Implementation of Bio-Risk Management System in a National Clinical and Medical Referral Centre Laboratories
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Designs
2.2. Subjects Study
2.3. Instruments and Data Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Biorisk Management: Laboratory Biosecurity Guidance; WHO: Geneva, Switzerland, 2006; pp. 1–41. [Google Scholar]
- Coelho, A.C.; Díez, J.G. Biological Risks and Laboratory-Acquired Infections: A Reality That Cannot Be Ignored in Health Biotechnology. Front. Bioeng. Biotechnol. 2015, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gronvall, G.K. National-Level Biosafety Norms Needed for Dual-Use Research. Front. Psychol. 2014, 2, 1–2. [Google Scholar] [CrossRef]
- Hegde, V.; Rd, K.; Gs, A. Biomedical Waste Management. J. Oral Maxillofac. Pathol. 2007, 11, 5–9. [Google Scholar] [CrossRef]
- Bathula, S.R.; Rakhimol, A. Global Trends in Biorisk Management. BioRisk 2017, 2017, 1–23. [Google Scholar] [CrossRef]
- Ogaro, H.M.; Kiiyukia, C.; Mbatha, S.; Ngayo, M.O. Biorisk Status: A Comparative Assessment of Private and Public Medical Diagnostic Laboratories in Western Kenya. Appl. Biosaf. 2018, 23, 47–54. [Google Scholar] [CrossRef]
- Juma, B.W.; Wadegu, M.; Makio, A.; Kirera, R.; Eyase, F.; Awinda, G.; Kamanza, J.; Schnabel, D.; Wurapa, E.K. A Survey of Biosafety and Biosecurity Practices in the United States Army Medical Research Unit-Kenya (USAMRU-K). Appl. Biosaf. 2014, 19, 28–34. [Google Scholar] [CrossRef]
- Samuel, S.; Kayode, O.; Musa, O.; Nwigwe, G.; Aboderin, A.; Salami, T.A.; Taiwo, S. Nosocomial Infections and the Challenges of Control in Developing Countries. Afr. J. Clin. Exp. Microbiol. 2010, 11, 102–110. [Google Scholar] [CrossRef]
- Elduma, A.H. Assessment of Biosafety Precautions in Khartoum State Diagnostic Laboratories, Sudan. Pan Afr. Med. J. 2012, 11, 19. [Google Scholar] [CrossRef]
- Main, C.L.; Carusone, S.C.; Davis, K.; Loeb, M. Compliance With Personal Precautions Against Exposure to Bloodborne Pathogens among Laboratory Workers: A Canadian Survey. Infect. Control Hosp. Epidemiol. 2008, 29, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Luksamijarulkul, P.; Kiennukul, N.; Utrarachkij, F.; Vatanasomboon, P. Current Situation of Biosafety Practices in Selected Hospital Laboratories, Bangkok. Asia J Public Health 2010, 1, 20–25. [Google Scholar]
- National Institutes of Health. NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (2013 Revision). Biosafety 2014, 3, 1–18. [Google Scholar] [CrossRef]
