Micro-Vesicles of Moringa oleifera Seeds in Heterozygous Rats for DAT Gene: Effects of Oral Intake on Behavioral Profile and Hematological Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Experiment
2.1.1. Ethical Note
2.1.2. Experimental Groups
- (1)
- Wild-type (WT) control group (n = 5), which had daily access to 70 mL of water;
- (2)
- WT group treated with microvesicles (n = 5), which had daily access to 70 mL of a solution made by water and microvesicles of Moringa;
- (3)
- Heterozygous (DATHET) control group (n = 4), which had daily access to 70 mL of water;
- (4)
- DATHET group treated with microvesicles (n = 6), which had daily access to 70 mL of a solution made by water and microvesicles of Moringa.
2.1.3. Blood Sampling and Treatment
2.2. Preparation and Administration of Microvesicles
2.2.1. Preparation of Aqueous Seed Extract and Purification of Microvesicles
2.2.2. Oral Treatment Protocol
2.3. Behavioral Tests
2.3.1. Forced Swimming Test
- -
- “Swimming” (active swimming);
- -
- “Struggling/Climbing” (powerful attempts to go out from the water, trying to climb along the cylinder walls, by using its four paws);
- -
- “Diving” (underwater immersion);
- -
- “Floating” (lack of movements except little movements with paws needed to maintain just nose and eyes above the water surface, in order to just allow to breathe with nose).
2.3.2. Spontaneous Locomotor Activity
2.4. Body Weight
2.5. Statistical Analysis
3. Results
3.1. Behavioral Test
