Preterm Deliveries in Women with Uterine Myomas: The Japan Environment and Children’s Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
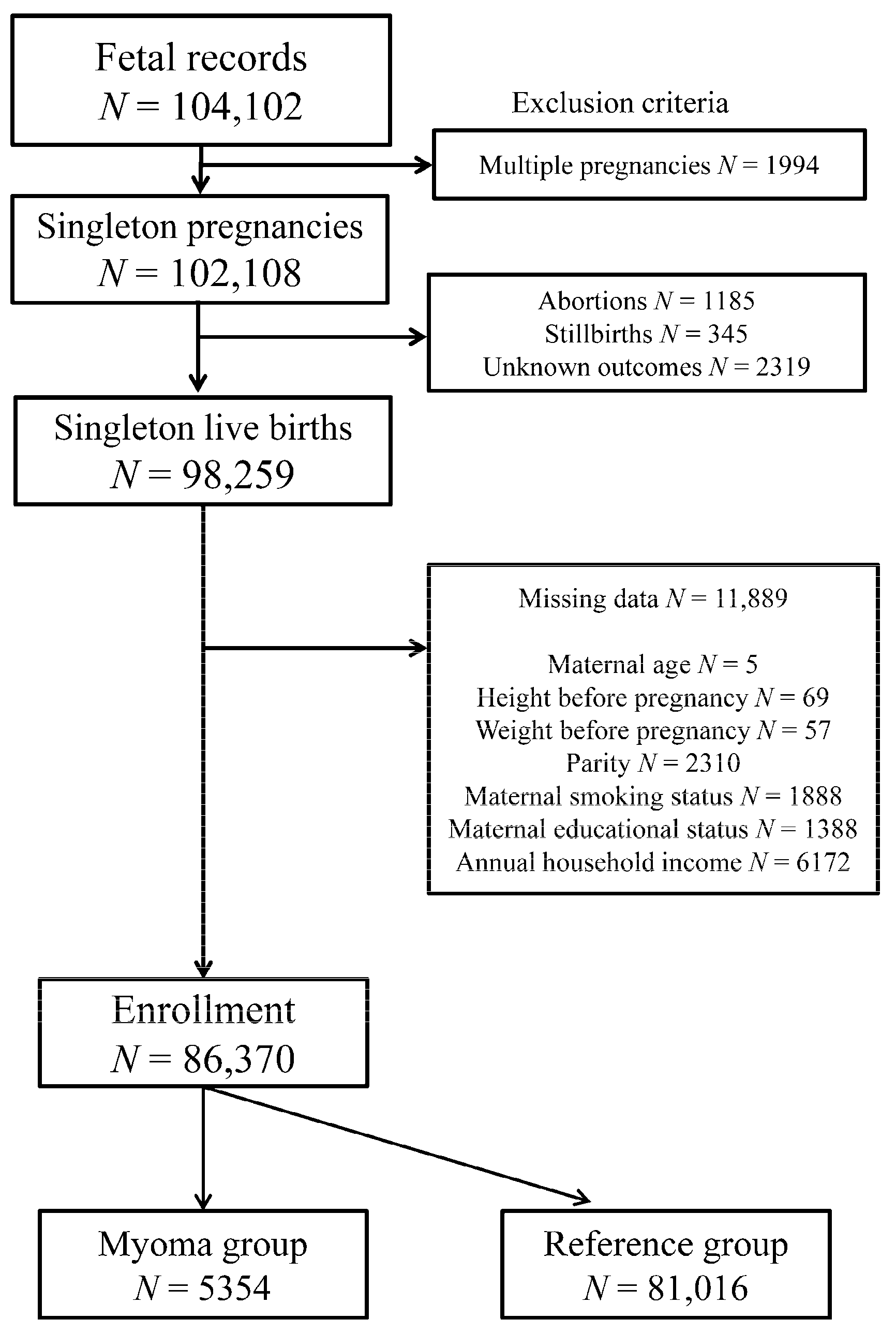
2.2. Data Collection
2.3. Exposure Variables
2.4. Obstetric Outcomes and Confounding Factors
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
4. Discussion
4.1. Main Findings
4.2. Interpretation
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stout, M.J.; Odibo, A.O.; Graseck, A.S.; Macones, G.A.; Crane, J.P.; Cahill, A.G. Leiomyomas at routine second-trimester ultrasound examination and adverse obstetric outcomes. Obstet. Gynecol. 2010, 116, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Girault, A.; Le Ray, C.; Chapron, C.; Goffinet, F.; Marcellin, L. Leiomyomatous uterus and preterm birth: An exposed/unexposed monocentric cohort study. Am. J. Obstet. Gynecol. 2018, 219, 410.e1–410.e7. [Google Scholar] [CrossRef]
- Coronado, G.D.; Marshall, L.M.; Schwartz, S.M. Complications in pregnancy, labor, and delivery with uterine leiomyomas: A population-based study. Obstet. Gynecol. 2000, 95, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Exacoustòs, C.; Rosati, P. Ultrasound diagnosis of uterine myomas and complications in pregnancy. Obstet. Gynecol. 1993, 82, 97–101. [Google Scholar] [CrossRef]
- Davis, J.L.; Ray-Mazumder, S.; Hobel, C.J.; Baley, K.; Sassoon, D. Uterine leiomyomas in pregnancy: A prospective study. Obstet. Gynecol. 1990, 75, 41–44. [Google Scholar]
- Qidwai, G.I.; Caughey, A.B.; Jacoby, A.F. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet. Gynecol. 2006, 107, 376–382. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, H.C.; Chen, S.F.; Lin, H.C. Increased risk of preterm births among women with uterine leiomyoma: A nationwide population-based study. Hum. Reprod. 2009, 24, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Wen, S.W.; Smith, G.; Yang, Q.; Walker, M. Epidemiology of preterm birth and neonatal outcome. Semin. Fetal Neonatal Med. 2004, 9, 429–435. [Google Scholar] [CrossRef] [PubMed]
- López Bernal, A. Overview. Preterm labour: Mechanisms and management. BMC Pregnancy Childbirth 2007, 7, S2. [Google Scholar] [CrossRef]
- Ananth, C.V.; Vintzileos, A.M. Epidemiology of preterm birth and its clinical subtypes. J. Matern. Fetal Neonatal Med. 2006, 19, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.P.; Kay, H.H.; Mahony, B.S. The clinical significance of uterine leiomyomas in pregnancy. Am. J. Obstet. Gynecol. 1989, 160, 1212–1216. [Google Scholar] [CrossRef]
- Vergani, P.; Ghidini, A.; Strobelt, N.; Roncaglia, N.; Locatelli, A.; Lapinski, R.H.; Mangioni, C. Do uterine leiomyomas influence pregnancy outcome? Am. J. Perinatol. 1994, 11, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan Environment and Children’s Study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef]
- Lencki, S.G.; Maciulla, M.B.; Eglinton, G.S. Maternal and umbilical cord serum interleukin levels in preterm labor with clinical chorioamnionitis. Am. J. Obstet. Gynecol. 1994, 170, 1345–1351. [Google Scholar] [CrossRef]
