.S.; Lung, S.C.C.; Chang, T.Y.; Tu, H.H.; Chang, L.T. Investigation of Microenvironmental Exposures to Particle-Bound Polycyclic Aromatic Hydrocarbons for Elementary School Children. Int. J. Environ. Res. Public Health 2019, 16, 4390. [Google Scholar] [CrossRef]
- Sopian, N.A.; Jalaludin, J. The application of biomarker in determining genotoxic potential of polyaromatic hydrocarbon exposure among children. Ann. Trop. Med. Public Health 2017, 10, 533–543. [Google Scholar]
- Jasso-pineda, Y.; Díaz-barriga, F.; Yáñez-estrada, L.; Pérez-vázquez, F.J.; Pérez-maldonado, I.N. DNA damage in Mexican children living in high-risk contaminated scenarios. Sci. Total Environ. 2015, 519, 38–48. [Google Scholar] [CrossRef]
- Busso, T.; Carreras, H.; Amarillo, A.C. Exposure to polycyclic aromatic hydrocarbons in urban environments: Health risk assessment by age groups. Environ. Pol. 2014, 195, 157–162. [Google Scholar]
- Pelallo-martınez, N.A.; Carrizales-ya, L.B.L.; Martı, F. Genotoxic and Hematological Effects in Children Exposed to a Chemical Mixture in a Petrochemical Area in Mexico. Arch. Environ. Contam. Toxico. 2014, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ruchirawat, M.; Settachan, D.; Navasumrit, P. Assessment of potential cancer risk in children exposed to urban air pollution in Bangkok, Thailand. Toxicol. Lett. 2007, 168, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huo, X.; Wang, Q.; Wang, C.; Hylkema, M.N.; Xu, X. PM2.5-Bound PAHs Exposure Linked with Low Plasma Insulin-like Growth Factor 1 Levels and Reduced Child Height. Environ. Int. 2020, 138, 105660. [Google Scholar] [CrossRef]
- Chen, L.; Hu, G.; Fan, R.; Lv, Y.; Dai, Y.; Xu, Z. Association of PAHs and BTEX Exposure with Lung Function and Respiratory Symptoms among a Nonoccupational Population near the Coal Chemical Industry in Northern China. Environ. Int. 2018, 120, 480–488. [Google Scholar] [CrossRef]
- Poursafa, P.; Dadvand, P.; Mehdi, M.; Hajizadeh, Y.; Ebrahimpour, K.; Mansourian, M.; Pourzamani, H.; Sunyer, J.; Kelishadi, R. Association of Polycyclic Aromatic Hydrocarbons with Cardiometabolic Risk Factors and Obesity in Children. Environ. Int. 2018, 118, 203–210. [Google Scholar] [CrossRef]
- Bortey-Sam, N.; Ikenaka, Y.; Akoto, O.; Nakayama, S.M.M.; Asante, K.A.; Baidoo, E.; Obirikorang, C.; Saengtienchai, A.; Isoda, N.; Nimako, C.; et al. Oxidative Stress and Respiratory Symptoms Due to Human Exposure to Polycyclic Aromatic Hydrocarbons (PAHs) in Kumasi, Ghana. Environ. Pollut. 2017, 228, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.S.; Rossato, J.M.; Vaz Rocha, J.A.; Vargas, V.M. Characterisation of an area of reference for inhalable particulate matter (PM2.5) associated with genetic biomonitoring in children. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 778, 44–55. [Google Scholar] [CrossRef]
- Ceretti, E.; Feretti, D.; Viola, G.C.V.; Zerbini, I.; Limina, R.M.; Zani, C.; Capelli, M.; Lamera, R.; Donato, F.; Gelatti, U. DNA Damage in Buccal Mucosa Cells of Pre-School Children Exposed to High Levels of Urban Air Pollutants. PLoS ONE 2014, 9, e96524. [Google Scholar] [CrossRef] [PubMed]
- Sopian, N.A.; Jalaludin, J.; Mayusi, T.Z.A.T.; Latif, M.T. Increased Chromosomal Damage among Children in Proximity to Industrial Zone. Aerosol Air Qual. Res. 2020, 20, 944–955. [Google Scholar] [CrossRef]
- Sánchez-Guerra, M.; Pelallo-Martínez, N.; Díaz-Barriga, F.; Rothenberg, S.J.; Hernández-Cadena, L.; Faugeron, S.; Oropeza-Hernándeze, L.F.; Guaderrama-Díaz, M.; Quintanilla-Vega, B. Environmental polycyclic aromatic hydrocarbon (PAH) exposure and DNA damage in Mexican children. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 742, 66–71. [Google Scholar] [CrossRef]