- Farradika, Y.; Lisdawati, V.; Roehaeni, R.; Suwandono, A. Dominant Factors Associated with Biosafety Facility and Equipment in Laboratories: An Indonesian 2011 Study. Health Sci. J. Indones. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Tun, T.; Wah, L.K. BSL2 Audit and Certification Program: An Effort to Harmonize and to Raise Standards in Both Laboratory Infrastructure and Biosafety Practices in Singapore. Biomed. Sci. Lett. 2016, 22, 65–74. [Google Scholar] [CrossRef][Green Version]
- Oladeinde, B.H.; Omoregie, R.; Odia, I.; Osakue, E.O.; Imade, O.S. Biorisk Assessment of Medical Diagnostic Laboratories in Nigeria. Saf. Health Work 2013, 4, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.G.; Mathews, H.; Alderman, L.M. Biosafety Officers, Behavioral Compliance Strategies, and Their Effects on Laboratory Practices. Appl. Biosaf. 2007, 12, 75–78. [Google Scholar] [CrossRef]
- Mourya, D.T.; Sapkal, G.; Yadav, P.D.; Belani, S.K.M.; Shete, A.; Gupta, N. Biorisk Assessment for Infrastructure & Biosafety Requirements for the Laboratories Providing Coronavirus SARS-CoV-2/(COVID-19) Diagnosis. Indian J. Med. Res. 2020, 151, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.M.L.; Morel, C.M. The COVID-19 Pandemics and the Relevance of Biosafety Facilities for Metagenomics Surveillance, Structured Disease Prevention and Control. Biosaf. Health 2020, 7–9. [Google Scholar] [CrossRef]
- Susanti, I.; Susilarini, N.K.; Setiawaty, V. Assessment of Biorisk Management Implementation in NIHRD Laboratory as National Referral Laboratory of Emerging Infectious Diseases in Indonesia. Health Sci. J. Indones. 2018, 9, 70–75. [Google Scholar] [CrossRef]
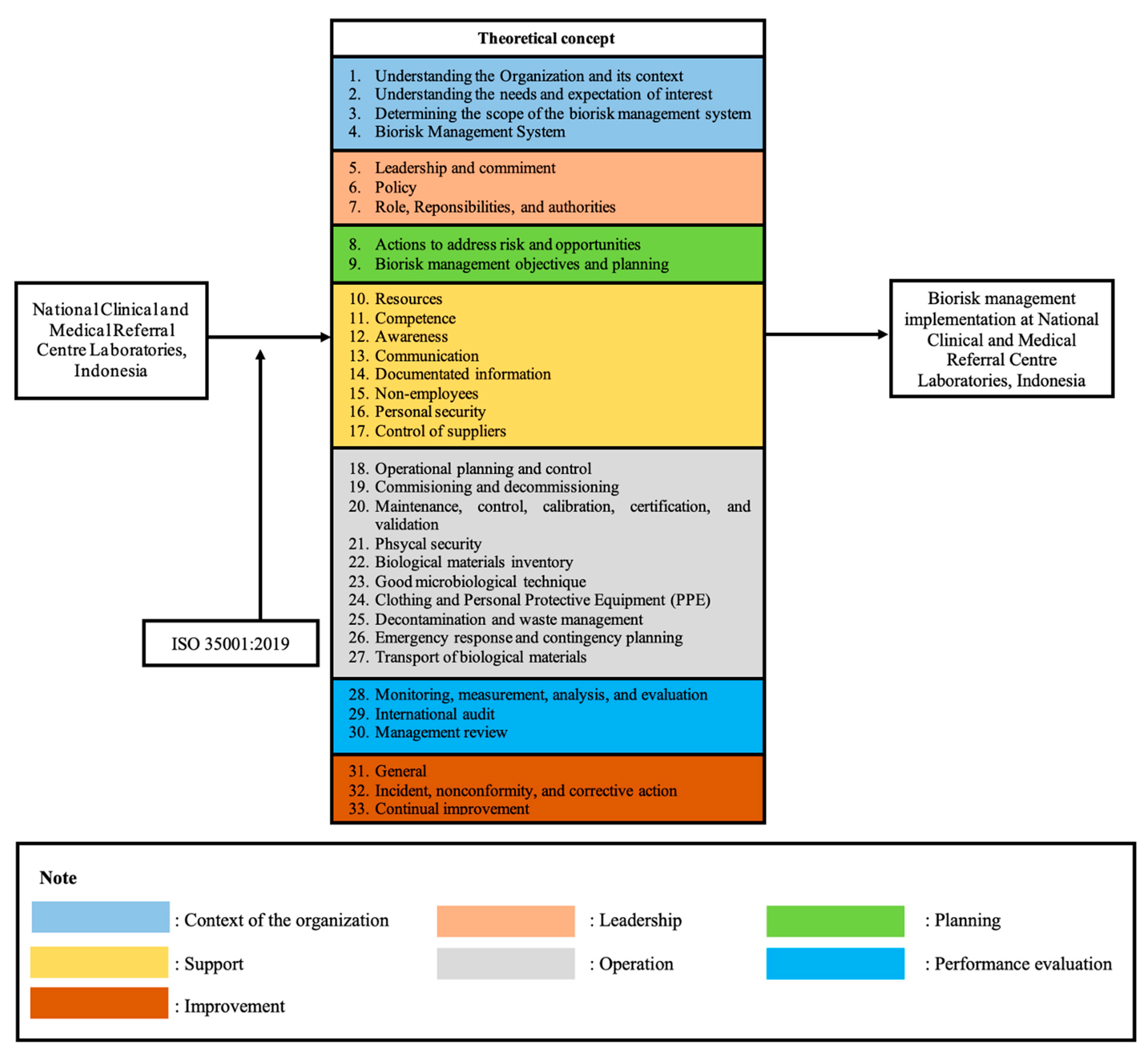
- ISO 35001: 2019. Biorisk Management for Laboratories and Other Related Organisations; ISO: Geneva, Switzerland, 2020; Volume 2019. [Google Scholar]
- Beeckman, D.S.A.; Rüdelsheim, P. Biosafety and Biosecurity in Containment: A Regulatory Overview. Front. Bioeng. Biotechnol. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Naroeni, A.; Bachtiar, E.W.; Ibrahim, F.; Bela, B.; Kusminanti, Y.; Pujiriani, I.; Lestari, F. Challenges in Implementing a Biorisk Management Program at Universitas Indonesia: A Checklist Tool for Biorisk Management. Health Secur. 2016, 14. [Google Scholar] [CrossRef]
- Nulens, E.; Voss, A. Laboratory Diagnosis and Biosafety Issues of Biological Warfare Agents. Clin. Microbiol. Infect. 2002, 8, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Bakanidze, L.; Imnadze, P.; Perkins, D. Biosafety and Biosecurity as Essential Pillars of International Health Security and Cross-Cutting Elements of Biological Nonproliferation. BMC Public Health 2010, 10 (Suppl. S1). [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Song, H.; Wang, J.; Li, Z.; Xu, S.; Ji, X.; Hou, X.; Xu, J. Biosafety and Biosecurity. J. Biosaf. Biosecur. 2019, 1, 15–18. [Google Scholar] [CrossRef]
