An Improved Stress-Scale Specifically Designed to Measure Stress of Women with Newly Diagnosed Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Original NDBCSS
2.2. Participants
2.3. Statistical Analyses
3. Results
3.1. Clinical and Demographic Characteristics
3.2. Confirmatory Factor Analysis of the Original Four-Factor Structure of the NDBCSS
3.3. Model Improvement of the NDBCSS by Exploratory Factor Analyses
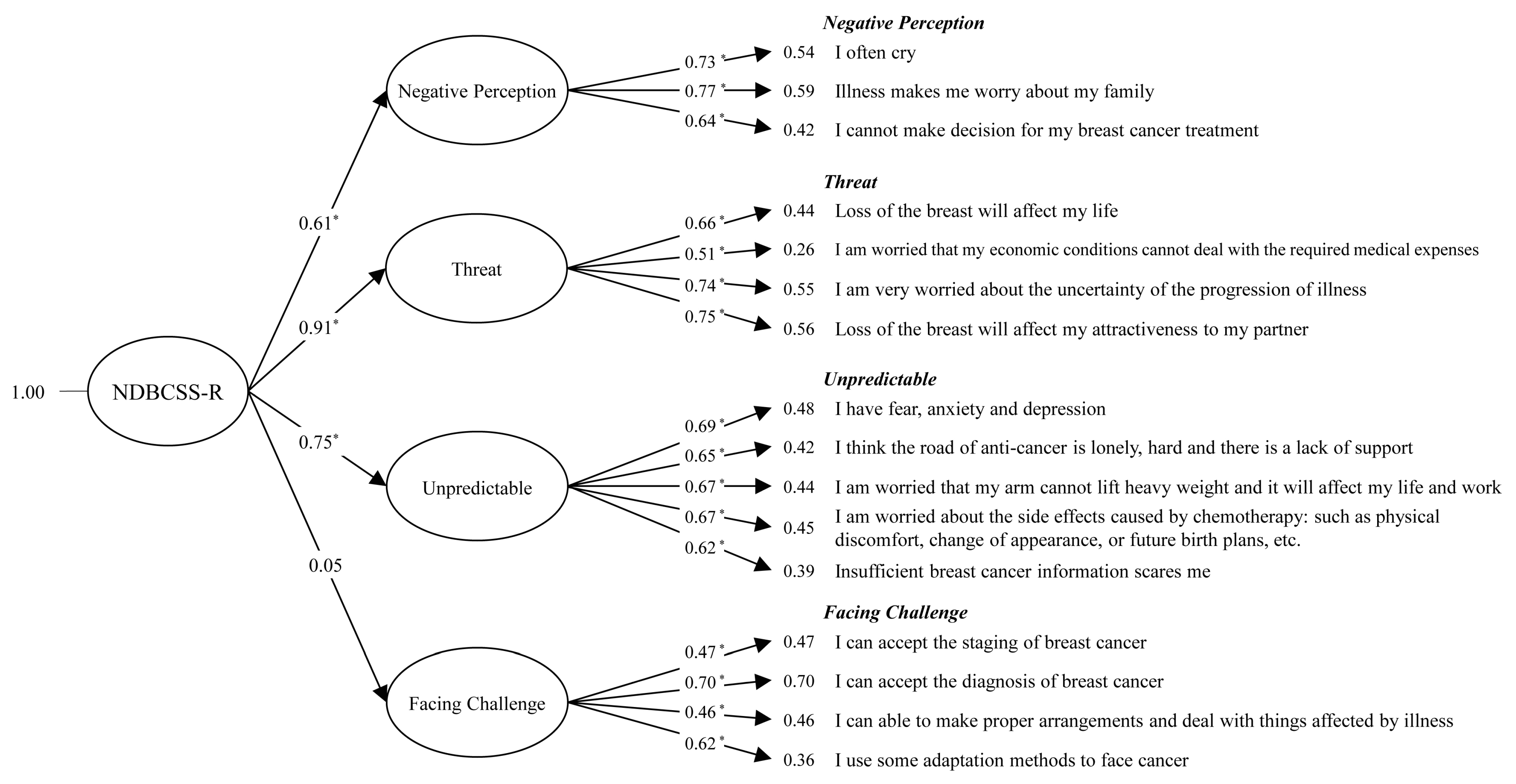
3.4. Confirmatory Factor Analysis of the New NDBCSS-R
3.5. Analysis of the Inter-Relationship between the Four-Factor Structures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banas, T.; Juszczyk, G.; Pitynski, K.; Nieweglowska, D.; Ludwin, A.; Czerw, A. Incidence and mortality rates in breast, corpus uteri, and ovarian cancers in Poland (1980-2013): An analysis of population-based data in relation to socioeconomic changes. Oncotargets 2016, 9, 5521–5530. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Beaudry, R.I.; Paterson, D.I.; Mackey, J.R.; Haykowsky, M.J. Curing breast cancer and killing the heart: A novel model to explain elevated cardiovascular disease and mortality risk among women with early stage breast cancer. Prog. Cardiovasc. Dis. 2019, 62, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Qi, X.; Cai, D.A.; Han, X. Modeling posttraumatic growth among cancer patients: The roles of social support, appraisals, and adaptive coping. Psychooncology 2018, 27, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Lu, Y.W.; Yang, C.C. Breast cancer trend in Taiwan. MOJ Women’s Health 2017, 6, 376–379. [Google Scholar] [CrossRef]
- Choi, S.W.; Ryu, S.Y.; Han, M.A.; Park, J. Higher breast cancer prevalence associated with higher socioeconomic status in the South Korean population; Has it resulted from overdiagnosis? PLoS ONE 2018, 13, e0200484. [Google Scholar] [CrossRef]
- Mubarik, S.; Malik, S.S.; Wang, Z.; Li, C.; Fawad, M.; Yu, C. Recent insights into breast cancer incidence trends among four Asian countries using age-period-cohort model. Cancer Manag. Res. 2019, 11, 8145–8155. [Google Scholar] [CrossRef]
- Gibbons, A.; Groarke, A.; Sweeney, K. Predicting general and cancer-related distress in women with newly diagnosed breast cancer. BMC Cancer 2016, 16, 935. [Google Scholar] [CrossRef]
- Kaplan, H.G.; Malmgren, J.A.; Atwood, M.K.; Calip, G.S. Effect of treatment and mammography detection on breast cancer survival over time: 1990–2007. Cancer 2015, 121, 2553–2561. [Google Scholar] [CrossRef]
- Ando, N.; Iwamitsu, Y.; Kuranami, M.; Okazaki, S.; Nakatani, Y.; Yamamoto, K.; Watanabe, M.; Miyaoka, H. Predictors of psychological distress after diagnosis in breast cancer patients and patients with benign breast problems. Psychosomatics 2011, 52, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.B.; Kang, E.C.; Jeon, D.W.; Kim, T.H.; Moon, J.J.; Kim, S.J.; Choi, J.M.; Jung, D.U. Associations Among Plasma Stress Markers and Symptoms of Anxiety and Depression in Patients with Breast Cancer Following Surgery. Psychiatry Investig. 2018, 15, 133–140. [Google Scholar] [CrossRef]