- Kyrtopoulos, S.; Georgiadis, P.; Autrup, H.; Demopoulos, N.; Farmer, P.; Haugen, A.; Katsouyanni, K.; Lambert, B.; Ovrebo, S.; Sram, R.; et al. Biomarkers of genotoxicity of urban air pollution: Overview and descriptive data from a molecular epidemiology study on populations exposed to moderate-to- low levels of polycyclic aromatic hydrocarbons: The AULIS project. Mutat. Res. 2001, 496, 207–228. [Google Scholar] [CrossRef]
- Hrelia, P.; Maffei, F.; Angelini, S.; Forti, G.C. A molecular epidemiological approach to health risk assessment of urban air pollution. Toxicol. Lett. 2004, 149, 261–267. [Google Scholar] [CrossRef]
- Suhaimi, N.F.; Jalaludin, J. Biomarker as a Research Tool in Linking Exposure to Air Particles and Respiratory Health. BioMed Res. Int. 2015, 2015, 962853. [Google Scholar] [CrossRef] [PubMed]
- Daud, S.A.M.; Jalaludin, J.; Sopian, N.A. Air pollutants exposure and frequency of micronuclei (MN) among primary school children nearby industrial Area. Malays. J. Med. Health Sci. 2018, 14, 56–62. [Google Scholar]
- Kamaruddin, A.S.; Jalaludin, J.; Hamedon, T.R.; Hisamuddin, N.H. FeNO as a Biomarker for Airway Inflammation Due to Exposure to Air Pollutants among School Children Nearby Industrial Areas in Terengganu. Pertanika J. Sci. Technol. 2019, 27, 589–600. [Google Scholar]
- Seifi, M.; Niazi, S.; Johnson, G.; Nodehi, V.; Yunesian, M. Exposure to ambient air pollution and risk of childhood cancers: A population-based study in Tehran, Iran. Sci. Total Environ. 2019, 646, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Jalaludin, J.; Noh, S.N.S.; Suhaimi, N.F.; Akim, A.M. Tumor necrosis factor-alpha as biomarkers of exposure to indoor pollutants among primary school children in Klang Valley. Am. J. Appl. Sci. 2014, 11, 1616–1630. [Google Scholar] [CrossRef]
- Hisamuddin, N.H.; Jalaludin, J. Traffic Related Air Pollution and Its Impact on Respiratory Health among Children. Malays. J. Med. Health Sci. 2019, 15, 103–106. [Google Scholar]
- Ceppi, M.; Biasotti, B.; Fenech, M.; Bonassi, S. Human population studies with the exfoliated buccal micronucleus assay: Statistical and epidemiological issues. Mutat. Res. Rev. Mutat. Res. 2010, 705, 11–19. [Google Scholar] [CrossRef]
- Sinitsky, M.Y.; Druzhinin, V.G. The application of the cytokinesis-block micronucleus assay on peripheral blood lymphocytes for the assessment of genome damage in long-term residents of areas with high radon concentration. J. Radiat. Res. 2014, 55, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Latif, M.T.; Lim, C.H.; Amil, N.; Jaafar, S.A.; Dominick, D.; Mohd Nadzir, M.S.; Sahani, M.; Tahir, N.M. Seasonal effect and source apportionment of polycyclic aromatic hydrocarbons in PM2.5. Atmos. Environ. 2015, 106, 178–190. [Google Scholar] [CrossRef]
- Mirrezaei, M.A.; Orkomi, A.A. Gas flares contribution in total health risk assessment of BTEX in Asalouyeh, Iran. Process Safety Environ. Prot. 2020, 137, 223–237. [Google Scholar] [CrossRef]
- Nisbet, I.C.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Nadali, A.; Leili, M.; Bahrami, A.; Karami, M.; Afkhami, A. Phase distribution and risk assessment of PAHs in ambient air of Hamadan, Iran. Ecotox. Environ. Saf. 2021, 209, 111807. [Google Scholar] [CrossRef]
- Othman, M.; Latif, M.T.; Jamhari, A.A.; Abd Hamid, H.H.; Uning, R.; Khan, M.F.; Mohd Nadzir, M.S.; Sahani, M.; Abdul Wahab, M.I.; Chan, K.M. Spatial distribution of fine and coarse particulate matter during a southwest monsoon in Peninsular Malaysia. Chemosphere 2021, 262, 127767. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, R.; Yang, S.; Xu, L. Analysis and risk assessment of PM2.5-bound PAHs in a comparison of indoor and outdoor environments in a middle school: A case study in Beijing, China. Atmosphere 2020, 11, 904. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Exposure Factors Handbook: 2011 Edition; EPA/600/R-09/052F; National Center for Environmental Assessment: Washington, DC, USA, 2011. Available online: https://www.epa.gov/expobox/exposure-factors-handbook-chapter-6 (accessed on 5 February 2021).