- Gao, G.F. Biosafety and Health For a Better World: Biosafety Strategies to Protect Global Health. Biosaf. Health 2019, 1, 1–3. [Google Scholar] [CrossRef]
- Khripunov, I.; Smidovich, N.; Williams, D.M. Bio-Risk Management Culture: Concept, Model, Assessment Bio-Risk Management Culture: Concept, Model, Assessment. Available online: https://doi.org/10.1007/978-3-319-62108-1 (accessed on 26 February 2021).
- Appelt, S.; Jacob, D.; Rohleder, A.M.; Bråve, A.; Björndal, Å.S.; Di Caro, A.; Grunow, R. Assessment of Biorisk Management Systems in High Containment Laboratories, 18 Countries in Europe, 2016 and 2017. Eurosurveillance 2020, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Siengsanan-Lamont, J.; Kamolsiripichaiporn, S.; Ruanchaimun, S.; Patchimasiri, T.; Jongrakwattana, B.; Blacksell, S.D. Biosafety and Biosecurity Challenges Facing Veterinary Diagnostic Laboratories in Lower-Middle Income Countries in Southeast Asia: A Case Study of Thailand. Appl. Biosaf. 2019, 24, 220–230. [Google Scholar] [CrossRef]
- Kagirita, A.A.; Owalla, T.J.; Okwalinga, P.; Opio, J.; Baguma, A.; Mugasha, R.; Kakooza, F.; Ojwiya, A.; Aisu, S.; Eragu, R.; et al. Biorisk Management Practices in Public and Private Laboratories in Uganda: A Nationwide Baseline Survey. J. Bioterror Biodef 2018, 9. [Google Scholar] [CrossRef]
- Abad, X. CWA 15793: When the Biorisk Management Is the Core of a Facility. Biosafety 2014, 3, 1–5. [Google Scholar] [CrossRef]
- Brizee, S.; Passel, M.W.J.; van den Berg, L.M.; Feakes, D.; Izar, A.; Lin, K.T.B.; Podin, Y.; Yunus, Z.; Bleijs, D.A. Development of a Biosecurity Checklist for Laboratory Assessment and Monitoring. Appl. Biosaf. 2019, 24, 83–89. [Google Scholar] [CrossRef]
- Heckert, R.A.; Reed, J.C.; Gmuender, F.K.; Ellis, M.; Tonui, W. International Biosafety and Biosecurity Challenges: Suggestions for Developing Sustainable Capacity in Low-Resource Countries. Appl. Biosaf. 2011, 16, 223–230. [Google Scholar] [CrossRef]
- Khripunov, I. Bio Risk Management Culture; Compass: Georgia, GA, USA, 2016. [Google Scholar]
- European Committee for Standardization. Workshop Agreement: Laboratory Biorisk Management. Available online: https://www.aimst.edu.my/ibc/pdf/Guidelines/10.%20CWA%2015793,%202011.pdf (accessed on 26 February 2021).
