Tobacco Smoke and CYP1A2 Activity in a US Population with Normal Liver Enzyme Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Serum Liver Enzymes
2.3. Smoking Status Assessment
2.4. Assessment of Lifetime Smoke Intensity
2.5. Assessment of Serum Cotinine
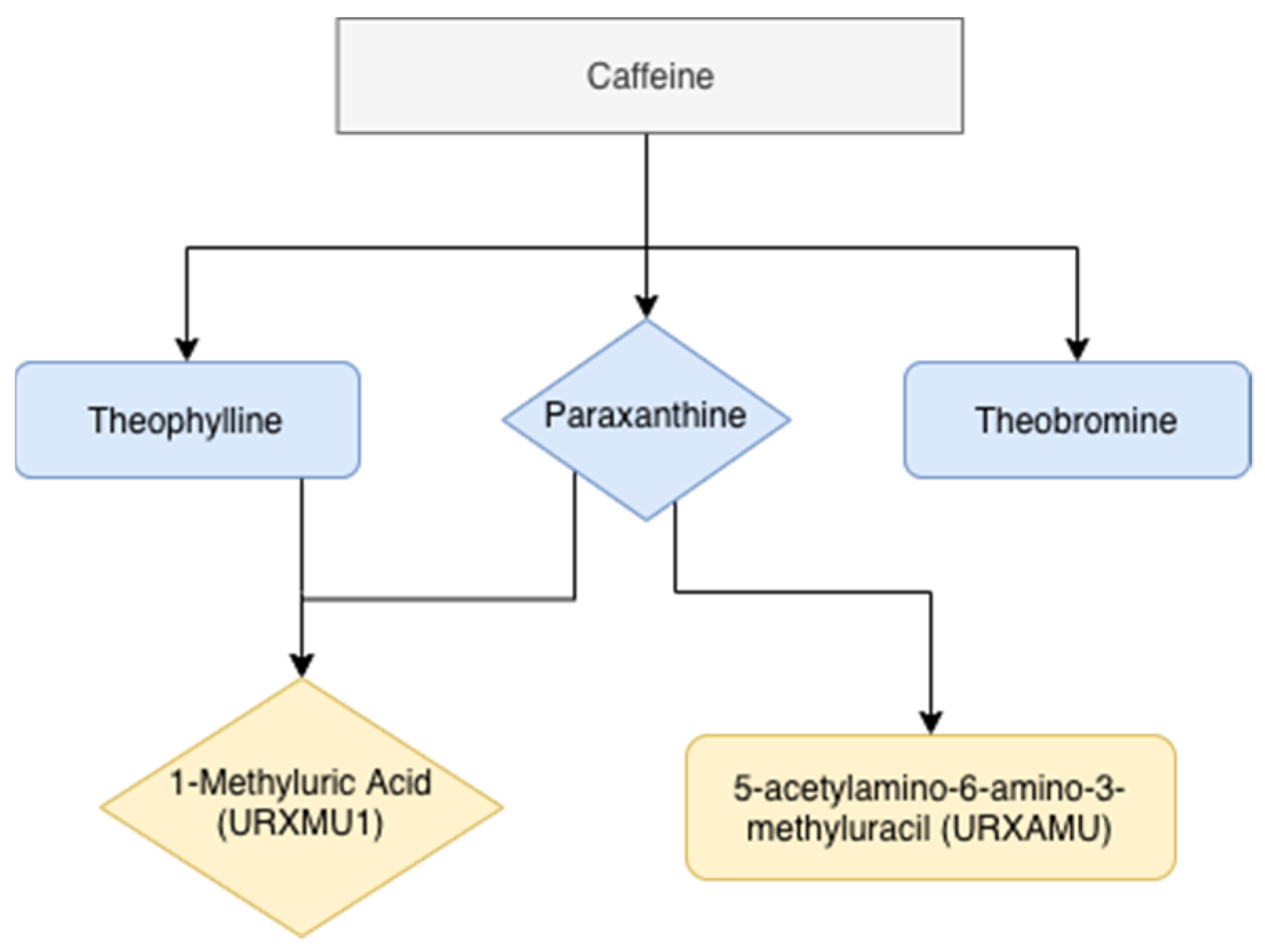
2.6. Caffeine Intake Assessment
2.7. Assessment of Urinary Caffeine Metabolites
2.8. Other Assessments
2.9. Statistical Analyses
3. Results
3.1. Demographic Characteristics by Smoking Status
3.2. Liver Enzyme Levels by Smoking Status in All the Participants in This Study
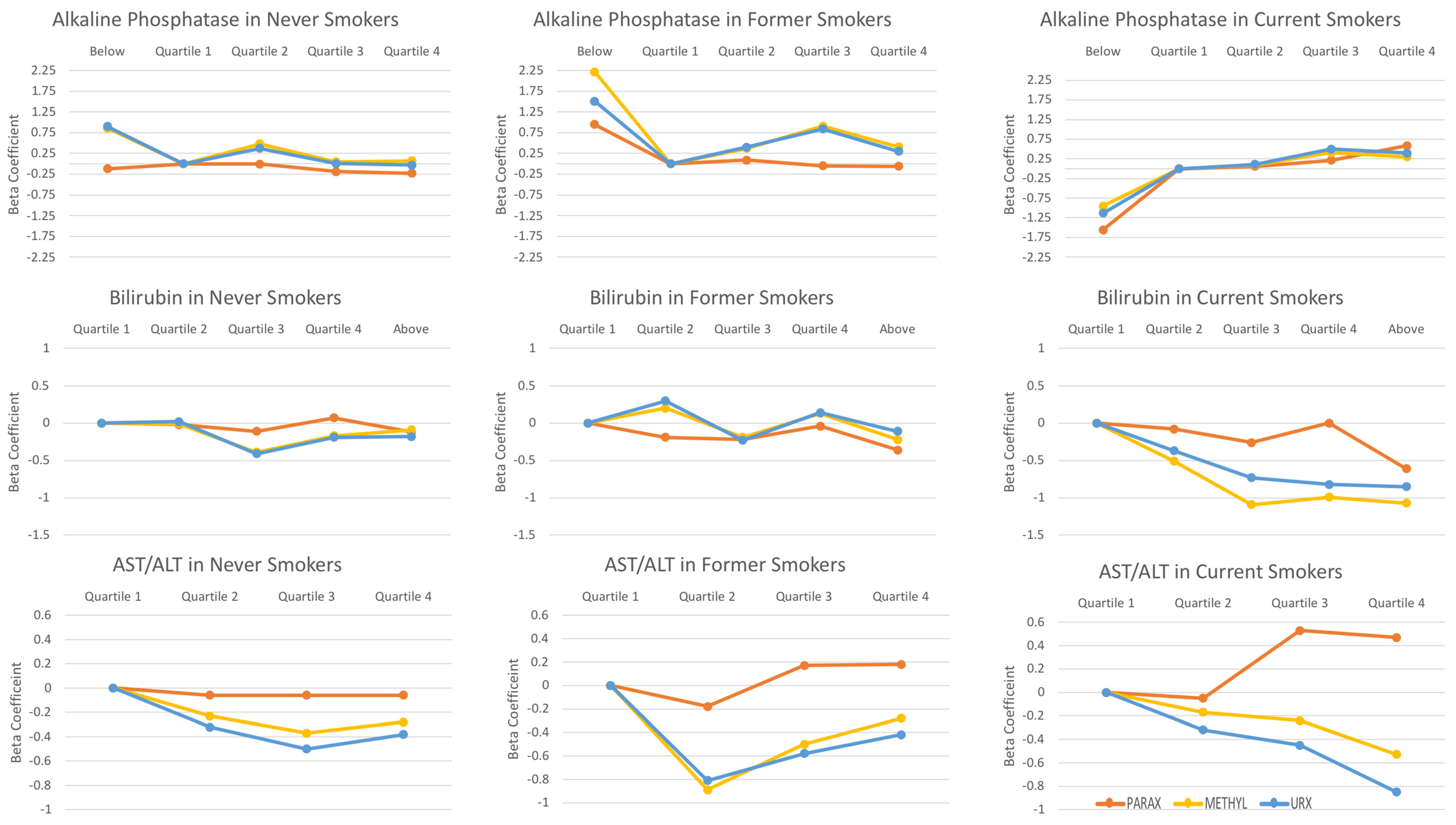
3.3. The Associations between Liver Enzymes and Day 1 Caffeine Metabolite Indices
3.4. The Associations between Liver Enzymes and Day 2 Caffeine Metabolites
3.5. The Associations between Serum Cotinine and Smoking Intensity and Caffeine Metabolite Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duseja, A.; Chalasani, N. Epidemiology and risk factors of nonalcoholic fatty liver disease (NAFLD). Hepatol. Int. 2013, 7, 755–764. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metab. Clin. Exp. 2016, 65, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Zein, C.O. Clearing the smoke in chronic liver diseases. Hepatology 2010, 51, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Baecker, A.; Liu, X.; La Vecchia, C.; Zhang, Z.F. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur. J. Cancer Prev. 2018, 27, 205–212. [Google Scholar] [CrossRef]