- Peng, C.; Chen, W.; Liao, X.; Wang, M.; Ouyang, Z.; Jiao, W.; Bai, Y. Polycyclic aromatic hydrocarbons in urban soils of Beijing: Status, sources, distribution and potential risk. Environ. Pol. 2011, 159, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, N.; Liang, W.; Chen, X.; Hou, R.; Kang, Y.; Guo, Q.; Cao, S.; Duan, X. Polycycl. Aromatic hydrocarbon exposure of children in typical household coal combustion environments: Seasonal variations, sources, and carcinogenic risks. Int. J. Environ. Res. Public Health 2020, 17, 6520. [Google Scholar] [CrossRef]
- Tarafdar, A.; Oh, M.J.; Nguyen-Phuong, Q.; Kwon, J.H. Profiling and potential cancer risk assessment on children exposed to PAHs in playground dust/soil: A comparative study on poured rubber surfaced and classical soil playgrounds in Seoul. Environ. Geochem. Health 2020, 42, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M. OpenComet: An automated tool for comet assay image analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zhang, X.; Li, J.; Zhao, T.; Gao, Y.; Jiang, P.; Li, Y.; Chen, X.; Wang, W. Characteristics of PM2.5-bound PAHs at an Urban Site and a Suburban Site in Jinan in North China Plain. Aerosol Air Qual. Res. 2019, 19, 871–884. [Google Scholar] [CrossRef]
- Mohamad, N.; Latif, M.T.; Khan, M.F. Source apportionment and health risk assessment of PM10 in a naturally ventilated school in a tropical environment. Ecotox. Environ. Saf. 2016, 124, 351–362. [Google Scholar] [CrossRef]
- Yabueng, N.; Wiriya, W.; Chantara, S. Influence of zero-burning policy and climate phenomena on ambient PM2.5 patterns and PAHs inhalation cancer risk during episodes of smoke haze in Northern Thailand. Atmos. Environ. 2020, 232, 117485. [Google Scholar] [CrossRef]
- Richmond-bryant, J.; Saganich, C.; Bukiewicz, L.; Kalin, R. Associations of PM2.5 and black carbon concentrations with traffic, idling, background pollution, and meteorology during school dismissals. Sci. Total Environ. 2009, 407, 3357–3364. [Google Scholar] [CrossRef]
- Xu, H.; Guinot, B.; Cao, J.; Li, Y.; Niu, X.; Ho, K.F.; Shen, Z.; Liu, S.; Zhang, T.; Lei, Y.; et al. Source, health risk and composition impact of outdoor very fine particles (VFPs) to school indoor environment in Xi’an, North western China. Sci. Total Environ. 2018, 612, 238–246. [Google Scholar] [CrossRef]
- Di Gilio, A.; Farella, G.; Marzocca, A.; Giua, R.; Assennato, G.; Tutino, M.; de Gennaro, G. Indoor/Outdoor Air Quality Assessment at School near the Steel Plant in Taranto (Italy). Adv. Meteorol. 2017, 2017, 1526209. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Madureira, J.; De Oliveira, E.; Delerue-matos, C.; Morais, S.; Pereira, C. Polycyclic aromatic hydrocarbons in primary school environments: Levels and potential risks. Sci. Total Environ. 2017, 575, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Hassanvand, M.S.; Naddafi, K.; Faridi, S.; Nabizadeh, R.; Sowlat, M.H.; Momeniha, F.; Gholampour, A.; Arhami, M.; Kashani, H.; Zare, A.; et al. Characterisation of PAHs and metals in indoor/outdoor PM10/PM2.5/PM1 in a retirement home and a school dormitory. Sci. Total Environ. 2015, 527–528, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, X.; Ji, H.; Li, C.; Ding, H.; Briki, M.; Tang, L.; Zhang, Y. Potential threat of heavy metals and PAHs in PM2.5 in different urban functional areas of Beijing. Atmos. Res. 2016, 178–179, 6–16. [Google Scholar] [CrossRef]