- World Health Organization. Responsible Life-Sciences Research for Global Health Security: A Guidance Document. Available online: https://apps.who.int/iris/handle/10665/70507 (accessed on 26 February 2021).
- Sharif, D.S.K.; Kimani, D.F. Laboratory Biosafety and Biosecurity Policy Guidelines; Ministry of Public Health and Sanitation & Ministry of Medical Services: Kenya, Republic of Kenya, 2019. [Google Scholar]
- Bhore, S.J. Highlights of Biosafety and Biosecurity Month (BBM) at the AIMST University and Perspectives on Biorisk Management. Bioinformation 2019, 15, 568–571. [Google Scholar] [CrossRef]
- Stroot, P.; Jenal, U. A New Approach Contributing to BWC Compliance via Biosafety, Biosecurity, and Biorisk Management. Nonprolif. Rev. 2011, 18, 545–555. [Google Scholar] [CrossRef]
- Gentilli, S.M.; Potts, J.M.; Clarkson, A.J.; Jacobi, H.B. An Overview of the NIH Biorisk Management Program. Appl. Biosaf. 2016, 21, 26–33. [Google Scholar] [CrossRef][Green Version]
- Lin, K.; Liu, M.; Ma, H.; Pan, S.; Qiao, H.; Gao, H. Laboratory Biosafety Emergency Management for SARS-CoV-2. J. Biosaf. Biosecur. 2020, 2, 99–101. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.; Xu, M. Biohazard Levels and Biosafety Protection for Mycobacterium Tuberculosis Strains with Different Virulence. Biosaf. Health 2020, 2, 135–141. [Google Scholar] [CrossRef]
- Sundqvist, B.; Bengtsson, U.A.; Wisselink, H.J.; Peeters, B.P.H.; Van Rotterdam, B.; Kampert, E.; Bereczky, S.; Olsson, N.G.J.; Szekely Björndal, Å; Zini, S.; et al. Harmonization of European Laboratory Response Networks by Implementing CWA 15793: Use of a Gap Analysis and an “Insider” Exercise as Tools. Biosecur. Bioterror. 2013, 11 (Suppl. S1). [Google Scholar] [CrossRef]
- Zhai, P.; Wang, R.; Zhou, Y.; Hu, D.; Li, J.; Zhou, Y. Enhancing the Capabilities of Biosafety Laboratories through the Established Accreditation System: Development of the Biosafety Laboratory Accreditation System in China. J. Biosaf. Biosecur. 2019, 1, 86–89. [Google Scholar] [CrossRef]
| Laboratory Name | Type of Laboratory | Type of BSL (Biosafety Level) |
|---|---|---|
| Lab 1 | Pathology Anatomy | BSL 1 |
| Lab 2 | Haematology | BSL 1 |
| Lab 3 | Cytogenetic | BSL 1 |
| Lab 4 | Molecular | BSL 3 |
| Lab 5 | Microbiology | BSL 2 |
| Lab 6 | Urinalysis | BSL 1 |
| Lab 7 | Automation | BSL 1 |
| Lab 8 | Immuno-serum | BSL 1 |
| No | Element | Sub-Clauses (Number of Items) | Description | Total of Items |
|---|---|---|---|---|
| 1. | Context and Organisations (Clause 4) |