- Gu, L.; Gonzalez, F.J.; Kalow, W.; Tang, B.K. Biotransformation of caffeine, paraxanthine, theobromine and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenetics 1992, 2, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kot, M.; Daniel, W.A. Caffeine as a marker substrate for testing cytochrome P450 activity in human and rat. Pharm. Rep. 2008, 60, 789–797. [Google Scholar]
- Rodenburg, E.M.; Eijgelsheim, M.; Geleijnse, J.M.; Amin, N.; Van Duijn, C.M.; Hofman, A.; Uitterlinden, A.G.; Stricker, B.H.; Visser, L.E. CYP1A2 and coffee intake and the modifying effect of sex, age, and smoking. Am. J. Clin. Nutr. 2012, 96, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Fuhr, U.; Rost, K.L. Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics 1994, 4, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tantcheva-Poór, I.; Zaigler, M.; Rietbrock, S.; Fuhr, U. Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharm. Genom. 1999, 9, 131–144. [Google Scholar]
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. Biol. Fate Chem. 2009, 37, 2087–2094. [Google Scholar] [CrossRef]
- Donato, M.T.; Lahoz, A.; Jimenez, N.; Pérez, G.; Serralta, A.; Mir, J.; Castell, J.V.; Gómez-Lechón, M.J. Potential impact of steatosis on cytochrome P450 enzymes of human hepatocytes isolated from fatty liver grafts. Drug Metab. Dispos. 2006, 34, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Kotronen, A.; Westerbacka, J.; Puig, O.; Arkkila, P.; Kiviluoto, T.; Laitinen, S.; Kolak, M.; Fisher, R.M.; Hamsten, A.; et al. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1281–G1287. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Aklillu, E.; McDonagh, E.M.; Klein, T.E.; Altman, R.B. PharmGKB summary: Caffeine pathway. Pharm. Genom. 2012, 22, 389–395. [Google Scholar] [CrossRef]