- Kyozuka, H.; Fujimori, K.; Hosoya, M.; Yasumura, S.; Yokoyama, T.; Sato, A.; Hashimoto, K.; Japan Environment and Children’s Study (JECS) Group. The effect of maternal age at the first childbirth on gestational age and birth weight: The Japan Environment and Children’s Study (JECS). J. Epidemiol. 2019, 29, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ancel, P.Y. Preterm labor: Pathophysiology, risk factors and outcomes. J. Gynecol. Obstet. Biol. Reprod. 2002, 31, 5S10–5S21. [Google Scholar]
- Goldenberg, R.L.; Goepfert, A.R.; Ramsey, P.S. Biochemical markers for the prediction of preterm birth. Am. J. Obstet. Gynecol. 2005, 192, S36–S46. [Google Scholar] [CrossRef]
- Naeye, R.L.; Peters, E.C. Causes and consequences of premature rupture of fetal membranes. Lancet 1980, 1, 192–194. [Google Scholar] [CrossRef]
- Menon, R.; Richardson, L.S. Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin. Perinatol. 2017, 41, 409–419. [Google Scholar] [CrossRef]
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T.N.K.; Chorioamnionitis Workshop Participants. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: Summary of a workshop. Obstet. Gynecol. 2016, 127, 426–436. [Google Scholar] [CrossRef]
- Roberts, D.; Brown, J.; Medley, N.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2017, 3, CD004454. [Google Scholar] [CrossRef] [PubMed]
- Jarde, A.; Lutsiv, O.; Beyene, J.; McDonald, S.D. Vaginal progesterone, oral progesterone, 17-OHPC, cerclage, and pessary for preventing preterm birth in at-risk singleton pregnancies: An updated systematic review and network meta-analysis. BJOG 2019, 126, 556–567. [Google Scholar] [CrossRef]
- Duley, L.; Gülmezoglu, A.M.; Henderson-Smart, D.J.; Chou, D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst. Rev. 2010, 2010, CD000025. [Google Scholar] [CrossRef]
- Ciavattini, A.; Clemente, N.; Delli Carpini, G.; Di Giuseppe, J.; Giannubilo, S.R.; Tranquilli, A.L. Number and size of uterine fibroids and obstetric outcomes. J. Matern. Fetal Neonatal Med. 2015, 28, 484–488. [Google Scholar] [CrossRef]
- Marzano, S.; Padula, F.; Meloni, P.; Anceschi, M.M. Preterm delivery at low gestational age: Risk factors for short latency. A multivariated analysis. J. Prenat. Med. 2008, 2, 15–18. [Google Scholar] [PubMed]
- Zullo, F.; Spagnolo, E.; Saccone, G.; Acunzo, M.; Xodo, S.; Ceccaroni, M.; Berghella, V. Endometriosis and obstetrics complications: A systematic review and meta-analysis. Fertil. Steril. 2017, 108, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, F.; Arcuri, F.; de Ziegler, D.; Chapron, C. Inflammation: A link between endometriosis and preterm birth. Fertil. Steril. 2012, 98, 36–40. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total Participants (n = 86,370) | Myoma Group (n = 5354) | Reference Group (n = 81,016) | p-Value |
|---|---|---|---|---|
| Maternal age, years mean (SD) | 31.3 (4.9) | 35.1 (4.1) | 31.1 (4.9) | <0.001 |
| Maternal BMI before pregnancy, kg/m2 mean (SD) | 21.2 (3.3) | 21.9 (3.5) | 21.2 (3.3) | <0.001 |
| Nulliparous, % (n) | 39.9 (34,478) | 47.9 (2563) | 39.4 (31,915) | <0.001 |