| The goals and mandates of the organisation, its objectives, and the boundaries of its work must be clearly defined and communicated throughout the organisation. The organisation must determine external and internal issues that are relevant to its objectives and which affect the ability to achieve the expected results of bio-risk management system. | 7 |
| 2. | Leadership (Clause 5) |
| Top management must demonstrate leadership and commitment with respect to the bio-risk management system | 50 |
| 3. | Planning (Clause 6) |
| Actions are needed to identify, assess, and prioritize bio-risk, implement measures to mitigate bio-risk, integrate these actions into the organisation’s bio-risk management system processes, and evaluate the effectiveness of these measures. | 11 |
| 4. | Support (Clause 7) |
| The organisation shall determine and provide the necessary support items for the establishment, implementation, maintenance, evaluation, and continuous improvement of the bio-risk management system. | 55 |
| 5. | Operation (Clause 8) |
| The organisation shall carry out the operations required for the implementation of the bio-risk management system. | 43 |
| 6. | Performance Evaluation (Clause 9) |
| The organisation must carry out the necessary performance evaluations to enhance the performance of the bio-risk management system | 23 |
| 7. | Improvement (Clause 10) |
| Organisations must determine opportunities for improvement from performance evaluation and implement the necessary actions to achieve the desired results of the bio-risk management system | 13 |
| Total Checklist Items | 202 | |||
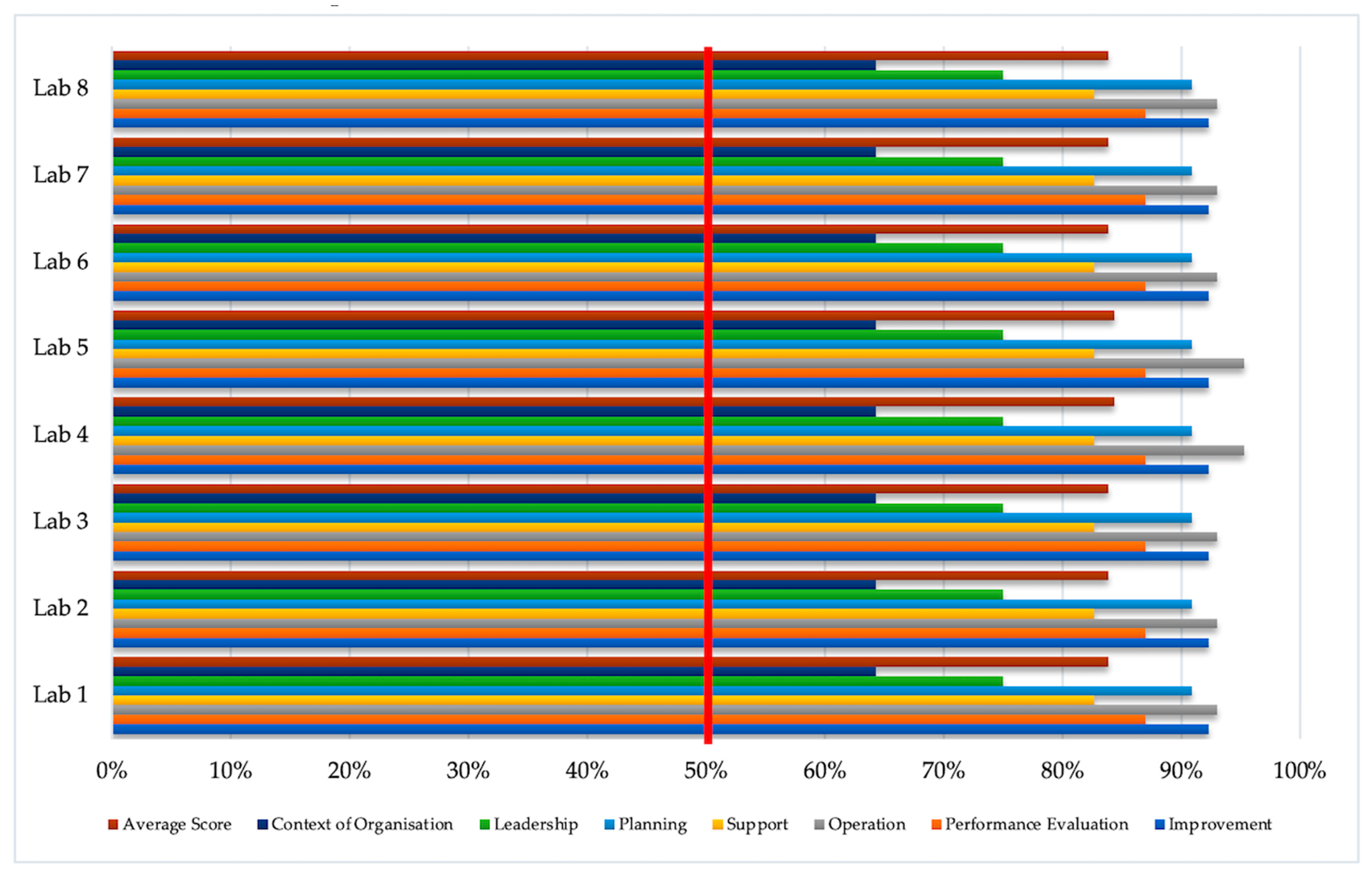
| No | Element | Sub-Clauses | Number of Items | Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 5 | Lab 6 | Lab 7 | Lab 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (%) | T (%) | T (%) | T (%) | T (%) | T (%) | T (%) | T (%) | |||||
| 1 | Context and Organisations | 4.2 | Understanding the Organisation and its context | 2 | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) |
| (Clause 4) | 4.3 | Understanding the needs and expectations of interested parties | 2 | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | |
| 4.4 | Determining the scope of bio-risk management systems | 2 | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | 1.5 (75) | ||
| 4.5 | Bio-risk management system | 1 | 0.5 (50) | 0.5 (50) | 0.5 (50) | 0.5 (50) | 0.5 (50) | 0.5 (50) | 0.5 (50) | 0.5 (50) | ||
| 2 | Leadership | 5.1 | Leadership and commitment | 10 | 8.5 (85) | 8.5 (85) | 8.5 (85) | 8.5 (85) | 8.5 (85) | 8.5 (85) | 8.5 (85) | 8.5 (85) |
| (Clause 5) | 5.2 | Policy | 8 | 6.5 (81) | 6.5 (81) | 6.5 (81) | 6.5 (81) | 6.5 (81) | 6.5 (81) | 6.5 (81) | 6.5 (81) | |
| 5.3 | Roles, responsibilities, and authorities | 32 | 22.5 (70) | 22.5 (70) | 22.5 (70) | 22.5 (70) | 22.5 (70) | 22.5 (70) | 22.5 (70) | 22.5 (70) | ||