- Ana, G.R.E.E.; Sridhar, M.K.C.; Emerole, G.O. Polycyclic aromatic hydrocarbon burden in ambient air in selected Niger Delta communities in Nigeria. J. Air Waste Manag. Assoc. 2012, 62, 18–25. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Zhang, S.; Wan, C.; Yue, D.; Ye, Y.; Wang, X. Source diagnostics of polycyclic aromatic hydrocarbons in urban road runoff, dust, rain and canopy throughfall. Environ. Pollut. 2008, 153, 594–601. [Google Scholar] [CrossRef]
- Bucheli, T.D.; Blum, F.; Desaules, A.; Gustafsson, O. Polycyclic aromatic hydrocarbons, black carbon, and molecular markers in soils of Switzerland. Chemosphere 2004, 56, 1061–1076. [Google Scholar] [CrossRef]
- Akyuz, M.; Cabuk, H. Meteorological variations of PM2.5/PM10 concentrations and particle-associated polycyclic aromatic hydrocarbons in the atmospheric environment of Zonguldak, Turkey. J. Hazard. Mater. 2009, 170, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Fang, G.C.; Wu, Y.S.; Chen, M.H.; Ho, T.T.; Huang, S.H.; Rau, J.Y. Polycyclic aromatic hydrocarbons study in Taichung, Taiwan, during 2002–2003. Atmos. Environ. 2004, 38, 3385–3391. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lohmann, R.; Yu, N.; Zhang, C.; Gao, Y.; Zhao, J.; Ma, L. Source apportionment of gaseous and particulate PAHs from traffic emission using tunnel measurements in Shanghai, China. Atmos. Environ. 2015, 107, 129–136. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, Y.C.; Shangdiar, S.; Chou, F.C.; Sheu, Y.T.; Cheng, P.C. Assessment of PM2.5 and PAH content in PM2.5 emitted from mobile source gasoline-fueled vehicles in concomitant with the vehicle model and mileages. Chemosphere 2019, 226, 502–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.S.; Xu, J.; Tian, Y.Z.; Shi, G.L.; Feng, Y.C. Potential source contributions and risk assessment of PAHs in sediments from Taihu Lake, China: Comparison of three receptor models. Water Res. 2012, 46, 3065–3073. [Google Scholar] [CrossRef]
- Kwon, H.O.; Choi, S.D. Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea. Sci. Total Environ. 2014, 470–471, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, L.; Xue, N.; Li, F.; Li, Y.; Vogt, R.D.; Cong, X.; Yan, Y.; Liu, B. Source apportionment of polycyclic aromatic hydrocarbons in soils of Huanghuai Plain, China: Comparison of three receptor models. Sci. Total Environ. 2013, 443, 31–39. [Google Scholar] [CrossRef]
- Thang, P.Q.; Kim, S.J.; Lee, S.J.; Ye, J.; Seo, Y.K.; Baek, S.O.; Choi, S.D. Seasonal characteristics of particulate polycyclic aromatic hydrocarbons (PAHs) in a petrochemical and oil refinery industrial area on the west coast of South Korea. Atmos. Environ. 2019, 198, 398–406. [Google Scholar] [CrossRef]
- Freeman, D.J.; Cattell, F.C. Woodburning as a source of atmospheric polycyclic aromatic hydrocarbons. Environ. Sci. Tech. 2019, 24, 1581–1585. [Google Scholar] [CrossRef]