| Maternal smoking during pregnancy, % (n) | 4.7 (4036) | 3.0 (162) | 4.8 (3874) | <0.001 |
| Maternal educational status, % (n) | ||||
| <10 years | 4.5 (3909) | 2.1 (112) | 4.7 (3797) | <0.001 |
| 10–12 years | 30.8 (26,573) | 24.8 (1328) | 31.2 (25,245) | <0.001 |
| 13–16 years | 63.2 (54,586) | 70.7 (3783) | 62.7 (50,803) | <0.001 |
| ≥17 years | 1.5 (1302) | 2.4 (131) | 1.4 (1171) | <0.001 |
| Annual household income, % (n) | ||||
| <2,000,000 JPY | 5.6 (4878) | 2.8 (151) | 5.8 (4727) | <0.001 |
| 2,000,000–5,999,999 JPY | 67.6 (58,392) | 62.3 (3336) | 68.0 (55,056) | <0.001 |
| 6,000,000–9,999,999 JPY | 22.5 (19,404) | 28.6 (1530) | 22.1 (17,874) | <0.001 |
| ≥10,000,000 JPY | 4.3 (3696) | 6.3 (337) | 4.1 (3359) | <0.001 |
| PTB <37 weeks, % (n) | 4.5 (3901) | 6.2 (333) | 4.4 (3568) | <0.001 |
| PTB <34 weeks, % (n) | 0.9 (752) | 1.5 (79) | 0.8 (673) | <0.001 |
| pPROM, % (n) | 1.1 (959) | 1.8 (98) | 1.1 (861) | <0.001 |
| II, % (n) | 0.6 (493) | 0.7 (39) | 0.6 (454) | 0.114 |
| Obstetric Outcomes | PTB | PTB | pPROM | II |
|---|---|---|---|---|
| <37 Weeks | <34 Weeks | |||
| Myoma Group | Odds Ratios (95% CI) | |||
| cORs | 1.44 (1.28–1.62) | 1.79 (1.41–2.26) | 1.74 (1.41–2.14) | 1.30 (0.94–1.81) |
| aORs | 1.37 (1.22–1.54) | 1.61 (1.27–2.05) | 1.65 (1.33–2.04) | 1.05 (0.75–1.46) |
| Obstetric Outcomes | Myoma Group | Reference Group | ||
|---|---|---|---|---|
| PTB | pPROM | PTB | pPROM | |
| aORs | ||||
| II (–) | Ref | Ref | Ref | Ref |
| II (+) | 1.81 (0.64–5.15) | 1.29 (0.17–9.54) | 3.16 (2.39–4.19) | 5.54 (3.72–8.25) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, T.; Kyozuka, H.; Endo, Y.; Fukuda, T.; Yasuda, S.; Yamaguchi, A.; Sato, A.; Ogata, Y.; Shinoki, K.; Hosoya, M.; et al. Preterm Deliveries in Women with Uterine Myomas: The Japan Environment and Children’s Study. Int. J. Environ. Res. Public Health 2021, 18, 2246. https://doi.org/10.3390/ijerph18052246
Murata T, Kyozuka H, Endo Y, Fukuda T, Yasuda S, Yamaguchi A, Sato A, Ogata Y, Shinoki K, Hosoya M, et al. Preterm Deliveries in Women with Uterine Myomas: The Japan Environment and Children’s Study. International Journal of Environmental Research and Public Health. 2021; 18(5):2246. https://doi.org/10.3390/ijerph18052246
Chicago/Turabian StyleMurata, Tsuyoshi, Hyo Kyozuka, Yuta Endo, Toma Fukuda, Shun Yasuda, Akiko Yamaguchi, Akiko Sato, Yuka Ogata, Kosei Shinoki, Mitsuaki Hosoya, and et al. 2021. "Preterm Deliveries in Women with Uterine Myomas: The Japan Environment and Children’s Study" International Journal of Environmental Research and Public Health 18, no. 5: 2246. https://doi.org/10.3390/ijerph18052246
APA StyleMurata, T., Kyozuka, H., Endo, Y., Fukuda, T., Yasuda, S., Yamaguchi, A., Sato, A., Ogata, Y., Shinoki, K., Hosoya, M., Yasumura, S., Hashimoto, K., Nishigori, H., Fujimori, K., & Children’s Study Group, T. J. E. (2021). Preterm Deliveries in Women with Uterine Myomas: The Japan Environment and Children’s Study. International Journal of Environmental Research and Public Health, 18(5), 2246. https://doi.org/10.3390/ijerph18052246