- Osborne, R.H.; Elsworth, G.R.; Hopper, J.L. Age-specific norms and determinants of anxiety and depression in 731 women with breast cancer recruited through a population-based cancer registry. Eur. J. Cancer (Oxf. Engl. 1990) 2003, 39, 755–762. [Google Scholar] [CrossRef]
- Hinz, A.; Krauss, O.; Hauss, J.P.; Höckel, M.; Kortmann, R.D.; Stolzenburg, J.U.; Schwarz, R. Anxiety and depression in cancer patients compared with the general population. Eur. J. Cancer Care 2010, 19, 522–529. [Google Scholar] [CrossRef]
- Bertero, C.M. Affected self-respect and self-value: The impact of breast cancer treatment on self-esteem and QoL. Psychooncology 2002, 11, 356–364. [Google Scholar] [CrossRef]
- Helms, R.L.; O’Hea, E.L.; Corso, M. Body image issues in women with breast cancer. Psychol. Health Med. 2008, 13, 313–325. [Google Scholar] [CrossRef]
- Hill, J.; Holcombe, C.; Clark, L.; Boothby, M.R.; Hincks, A.; Fisher, J.; Tufail, S.; Salmon, P. Predictors of onset of depression and anxiety in the year after diagnosis of breast cancer. Psychol. Med. 2011, 41, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Piot-Ziegler, C.; Sassi, M.L.; Raffoul, W.; Delaloye, J.F. Mastectomy, body deconstruction, and impact on identity: A qualitative study. Br. J. Health Psychol. 2010, 15, 479–510. [Google Scholar] [CrossRef] [PubMed]
- Ciambella, C.C.; Taneja, C.; Dizon, D.S.; Wiggins, D.L.; Emmick, C.M.; Leonard, K.L.; Lopresti, M.L.; Witherby, S.; Cabral, D.; Snow, S.; et al. Distress: Characterizing What Causes the Thermometer to Shift in Patients with Newly Diagnosed Breast Cancer Attending a Multidisciplinary Clinic. Ann. Surg. Oncol. 2019, 26, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, J.P.; Choppala, S.; Hamann, H.A.; Gjerde, J. Uncertainty during the transition from cancer patient to survivor. Cancer Nurs. 2009, 32, E8–E14. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, M.; Johansson, B.; Nordin, K.; Bergkvist, L.; Ahlgren, J.; Lidin-Lindqvist, A.; Lambe, M.; Lampic, C. Health-related quality of life among women with breast cancer—A population-based study. Acta Oncol. (Stock. Swed.) 2011, 50, 1015–1026. [Google Scholar] [CrossRef]
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Rosner, B.; Chen, W.Y.; Kawachi, I.; Colditz, G.A.; Holmes, M.D. Functional impact of breast cancer by age at diagnosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 1849–1856. [Google Scholar] [CrossRef]