- Slezakova, K.; Oliveira, M.; Madureira, J.; de Oliveira Fernandes, E.; Delerue-matos, C.; Morais, S.; do Carmo Pereira, M. Polycyclic aromatic hydrocarbons (PAH) in Portuguese educational settings: A comparison between preschools and elementary schools. J. Toxicol. Environ. Health A 2017, 80, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Jamhari, A.A.; Sahani, M.; Latif, M.T.; Chan, K.M.; Seng, H.; Khan, M.F.; Mohd Tahir, N. Concentration and source identification of polycyclic aromatic hydrocarbons (PAHs) in PM10 of urban, industrial and semi-urban areas in Malaysia. Atmos. Environ. 2014, 86, 16–27. [Google Scholar] [CrossRef]
- Byambaa, B.; Yang, L.; Matsuki, A.; Nagato, E.G.; Gankhuyag, K.; Chuluunpurev, B.; Banzragch, L.; Chonokhuu, S.; Tang, N.; Hayakawa, K. Sources and characteristics of polycyclic aromatic hydrocarbons in ambient total suspended particles in Ulaanbaatar City, Mongolia. Int. J. Environ. Res. Public Health 2019, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Grmasha, R.A.; Al-sareji, O.J.; Salman, J.M.; Hashim, K.S. Polycyclic aromatic hydrocarbons (PAHs) in urban street dust within three land-uses of Babylon governorate, Iraq: Distribution, sources, and health risk assessment. J. King Saud Uni. Engin. Sci. 2020. [Google Scholar] [CrossRef]
- Fandi, N.F.M.; Jalaludin, J.; Latif, M.T.; Hamid, H.H.A.; Awang, M.F. BTEX exposure assessment and inhalation health risks to traffic policemen in the Klang valley region, malaysia. Aerosol Air Qual. Res. 2020, 20, 1922–1937. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K.; Mahato, M.K. Monte Carlo simulation-based probabilistic health risk assessment of metals in groundwater via ingestion pathway in the mining areas of Singhbhum copper belt, India. Int. J. Environ. Res. Public Health 2020, 30, 447–460. [Google Scholar] [CrossRef]
- Coronas, M.V.; Pereira, T.S.; Rocha, J.A.V.; Lemos, A.T.; Fachel, J.M.G.; Salvadori, D.M.F.; Vargas, V.M.F. Genetic biomonitoring of an urban population exposed to mutagenic airborne pollutants. Environ. Int. 2009, 35, 1023–1029. [Google Scholar] [CrossRef]
- Aksu, İ.; Anlar, H.G.; Taner, G.; Bacanl, M.; Servet, İ.; Tutkun, E. Assessment of DNA damage in welders using comet and micronucleus assays. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 843, 40–45. [Google Scholar] [CrossRef]
- Zalata, A.; Yahia, S.; El-bakary, A.; Elsheikha, H.M. Increased DNA damage in children caused by passive smoking as assessed by comet assay and oxidative stress. Mutat. Res. 2007, 629, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Beyoglu, D.; Ozkozaci, T.; Akici, N.; Omurtag, G.Z.; Akici, A.; Ceran, O.; Sardas, S. Assessment of DNA damage in children exposed to indoor tobacco smoke. Int. J. Hyg. Environ. Health 2010, 213, 40–44. [Google Scholar] [CrossRef]
- Gajski, G.; Geri, M.; Orescanin, V.; Garaj-vrhovac, V. Cytogenetic status of healthy children assessed with the alkaline comet assay and the cytokinesis-block micronucleus cytome assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 750, 55–62. [Google Scholar] [CrossRef]
- Xu, P.; Chen, Z.; Chen, Y.; Feng, L.; Wu, L.; Xu, D.; Wang, X.; Lou, X.; Lou, J. Body burdens of heavy metals associated with epigenetic damage in children living in the vicinity of a municipal waste incinerator. Chemosphere 2019, 229, 160–168. [Google Scholar] [CrossRef]