- Faber, M.S.; Fuhr, U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin. Pharmacol. Ther. 2004, 76, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). National Health and Nutrition Examination Laboratory Procedures Manual; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2009. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/manuals/lab.pdf (accessed on 21 February 2021).
- Church, T.R.; Anderson, K.E.; Le, C.; Zhang, Y.; Kampa, D.M.; Benoit, A.R.; Yoder, A.R.; Carmella, S.G.; Hecht, S.S. Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers 2010, 15, 345–352. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Sharp, D.S. Inverse relation between serum cotinine concentration and blood pressure in cigarette smokers. Circulation 1989, 80, 1309–1312. [Google Scholar] [CrossRef]
- Jacob, P., 3rd; Yu, L.; Wilson, M.; Benowitz, N.L. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: Absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol. Mass. Spectrom. 1991, 20, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Blanton, C.A.; Moshfegh, A.J.; Baer, D.J.; Kretsch, M.J. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J. Nutr. 2006, 136, 2594–2599. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2009. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/questionnaires.aspx?BeginYear=2009 (accessed on 21 February 2021).
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). Physical Activity: Data documentation, Codebook, and Frequencies: Centers for Disease Control and Prevention; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2011. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/PAQ_F.htm#Analytic_Notes (accessed on 21 February 2021).