- Ahmad, S.; Fergus, K.; McCarthy, M. Psychosocial issues experienced by young women with breast cancer: The minority group with the majority of need. Curr. Opin. Supportive Palliat. Care 2015, 9, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bidstrup, P.E.; Christensen, J.; Mertz, B.G.; Rottmann, N.; Dalton, S.O.; Johansen, C. Trajectories of distress, anxiety, and depression among women with breast cancer: Looking beyond the mean. Acta Oncol. (Stock. Swed.) 2015, 54, 789–796. [Google Scholar] [CrossRef]
- Kramer, J.R.; Ledolter, J.; Manos, G.N.; Bayless, M.L. Stress and metabolic control in diabetes mellitus: Methodological issues and an illustrative analysis. Ann. Behav. Med. A Publ. Soc. Behav. Med. 2000, 22, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.G.; Miller, D.B.; McCanlies, E.C.; Cawthon, R.M.; Andrew, M.E.; DeRoo, L.A.; Sandler, D.P. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Waldman, S.V.; Diez, J.C.; Arazi, H.C.; Linetzky, B.; Guinjoan, S.; Grancelli, H. Burnout, perceived stress, and depression among cardiology residents in Argentina. Acad. Psychiatry J. Am. Assoc. Dir. Psychiatr. Resid. Train. Assoc. Acad. Psychiatry 2009, 33, 296–301. [Google Scholar] [CrossRef]
- Herschbach, P.; Keller, M.; Knight, L.; Brandl, T.; Huber, B.; Henrich, G.; Marten-Mittag, B. Psychological problems of cancer patients: A cancer distress screening with a cancer-specific questionnaire. Br. J. Cancer 2004, 91, 504–511. [Google Scholar] [CrossRef]
- Thewes, B.; Butow, P.; Girgis, A.; Pendlebury, S. The psychosocial needs of breast cancer survivors; a qualitative study of the shared and unique needs of younger versus older survivors. Psychooncology 2004, 13, 177–189. [Google Scholar] [CrossRef]
- Bond, M.H. Oxford Handbook of Chinese Psychology; Oxford University Press Academic: Oxford, UK, 2010. [Google Scholar]
- Wong, P.T.P.; Wong, L.C.J. Handbook of Multicultural Perspectives on Stress and Coping; Springer: New York, NY, USA, 2006; pp. XXVI, 636. [Google Scholar]
- Chuang, W.L.; Chin, C.C. Information Needs of a Woman Newly Diagnosed with Breast Cancer. J. Heal. Sci. 2002, 4, 126–135. [Google Scholar] [CrossRef]
- Lee, T.Y.; Chen, H.H.; Yeh, M.L.; Li, H.L.; Chou, K.R. Measuring reliability and validity of a newly developed stress instrument: Newly Diagnosed Breast Cancer Stress Scale. J. Clin. Nurs. 2013, 22, 2417–2425. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Han, N.; Chen, Y. Reliability and validity analysis of Newly Diagnosed Breast Cancer Stress Scale. Chin. J. Mod. Nurs. 2018, 24, 916–919. [Google Scholar] [CrossRef]
- ΜAΡΙA, Χ. Στάθμιση του ερωτηματολογίου “ Κλίμακα Στρες σε Πρόσφατα Διαγνωσμένες με Καρκίνο του Μαστού [Stress Management and Health Promotion Intervention on Breast Cancer Patients]; ΕΘΝΙΚO ΚAΙ ΚAΠOΔΙΣΤΡΙAΚO ΠAΝΕΠΙΣΤHΜΙO AΘHΝΩΝ: Athens, Greece, 2019. [Google Scholar]