- Hofer, T.I.M.; Karlsson, H.L.; Mo, L. DNA oxidative damage and strand breaks in young healthy individuals: A gender difference and the role of lifestyle factors. Free Radic. Res. 2006, 40, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Setayesh, T.; Nersesyan, A.; Misik, M.; Ferk, F.; Langie, S.; Andrade, V.M.; Haslberger, A.; Knasmuller, S. Impact of obesity and overweight on DNA stability: Few facts and many hypotheses. Mutat. Res. Rev. Mutat. Res. 2018, 777, 64–91. [Google Scholar] [CrossRef]
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S.; Zaghloul, S.; Sawaya, W.; Ahmed, N.; Kannan, K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]
- Schoket, B. DNA damage in humans exposed to environmental and dietary polycyclic aromatic hydrocarbons. Mutat. Res. 1999, 424, 143–153. [Google Scholar] [CrossRef]
- Slyskova, J.; Lorenzo, Y.; Karlsen, A.; Carlsen, M.H.; Novosadova, V.; Blomhoff, R.; Vodicka, C.; Collins, A.R. Both genetic and dietary factors underlie individual differences in DNA damage levels and DNA repair capacity. DNA Repair 2014, 16, 66–73. [Google Scholar] [CrossRef]
- Fenech, M. Chapter 4—The Role of Nutrition in DNA Replication, DNA Damage Prevention and DNA Repair. In Principles of Nutrigenetics and Nutrigenomics; Caterina, R.D., Martinez, J.A., Kohlmeier, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 22–32. [Google Scholar]
- Naz, M.; Rehman, N.; Nazam, M.; Kamal, M.; Ganaie, M.A.; Awaad, A.S.; Alqasoumi, S.I. Comparative study of sub-chronic toxicities of mosquito repellents (coils, mats and liquids) on vital organs in Swiss albino mice. Saudi Pharm. J. 2019, 27, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.N.; Goswami, R.; Pal, A. The Insect repellents: A silent environmental chemical toxicant to the health. Environ. Toxicol. Pharmacol. 2017, 50, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Madhubabu, G.; Yenugu, S. Effect of continuous inhalation of allethrin-based mosquito coil smoke in the male reproductive tract of rats. Inhal. Toxicol. 2012, 24, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.; Hashim, J.H.; Jalaludin, J.; Hashim, Z.; Goldstein, B.D. Mosquito coil emissions and health implications. Environ. Health Perspect. 2003, 111, 1454–1460. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Leong, S.W.K.; Lam, K.K.; Hong, C.M.M.; Lee, D.K.M.; Chan, Y.T.F.; Benzie, I.F.F. Effects of incense smoke on human lymphocyte DNA. J. Toxicol. Environ. Health A 2009, 72, 369–373. [Google Scholar] [CrossRef]
- Chen, S.; Wong, R.; Shiu, L.; Chiou, M.; Lee, H. Exposure to Mosquito Coil Smoke Maybe a Risk Factor for Lung Cancer in Taiwan. J. Epidemiol. 2008, 18, 19–25. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, H.; Sun, Y.P.; Xie, H.K.; Zhou, C.C. Mosquito coil exposure associated with small cell lung cancer: A report of three cases. Oncol. Lett. 2015, 9, 1667–1671. [Google Scholar] [CrossRef]
- Gamboa, R.T.; Gamboa, A.R.; Bravo, A.H.; Ostrosky, W.P. Genotoxicity in child populations exposed to Polycyclic Aromatic Hydrocarbons (PAHs) in the air from Tabasco, Mexico. Int. J. Environ. Res. Public Health 2008, 5, 349–355. [Google Scholar] [CrossRef]