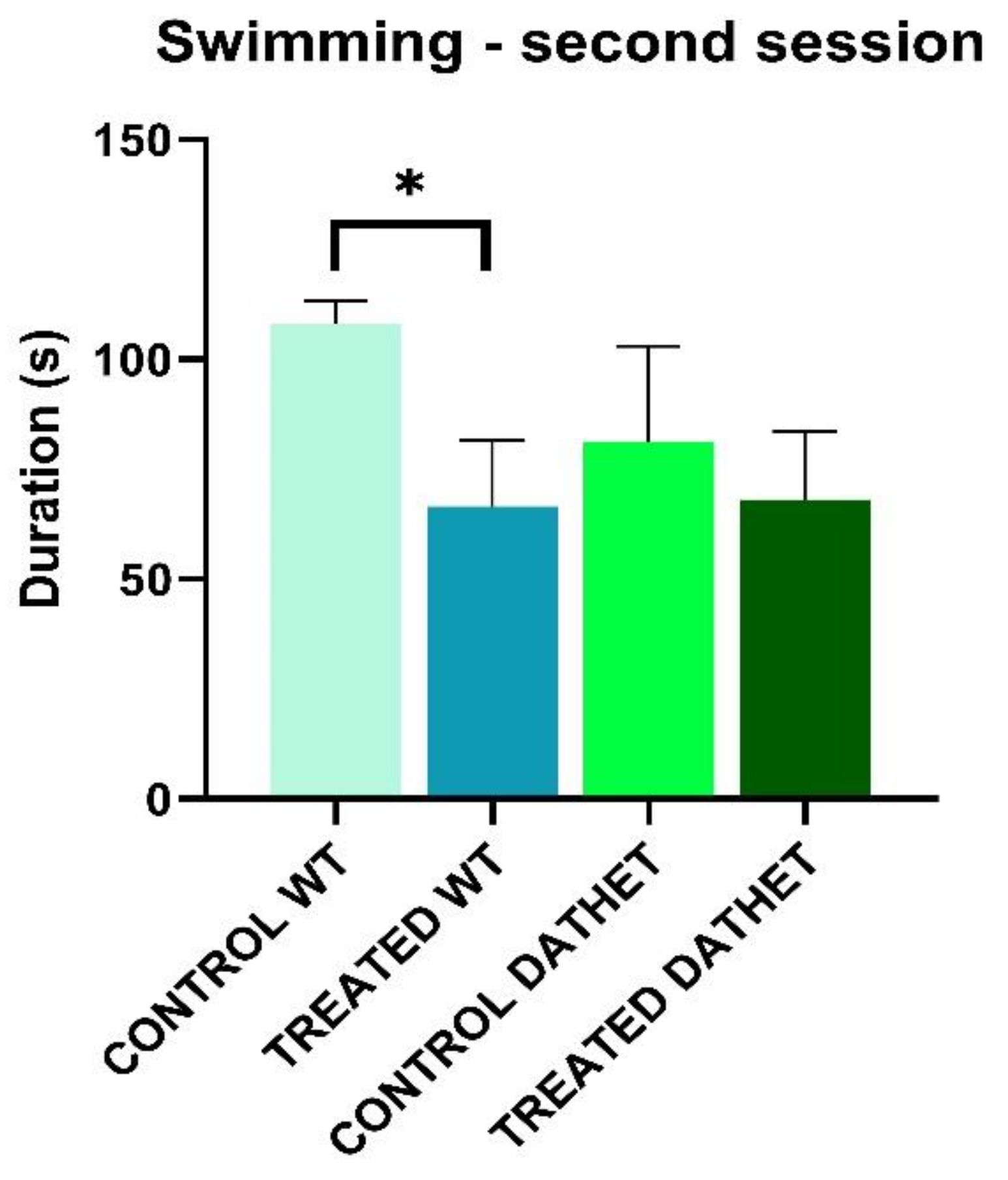
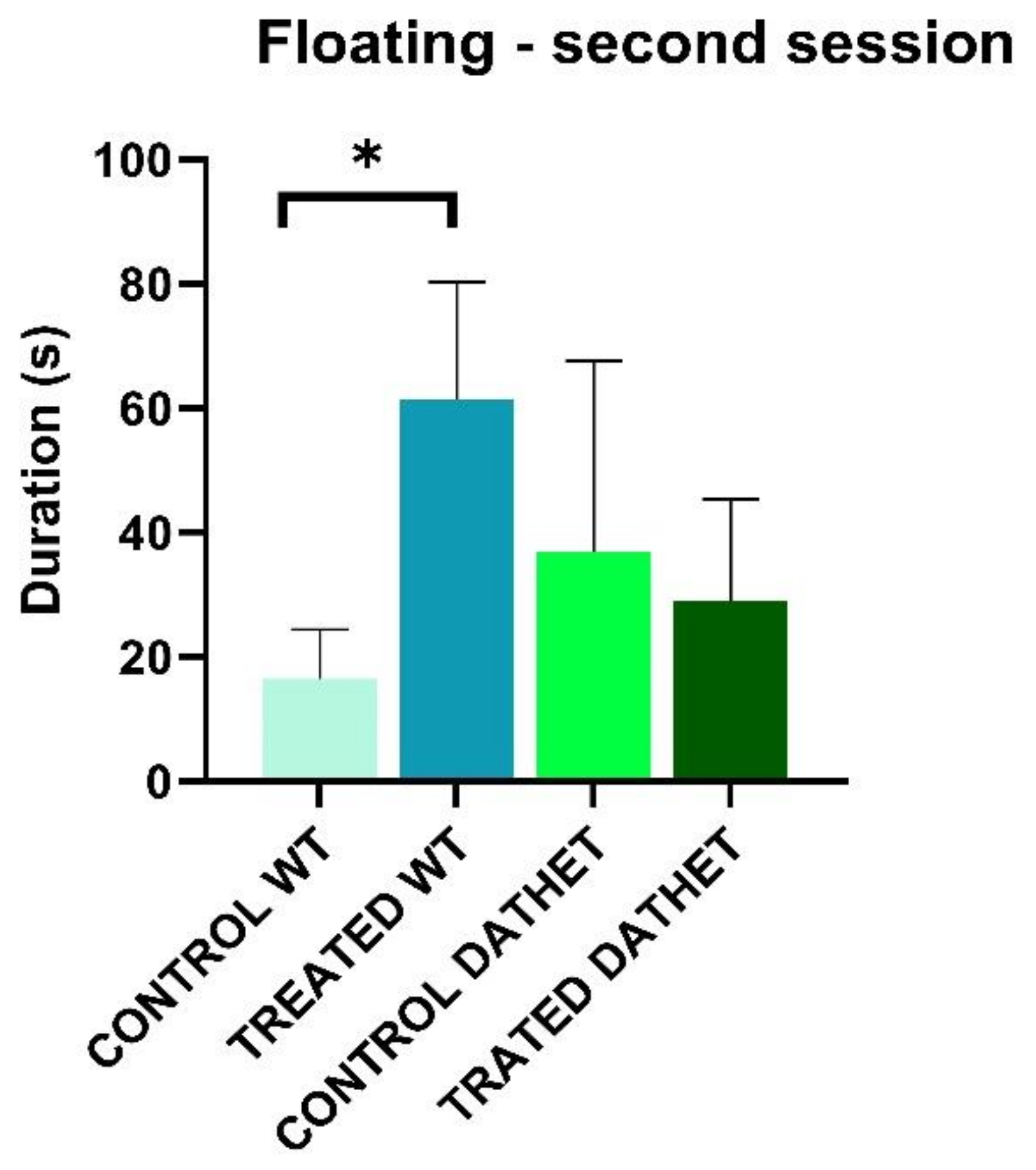
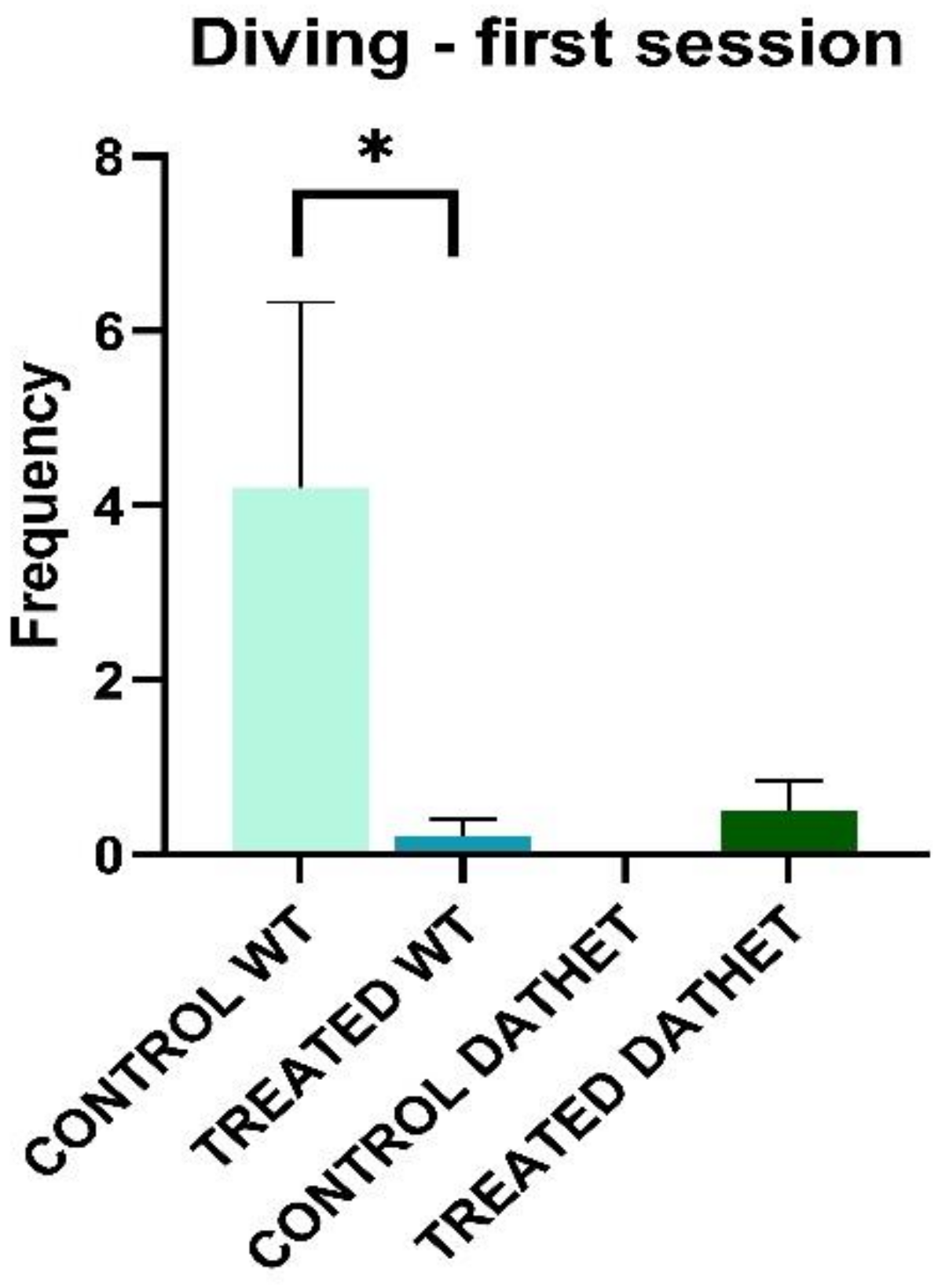
3.1.1. Forced Swimming Test (FST)
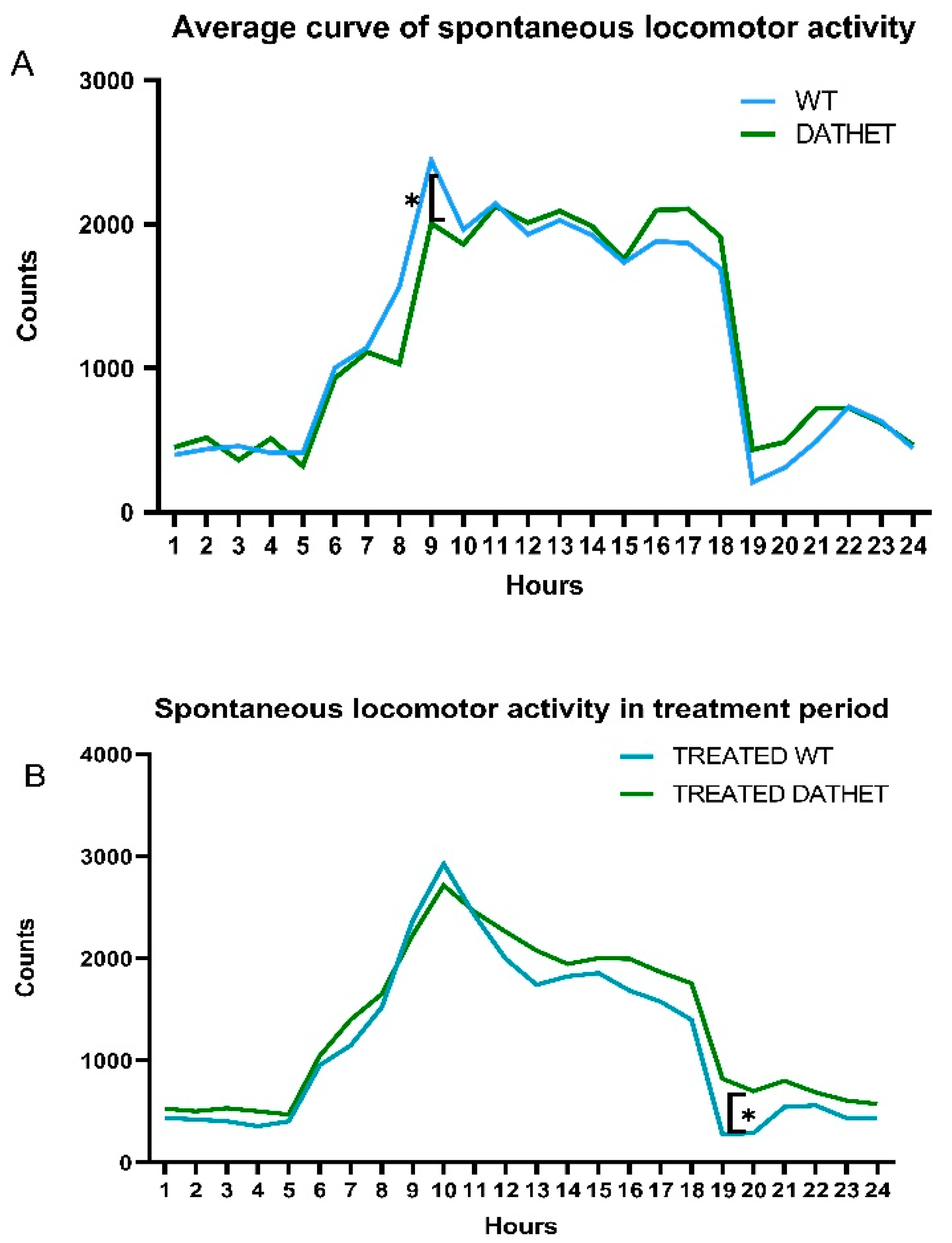
3.1.2. Spontaneous Locomotor Activity
3.2. Physiological and Hematological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Zhang, S.; Weber, J.; Baxter, D.; Galas, D. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010, 38, 7248–7259. [Google Scholar] [CrossRef]
- Balaj, B.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011. [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Wang, X.Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; Zhang, H.G. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 561–573. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. Peer J. 2018. [CrossRef]
- Zeng, J.; Gupta, V.; Jiang, Y.; Yang, B.; Gong, L.; Zhu, H. Cross-kingdom small RNAs among animals, plants and microbes. Cells 2019, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.; Singh, A. Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Hassan Gilani, A. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 2, 17–25. [Google Scholar] [CrossRef]
- Potestà, M.; Minutolo, A.; Gismondi, A.; Canuti, L.; Kenzo, M.; Roglia, V.; Macchi, F.; Grelli, S.; Canini, A.; Colizzi, V.; et al. Cytotoxic and apoptotic effects of different extracts of Moringa oleifera Lam on lymphoid and monocytoid cells. Exp. Ther. Med. 2019, 18, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Potestà, M.; Roglia, V.; Fanelli, M.L.; Pietrobono, E.; Gismondi, A.; Vumbaca, S.; Gildas Nguedia Tsangueu, R.; Canini, A.; Colizzi, V.; Grelli, S.; et al. Effect of microvesicles from Moringa oleifera containing miRNA on proliferation and apoptosis in tumor cell lines. Cell Death Discov. 2020, 6. [Google Scholar] [CrossRef]
- Sutalangka, C.; Wattanathorn, J.; Muchimapura, S.; Thukham-mee, W. Moringa oleifera mitigates memory impairment and neurodegeneration in animal model of age-related dementia. Oxid Med. Cell Longev. 2013. [CrossRef]
- Beaulieu, J.; Gainetdinov, R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Bannon, M.; Michelhaugh, S.; Wang, V.; Sacchetti, P. The human dopamine transporter gene: Organization, transcriptional regulation, and potential involvement in neuropsychiatric disorders. Eur. Neuropsycho Pharmacol. 2001, 11, 449–455. [Google Scholar] [CrossRef]
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronunced hyperactivity, cognitive dysfunctions, and BDNF dysregulation in dopamine transporter knock-out rats. J. Neurosci. 2018, 38, 1959–1972. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.; Wightman, R.; Caron, M. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef]
- Cinque, S.; Zoratto, F.; Poleggi, A.; Leo, D.; Cerniglia, L.; Cimino, S.; Tambelli, R.; Alleva, E.; Gainetdinov, R.; Laviola, G.; et al. Behavioral phenotyping of dopamine transporter knockout rats: Compulsive traits, motor stereotypies, and anhedonia. Front. Psychiatry 2018, 9, 43. [Google Scholar] [CrossRef]