- Li, X.L.; Wang, J.H.; He, S.L. Confirmatory factor analysis of the shortened dentine hypersensitivity experience questionnaire. Hua Xi Kou Qiang Yi Xue Za Zhi = Huaxi Kouqiang Yixue Zazhi West China J. Stomatol. 2018, 36, 267–270. [Google Scholar] [CrossRef]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 2nd ed.; Guilford Publications: New York, NY, USA, 2005. [Google Scholar]
- Bentler, P.M. Confirmatory Factor Analysis via Noniterative Estimation: A Fast, Inexpensive Method. J. Mark. Res. 1982, 19, 417–424. [Google Scholar] [CrossRef]
- Doll, W.J.; Xia, W.; Torkzadeh, G. A confirmatory factor analysis of the end-user computing satisfaction instrument. MIS Q. 1994, 18, 453–461. [Google Scholar] [CrossRef]
- MacCallum, R.C.; Hong, S. Power Analysis in Covariance Structure Modeling Using GFI and AGFI. Multivar. Behav. Res. 1997, 32, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Effendi, I.; Chaniago, S.; Lestari, I.; Nasib, N.; Chaniago, S.; Azzahra, A.s. Trust identification and smartphone purchase decisions (Structural equation modeling approach). Int. J. Civ. Eng. Technol. 2019, 10, 1020–1032. [Google Scholar]
- Hu, L.t.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Modeling Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- McDonald, R.P.; Ho, M.H. Principles and practice in reporting structural equation analyses. Psychol. Methods 2002, 7, 64–82. [Google Scholar] [CrossRef]
- Luo, B.; Xiao, S. Reliability and validity for Chinese version of the 9-item Shared Decision Making Questionnaire. Zhong Nan Da Xue Xue Bao. Yi Xue Ban J. Cent. South Univ. Med Sci. 2019, 44, 823–829. [Google Scholar] [CrossRef]
- Mulaik, S.A.; James, L.R.; Van Alstine, J.; Bennett, N.; Lind, S.; Stilwell, C.D. Evaluation of goodness-of-fit indices for structural equation models. Psychol. Bull. 1989, 105, 430–445. [Google Scholar] [CrossRef]
- Breivik, E.; Olsson, U.H. Adding Variables to Improve Fit: The Effect of Model Size on Fit Assessment in LISREL; Structural Equation Modeling: Present and Future. A Festschrifit in Honor of Karl Jöreskog; Scientific Software: Chicago, IL, USA, 2001; pp. 169–194. [Google Scholar]
- Abdollahi, A.; Panahipour, H.; Hosseinian, S.; Allen, K.A. The effects of perceived stress on hope in women with breast cancer and the role of psychological hardiness. Psychooncology 2019, 28, 1477–1482. [Google Scholar] [CrossRef]
- Andrykowski, M.A.; Cordova, M.J.; Studts, J.L.; Miller, T.W. Posttraumatic stress disorder after treatment for breast cancer: Prevalence of diagnosis and use of the PTSD Checklist-Civilian Version (PCL-C) as a screening instrument. J. Consult. Clin. Psychol. 1998, 66, 586–590. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Beckham, J.C.; Morey, R.A.; Calhoun, P.S. The validity and diagnostic efficiency of the Davidson Trauma Scale in military veterans who have served since September 11th, 2001. J. Anxiety Disord. 2009, 23, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Rochette, A.; Bravo, G.; Desrosiers, J.; St-Cyr Tribble, D.; Bourget, A. Adaptation process, participation and depression over six months in first-stroke individuals and spouses. Clin. Rehabil. 2007, 21, 554–562. [Google Scholar] [CrossRef]