- Ismail, I.; Jalaludin, J.; Abu Bakar, S.; Hisamuddin, N.H.; Suhaimi, N.F. Association of traffic-related air pollution (TRAP) with DNA damage and respiratory health symptoms among primary school children in Selangor. Asian J. Atmos. Environ. 2019, 13, 106–116. [Google Scholar] [CrossRef]
- Reyna-bensusan, N.; Wilson, D.C.; Smith, S.R. Uncontrolled burning of solid waste by households in Mexico is a significant contributor to climate change in the country. Environ. Res. 2018, 163, 280–288. [Google Scholar] [CrossRef]
- Wiriya, W.; Prapamontol, T.; Chantara, S. PM10-bound polycyclic aromatic hydrocarbons in Chiang Mai (Thailand): Seasonal variations, source identification, health risk assessment and their relationship to air-mass movement. Atmos. Res. 2013, 124, 109–122. [Google Scholar] [CrossRef]
- Courter, L.A.; Pereira, C.; Baird, W.M. Diesel exhaust influences carcinogenic PAH-induced genotoxicity and gene expression in human breast epithelial cells in culture. Mutat. Res. 2007, 625, 72–82. [Google Scholar] [CrossRef]
- Duan, H.; Leng, S.; Pan, Z.; Dai, Y.; Niu, Y.; Huang, C.; Bin, P.; Wang, Y.; Liu, Q.; Chen, W.; et al. Biomarkers measured by cytokinesis-block micronucleus cytome assay for evaluating genetic damages induced by polycyclic aromatic hydrocarbons. Mutat. Res. Gen. Toxicol. Environ. Mutag. 2009, 677, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Felipe, M.; Galv, D.O.; Regina, S.; De Medeiros, B.; Dreij, K. Genotoxicity and DNA damage signalling in response to complex mixtures of PAHs in biomass burning particulate matter from cashew. Environ. Pol. 2020, 256, 113381. [Google Scholar]
- Muthusamy, S.; Peng, C.; Ng, J.C. Genotoxicity evaluation of multi-component mixtures of polycyclic aromatic hydrocarbons (PAHs), arsenic, cadmium, and lead using flow cytometry-based micronucleus test in HepG2 cells. Mutat. Res. Gen. Toxicol. Environ. 2018, 827, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Pan, W.; Xue, Q.; Fu, J.; Liu, G.; Zheng, M.; Zhang, A. A novel computational solution to the health risk assessment of air pollution via joint toxicity prediction: A case study on selected PAH binary mixtures in particulate matters. Ecotox. Environ. Saf. 2019, 170, 427–435. [Google Scholar] [CrossRef] [PubMed]
| Variable | Unit | Distribution Mode | Exposed School | Comparative School |
|---|---|---|---|---|
| Toxicity equivalent concentration (TEQ) | ng m−3 | Logistic & Log-normal | 17.35 | 2.21, 2.51 |
| Inhalation rate (IR) | m3 day−1 | Constant | 12 | 12 |
| Exposure frequency (EF) | day year−1 | Constant | 250 | 250 |
| Exposure duration (ED) | year | Constant | 6 | 6 |
| Averaging time | days | Constant | 25,500 | 25,500 |
| Body weight (BW) | kg | Negative binomial | 0.206 | 0.307 |
| Cancer slope factor (CSF) | mg kg−1 day−1 | Constant | 3.85 | 3.85 |
| Principal Component (PC) | Species | Factor Loading | Eigenvalue | Variability (%) | Source |
|---|---|---|---|---|---|
| PC1 Exposed schools | NAP | 0.824 | 7.201 | 45.120 | Vehicle and coke oven |
| FLU | 0.829 | ||||
| PYR | 0.741 | ||||
| BbF | 0.827 | ||||
| CYR | 0.859 | ||||
| IcP | 0.889 | ||||
| BgP | 0.804 | ||||
| BaP | 0.630 | ||||
| PC2 Exposed schools | BkF | 0.828 | 1.782 | 14.836 | Gasoline |
| FLA | 0.728 | ||||
| BaA | 0.758 | ||||
| BbF | 0.293 | ||||
| BaP | 0.233 | ||||