- Adinolfi, A.; Zelli, S.; Leo, D.; Carbone, C.; Mus, L.; Illiano, P.; Alleva, E.; Gainetdinov, R.R.; Adriani, W. Behavioral of DAT-KO rats and evidence of asocial-like phenotype in DAT-HET rats: The potential involvement of norepinefhrine. Behav. Brain. 2019, 359, 516–527. [Google Scholar] [CrossRef]
- Carbone, C.; Brancato, A.; Adinolfi, A.; Lo Russo, S.L.M.; Alleva, E.; Cannizzaro, C.; Adriani, W. Motor transitions’ peculiarity of heterozygous DAT rats when offspring of an unconventional KOxWT mating. Neuroscience 2020, 433, 108–120. [Google Scholar] [CrossRef]
- Zelli, S.; Brancato, A.; Mattioli, F.; Pepe, M.; Alleva, E.; Carbone, C.; Cannizzaro, C.; Adriani, W. A new “sudden fright paradigm” to explore the role of (epi)genetic modulations of the DAT gene in fear-induced avoidance behavior. Genes Brain Behav. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Mariano, S.; Pardo, M.; Buccheri, C.; Illiano, P.; Adinolfi, A.; Lo Russo, S.L.M.; Alleva, E.; Carbone, C.; Adriani, W. Own or dam’s genotype? Classical colony breeding may bias spontaneous and stress- challenged activity in DAT-mutant rats. Dev. Psychobiol. 2020, 62, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Teruel, A.F.; Escorihuela, R.M.; García, E.; Zapata, A.; Tobeña, A. Struggling and Flumazenil effects in the Swimming Test are related to the level of anxiety in mice. Neuropsychobiology 1994, 29, 23–27. [Google Scholar] [CrossRef]