- Andersen, K.G.; Kehlet, H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J. Pain 2011, 12, 725–746. [Google Scholar] [CrossRef]
- Bodtcher, H.; Bidstrup, P.E.; Andersen, I.; Christensen, J.; Mertz, B.G.; Johansen, C.; Dalton, S.O. Fatigue trajectories during the first 8 months after breast cancer diagnosis. Qual. Life Res. 2015, 24, 2671–2679. [Google Scholar] [CrossRef]
- Hidding, J.T.; Beurskens, C.H.; van der Wees, P.J.; van Laarhoven, H.W.; Nijhuis-van der Sanden, M.W. Treatment related impairments in arm and shoulder in patients with breast cancer: A systematic review. PLoS ONE 2014, 9, e96748. [Google Scholar] [CrossRef]
- Mertz, B.G.; Bistrup, P.E.; Johansen, C.; Dalton, S.O.; Deltour, I.; Kehlet, H.; Kroman, N. Psychological distress among women with newly diagnosed breast cancer. Eur. J. Oncol. Nurs. 2012, 16, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Ferguson, D.W.; Gill, J.; Paul, J.; Symonds, P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 721–732. [Google Scholar] [CrossRef]
- Mertz, B.G.; Dunn-Henriksen, A.K.; Kroman, N.; Johansen, C.; Andersen, K.G.; Andersson, M.; Mathiesen, U.B.; Vibe-Petersen, J.; Dalton, S.O.; Envold Bidstrup, P. The effects of individually tailored nurse navigation for patients with newly diagnosed breast cancer: A randomized pilot study. Acta Oncol. (Stockh. Swed.) 2017, 56, 1682–1689. [Google Scholar] [CrossRef]
- Livsey, K.R.; Rossitch, J.C.; Tait, E.; Mannle, S.E.; Smith, R. Where Fear Begins: The Effect of a Nurse Navigator Home Visit to Decrease Distress in Newly Diagnosed Breast Cancer Patients. J. Oncol. Navig. Surviv. 2017, 8, 584–592. [Google Scholar]
- Li, P.; Guo, Y.J.; Tang, Q.; Yang, L. Effectiveness of nursing intervention for increasing hope in patients with cancer: A meta-analysis. Rev. Lat. Am. Enferm. 2018, 26, e2937. [Google Scholar] [CrossRef]

| Variables | N = 195 (%) |
|---|---|
| Age | |
| <45 | 49 (25.13) |
| 45–54 | 80 (41.00) |
| 55–64 | 45 (23.10) |
| ≥65 | 18 (9.20) |
| Marital status | |
| Unmarried (single, sivorced, separated and other) | 39 (20.00) |
| Married | 156 (80.00) |
| Education level | |
| High school and below | 111 (56.96) |
| College and above | 84 (43.10) |
| Income (NTD) | |
| <45,000 | 33 (25.00) |
| 45,000–69,999 | 35 (26.52) |
| 70,000–99,999 | 17 (12.88) |
| ≥100,000 | 47 (35.61) |
| Employment | |
| No | 104 (54.17) |
| Yes | 88 (45.83) |
| Tumor stage | |
| 0 | 58 (33.72) |
| 1 | 63 (36.63) |
| 2 | 45 (26.16) |
| 3 | 4 (2.33) |
| 4 | 2 (1.16) |
| Family history | |
| No | 152 (80.42) |
| Yes | 37 (19.58) |
| Mammographic x-ray | |
| No | 117 (61.9) |
| Yes | 72 (38.1) |