| 3 | Planning | 6.1 | Actions to address risks and opportunities | 6 | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) |
| (Clause 6) | 6.2 | Bio-risk management objectives and planning to achieve them | 5 | 4 (80) | 4 (80) | 4 (80) | 4 (80) | 4 (80) | 4 (80) | 4 (80) | 4 (80) | |
| 4 | Support | 7.1 | Resources | 8 | 6 (75) | 6 (75) | 6 (75) | 6 (75) | 6 (75) | 6 (75) | 6 (75) | 6 (75) |
| (Clause 7) | 7.2 | Competence | 14 | 8.5 (60.7) | 8.5 (60.7) | 8.5 (60.7) | 8.5 (60.7) | 8.5 (60.7) | 8.5 (60.7) | 8.5 (60.7) | 8.5 (60.7) | |
| 7.3 | Awareness | 7 | 5 (71.4) | 5 (71.4) | 5 (71.4) | 5 (71.4) | 5 (71.4) | 5 (71.4) | 5 (71.4) | 5 (71.4) | ||
| 7.4 | Communication | 6 | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | ||
| 7.5 | Documented information | 14 | 14 (100) | 14 (100) | 14 (100) | 14 (100) | 14 (100) | 14 (100) | 14 (100) | 14 (100) | ||
| 7.6 | Non-employees | 1 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | ||
| 7.7 | Personal security | 2 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | ||
| 7.8 | Control of suppliers | 3 | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | ||
| 5 | Operation | 8.1 | Operational planning and control | 10 | 8.5 (85) | 8.5 (85) | 8.5 (85) | 9.5 (85) | 9.5 (95) | 8.5 (85) | 8.5 (85) | 8.5 (85) |
| (Clause 8) | 8.2 | Commissioning and decommissioning | 2 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | |
| 8.3 | Maintenance, control, calibration, certification, and validation | 1 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | ||
| 8.4 | Physical security | 4 | 3.5 (87.5) | 3.5 (87.5) | 3.5 (87.5) | 3.5 (87.5) | 3.5 (87.5) | 3.5 (87.5) | 3.5 (87.5) | 3.5 (87.5) | ||
| 8.5 | Biological materials inventory | 3 | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | ||
| 8.6 | Good microbiological technique | 2 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | ||
| 8.7 | Clothing and personal protective equipment (PPE) | 4 | 4 (87.5) | 4 (87.5) | 4 (87.5) | 4 (87.5) | 4 (87.5) | 4 (87.5) | 4 (87.5) | 4 (87.5) | ||
| 8.8 | Decontamination and waste management | 6 | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | ||
| 8.9 | Emergency response and contingency planning | 8 | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | ||
| 8.10 | Transport biological materials | 3 | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | ||
| 6 | Performance Evaluation | 9.1 | Monitoring, measurement, analysis, and evaluation | 7 | 6 (87.5) | 6 (87.5) | 6 (87.5) | 6 (87.5) | 6 (87.5) | 6 (87.5) | 6 (87.5) | 6 (87.5) |
| (Clause 9) | 9.2 | Internal audit | 8 | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | |
| 9.3 | Management review | 8 | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) | ||
| 7 | Improvement | 10.1 | General | 1 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| (Clause 10) | 10.2 | Incident, non-conformity, and corrective action | 11 | 10.5 (95.5) | 10.5 (95.5) | 10.5 (95.5) | 10.5 (95.5) | 10.5 (95.5) | 10.5 (95.5) | 10.5 (95.5) | 10.5 (95.5) | |
| 10.3 | Continual improvement | 2 | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | ||
| No | Element | Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 5 | Lab 6 | Lab 7 | Lab 8 |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Context of Organisation | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| 2. | Leadership | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| 3. | Planning | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 4. | Support | 9.5 | 9.5 | 9.5 | 9.5 | 9.5 | 9.5 | 9.5 | 9.5 |
| 5. | Operation | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 3 |
| 6. | Performance Evaluation | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 7. | Improvement | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total | 32.5 | 32.5 | 32.5 | 31.5 | 31.5 | 32.5 | 32.5 | 32.5 | |
| Effectiveness | 83.9% | 83.9% | 83.9% | 84.4% | 84.4% | 83.9% | 83.9% | 83.9% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lestari, F.; Kadir, A.; Miswary, T.; Maharani, C.F.; Bowolaksono, A.; Paramitasari, D. Implementation of Bio-Risk Management System in a National Clinical and Medical Referral Centre Laboratories. Int. J. Environ. Res. Public Health 2021, 18, 2308. https://doi.org/10.3390/ijerph18052308
Lestari F, Kadir A, Miswary T, Maharani CF, Bowolaksono A, Paramitasari D. Implementation of Bio-Risk Management System in a National Clinical and Medical Referral Centre Laboratories. International Journal of Environmental Research and Public Health. 2021; 18(5):2308. https://doi.org/10.3390/ijerph18052308
Chicago/Turabian StyleLestari, Fatma, Abdul Kadir, Thariq Miswary, Cynthia Febrina Maharani, Anom Bowolaksono, and Debby Paramitasari. 2021. "Implementation of Bio-Risk Management System in a National Clinical and Medical Referral Centre Laboratories" International Journal of Environmental Research and Public Health 18, no. 5: 2308. https://doi.org/10.3390/ijerph18052308
APA StyleLestari, F., Kadir, A., Miswary, T., Maharani, C. F., Bowolaksono, A., & Paramitasari, D. (2021). Implementation of Bio-Risk Management System in a National Clinical and Medical Referral Centre Laboratories. International Journal of Environmental Research and Public Health, 18(5), 2308. https://doi.org/10.3390/ijerph18052308