- Bennie, J.J.; Duffy, J.P.; Inger, R.; Gaston, K.J. Biogeography of time partitioning in mammals. Proc. Natl. Acad. Sci. USA 2014, 111, 13727–13732. [Google Scholar] [CrossRef]
- Gyekye, A.; Frimpong-Manso, A.; Awortwe, C.; Antwi, D.; Nyarko, A. Micro- and macroelemental composition and safety evaluation of the nutraceutical Moringa Oleifera leaves. J. Toxicol. 2014. [CrossRef]
- Lee, K.; Coelho, M.; Sern, K.; Class, M.; Bocz, M.; Szumlinski, K. Anxiolytic effects of buspitrone and MTEP in the Porsolt Forced Swim test. Chronic Stress 2017, 1. [Google Scholar] [CrossRef]
- Pandey, A.; Dev Pandey, R.; Tripathi, P.; Gupta, P.P.; Haider, J.; Bhatt, S.; Singh, A.V. Moringa Oleifera Lam. (Sahijan)—A plant with a plethora of diverse therapeutic benefits: An updated retrospection. Med. Aromat. Plants 2012, 1, 101. [Google Scholar] [CrossRef]


| White Blood Cells Range Values = 3.0–8.0 103/mm3 | Lymphocytes Range Values = 1.2–26.4 103/mm3 | Granulocytes Range Values = 0.3–2.8 103/mm3 | |||
| WT | DATHET | WT | DATHET | WT | DATHET |
| 2.3 ± 0.33 | 1.7 ± 0.09 | 1.6 ± 0.23 | 1.2 ± 0.08 | 0.6 ± 0.09 | 0.5 ± 0.06 |
| Red Blood Cells Range Values = 7.0–9.0 106/mm3 | Hemoglobin Range Values = 15.0–18.0 g/dL | Hematocrit Range Values = 35.0–55.0 % | |||
| WT | DATHET | WT | DATHET | WT | DATHET |