| Self-awareness of health | |
| Well | 49 (25.39) |
| Not well | 144 (74.61) |
| Scales | Cronbach α | ||||
|---|---|---|---|---|---|
| All | Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
| Original NDBCSS | 0.82 | 0.76 | 0.65 | 0.76 | 0.79 |
| Improved NDBCSS-R | 0.84 | 0.75 | 0.76 | 0.79 | 0.79 |
| Item No. | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Negative Perception | Threat | Unpredictable | Facing Challenge | |
| S01 | 0.83 | 0.11 | 0.15 | −0.02 |
| S02 | 0.76 | 0.38 | −0.01 | −0.12 |
| S06 | 0.72 | 0.11 | 0.31 | 0.17 |
| S03 | 0.27 | 0.59 | 0.32 | −0.11 |
| S09 | 0.32 | 0.66 | 0.00 | 0.33 |
| S10 | 0.10 | 0.75 | 0.32 | 0.00 |
| S12 | 0.20 | 0.68 | 0.33 | −0.14 |
| S04 | 0.23 | 0.11 | 0.76 | −0.14 |
| S07 | −0.10 | 0.32 | 0.55 | 0.01 |
| S08 | 0.07 | 0.30 | 0.66 | 0.00 |
| S11 | 0.04 | 0.18 | 0.75 | −0.06 |
| S13 | 0.23 | 0.15 | 0.69 | 0.18 |
| S14 | −0.12 | −0.06 | −0.02 | 0.72 |
| S15 | −0.01 | −0.03 | 0.00 | 0.84 |
| S16 | 0.02 | 0.03 | −0.03 | 0.80 |
| S17 | 0.13 | 0.04 | 0.01 | 0.76 |
| Item | CMIN/DF | GFI | AGFI | NFI | RFI | IFI | TLI | CFI | RMSEA | RMR | PGFI | PNFI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guideline | 2–3 | >0.85 | >0.80 | >0.80 | >0.80 | >0.90 | >0.90 | >0.90 | <0.08 | <0.05 | >0.50 | >0.50 |
| Original (NDBCSS) | 3.30 | 0.80 | 0.73 | 0.72 | 0.66 | 0.79 | 0.74 | 0.78 | 0.11 | 0.06 | 0.59 | 0.60 |
| New (NDBCSS-R) | 2.48 | 0.92 | 0.81 | 0.87 | 0.83 | 0.92 | 0.90 | 0.92 | 0.08 | 0.04 | 0.63 | 0.65 |
| Negative Perception | Threat | Unpredictable | Facing Challenge | |
|---|---|---|---|---|
| Negative perception | - | 0.50 ** | 0.36 ** | 0.01 |
| Threat | - | 0.62 ** | 0.05 | |
| Unpredictable | - | 0.03 | ||
| Facing Challenge | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-Y.; Hsing, S.-C.; Li, C.-C. An Improved Stress-Scale Specifically Designed to Measure Stress of Women with Newly Diagnosed Breast Cancer. Int. J. Environ. Res. Public Health 2021, 18, 2346. https://doi.org/10.3390/ijerph18052346
Lee T-Y, Hsing S-C, Li C-C. An Improved Stress-Scale Specifically Designed to Measure Stress of Women with Newly Diagnosed Breast Cancer. International Journal of Environmental Research and Public Health. 2021; 18(5):2346. https://doi.org/10.3390/ijerph18052346
Chicago/Turabian StyleLee, Tso-Ying, Shih-Chun Hsing, and Chin-Ching Li. 2021. "An Improved Stress-Scale Specifically Designed to Measure Stress of Women with Newly Diagnosed Breast Cancer" International Journal of Environmental Research and Public Health 18, no. 5: 2346. https://doi.org/10.3390/ijerph18052346
APA StyleLee, T.-Y., Hsing, S.-C., & Li, C.-C. (2021). An Improved Stress-Scale Specifically Designed to Measure Stress of Women with Newly Diagnosed Breast Cancer. International Journal of Environmental Research and Public Health, 18(5), 2346. https://doi.org/10.3390/ijerph18052346