| PC3 Exposed schools | ANT | 0.948 | 1.526 | 15.103 | Wood combustion, diesel |
| PHE | 0.700 | ||||
| DbA | 0.558 | ||||
| NAP | 0.341 | ||||
| PYR | 0.335 | ||||
| PC1 Comparative schools | PHE | 0.853 | 7.130 | 47.040 | Vehicle |
| PYR | 0.931 | ||||
| BbF | 0.938 | ||||
| BaP | 0.907 | ||||
| IcP | 0.918 | ||||
| BgP | 0.832 | ||||
| PC2 Comparative schools | BaA | 0.941 | 2.857 | 27.27 | Diesel |
| BgP | 0.954 | ||||
| CYR | 0.714 | ||||
| PC3 Comparative schools | ANT | 0.977 | 1.080 | 9.34 | Wood combustion |
| NAP | 0.231 | ||||
| FLU | 0.258 |
| Variables | Exposed Group (n = 85) | Comparative Group (120) | ||
|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | |
| Age (year) | ||||
| 9 | 25.40 ± 4.12 | 0.409 | 18.44 ± 3.38 | 0.005 * |
| 10 | 26.70 ± 6.07 | 21.98 ± 4.50 | ||
| 11 | 27.96 ± 7.38 | 20.46 ± 4.08 | ||
| Exposure to tobacco smoke | ||||
| Yes | 27.24 ± 7.34 | 0.915 | 20.86 ± 474 | 0.674 |
| No | 27.08 ± 6.31 | 20.52 ± 3.91 | ||
| Grilled food | ||||
| Yes | 27.41 ± 7.33 | 0.804 | 21.14 ± 4.49 | 0.354 |
| No | 27.08 ± 6.16 | 20.37 ± 4.17 | ||
| Supplement | ||||
| Yes | 26.98 ± 6.76 | 0.646 | 20.53 ± 4.05 | 0.584 |
| No | 27.60 ± 6.56 | 21.06 ± 4.92 | ||
| Mosquito coil | ||||
| Yes | 30.05 ± 7.11 | 0.085 | 22.27 ± 4.32 | 0.034 * |
| No | 26.71 ± 6.46 | 20.20 ± 4.20 | ||
| Open burning | ||||
| Yes | 26.15 ± 6.33 | 0.350 | 20.56 ± 4.40 | 0.766 |
| No | 27.53 ± 6.73 | 20.79 ± 4.23 | ||
| Model | Adj R2 |
|---|---|
| Model 1 Tail moment = 12.892 + 0.054 (total outdoor PAHs)—2.415 (open burning) | 0.110 |
| Model 2 Tail moment = 14.120 + 0.170 (carcinogen outdoor PAHs)—1.870 (open burning) | 0.124 |
| Model 3 Tail moment = 13.345 + 0.076 (total indoor PAHs)—2.190 (open burning) | 0.115 |
| Model 4 Tail moment = 15.468 + 0.187 (carcinogen indoor PAHs)—2.328 (open burning) | 0.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopian, N.A.; Jalaludin, J.; Abu Bakar, S.; Hamedon, T.R.; Latif, M.T. Exposure to Particulate PAHs on Potential Genotoxicity and Cancer Risk among School Children Living Near the Petrochemical Industry. Int. J. Environ. Res. Public Health 2021, 18, 2575. https://doi.org/10.3390/ijerph18052575
Sopian NA, Jalaludin J, Abu Bakar S, Hamedon TR, Latif MT. Exposure to Particulate PAHs on Potential Genotoxicity and Cancer Risk among School Children Living Near the Petrochemical Industry. International Journal of Environmental Research and Public Health. 2021; 18(5):2575. https://doi.org/10.3390/ijerph18052575
Chicago/Turabian StyleSopian, Nor Ashikin, Juliana Jalaludin, Suhaili Abu Bakar, Titi Rahmawati Hamedon, and Mohd Talib Latif. 2021. "Exposure to Particulate PAHs on Potential Genotoxicity and Cancer Risk among School Children Living Near the Petrochemical Industry" International Journal of Environmental Research and Public Health 18, no. 5: 2575. https://doi.org/10.3390/ijerph18052575
APA StyleSopian, N. A., Jalaludin, J., Abu Bakar, S., Hamedon, T. R., & Latif, M. T. (2021). Exposure to Particulate PAHs on Potential Genotoxicity and Cancer Risk among School Children Living Near the Petrochemical Industry. International Journal of Environmental Research and Public Health, 18(5), 2575. https://doi.org/10.3390/ijerph18052575