| 8.5 ± 0.04 | 8.8 ± 0.13 | 15.0 ± 0.15 | 15.5 ± 0.2 | 40.2 ± 0.45 | 41.1 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buccheri, C.; Festucci, F.; Potestà, M.; Roglia, V.; Bernardini, R.; Minutolo, A.; Montesano, C.; Adriani, W. Micro-Vesicles of Moringa oleifera Seeds in Heterozygous Rats for DAT Gene: Effects of Oral Intake on Behavioral Profile and Hematological Parameters. Int. J. Environ. Res. Public Health 2021, 18, 2322. https://doi.org/10.3390/ijerph18052322
Buccheri C, Festucci F, Potestà M, Roglia V, Bernardini R, Minutolo A, Montesano C, Adriani W. Micro-Vesicles of Moringa oleifera Seeds in Heterozygous Rats for DAT Gene: Effects of Oral Intake on Behavioral Profile and Hematological Parameters. International Journal of Environmental Research and Public Health. 2021; 18(5):2322. https://doi.org/10.3390/ijerph18052322
Chicago/Turabian StyleBuccheri, Clelia, Fabiana Festucci, Marina Potestà, Valentina Roglia, Roberta Bernardini, Antonella Minutolo, Carla Montesano, and Walter Adriani. 2021. "Micro-Vesicles of Moringa oleifera Seeds in Heterozygous Rats for DAT Gene: Effects of Oral Intake on Behavioral Profile and Hematological Parameters" International Journal of Environmental Research and Public Health 18, no. 5: 2322. https://doi.org/10.3390/ijerph18052322
APA StyleBuccheri, C., Festucci, F., Potestà, M., Roglia, V., Bernardini, R., Minutolo, A., Montesano, C., & Adriani, W. (2021). Micro-Vesicles of Moringa oleifera Seeds in Heterozygous Rats for DAT Gene: Effects of Oral Intake on Behavioral Profile and Hematological Parameters. International Journal of Environmental Research and Public Health, 18(5), 2322. https://doi.org/10.3390/ijerph18052322