- Giglio, D. A new equation for estimating glomerular filtration rate in cancer patients. Chemotherapy 2014, 60, 63–72. [Google Scholar] [CrossRef]
- ABIM. ABIM Laboratory Test References Ranges—January 2020; ABIM: Philadelphia, PA, USA, 2020. [Google Scholar]
- Schreiner, A.D.; Rockey, D.C. Evaluation of abnormal liver tests in the adult asymptomatic patient. Curr. Opin. Gastroenterol. 2018, 34, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Kumagi, T.; Furukawa, S.; Tokumoto, Y.; Hirooka, M.; Abe, M.; Hiasa, Y.; Matsuura, B.; Onji, M. Non-alcoholic fatty liver disease: Factors associated with its presence and onset. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S4), 71–78. [Google Scholar] [CrossRef]
- Haghighi, S.; Amini, M.; Pournaghshband, Z.; Amini, P.; Hovsepian, S. Relationship between gamma-glutamyl transferase and glucose intolerance in first degree relatives of type 2 diabetics patients. J. Res. Med. Sci. 2011, 16, 123–129. [Google Scholar]
- Rybak, M.E.; Sternberg, M.R.; Pao, C.I.; Ahluwalia, N.; Pfeiffer, C.M. Urine excretion of caffeine and select caffeine metabolites is common in the U.S. population and associated with caffeine intake. J. Nutr. 2015, 145, 766–774. [Google Scholar] [CrossRef]
- Gawrieh, S.; Wilson, L.A.; Cummings, O.W.; Clark, J.M.; Loomba, R.; Hameed, B.; Abdelmalek, M.F.; Dasarathy, S.; Neuschwander-Tetri, B.A.; Kowdley, K.; et al. Histologic Findings of Advanced Fibrosis and Cirrhosis in Patients with Nonalcoholic Fatty Liver Disease Who Have Normal Aminotransferase Levels. Am. J. Gastroenterol. 2019, 114, 1626–1635. [Google Scholar] [CrossRef]
- Nebert, D.W.; Dalton, T.P.; Okey, A.B.; Gonzalez, F.J. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004, 279, 23847–23850. [Google Scholar] [CrossRef] [PubMed]
- Klomp, F.; Wenzel, C.; Drozdzik, M.; Oswald, S. Drug-Drug Interactions Involving Intestinal and Hepatic CYP1A Enzymes. Pharmaceutics 2020, 12, 1201. [Google Scholar] [CrossRef]
- Lieberman, D.; Phillips, D. Isolated" elevation of alkaline phosphatase: Significance in hospitalized patients. J. Clin. Gastroenterol. 1990, 12, 415–419. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Gu, J.; Yang, M.; Zhang, X.; Zhao, H.; Li, L. Serum alkaline phosphatase levels as a simple and useful test in screening for significant fibrosis in treatment-naive patients with hepatitis B e-antigen negative chronic hepatitis B. Eur. J. Gastroenterol. Hepatol. 2019, 31, 817. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.C.; Chan, K.M.; Lee, C.F.; Lee, Y.S.; Eldeen, F.Z.; Chou, H.S.; Lee, W.C.; Chen, M.F. Alkaline phosphatase: Does it have a role in predicting hepatocellular carcinoma recurrence? J. Gastrointest. Surg. 2011, 15, 1440–1449. [Google Scholar] [CrossRef]
- Marrero, J.A.; Fontana, R.J.; Su, G.L.; Conjeevaram, H.S.; Emick, D.M.; Lok, A.S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002, 36, 1349–1354. [Google Scholar] [CrossRef]
- Denaro, C.P.; Wilson, M.; Jacob, P., III; Benowitz, N.L. The effect of liver disease on urine caffeine metabolite ratios. Clin. Pharm. 1996, 59, 624–635. [Google Scholar] [CrossRef]
| (A) | ||||
|---|---|---|---|---|
| Characteristic | Never Smoker (<100 cig. Ever) (N = 812) | Former Smoker (>100 cig. Ever) (N = 348) | Current Smoker (>100 cig. Ever) (N = 305) | p-Value |
| Gender (N, %) | ≤0.0001 | |||
| Female | 489 (56.95) | 136 (39.98) | 146 (47.19) | |
| Male | 323 (43.05) | 212 (60.02) | 159 (52.81) | |
| Age (N, %) | ≤0.0001 | |||
| 18 to <28 | 136 (17.59) | 30 (11.53) | 61 (23.81) | |
| 28 to <38 | 158 (22.35) | 46 (16.61) | 66 (21.93) | |
| 38 to <48 | 127 (17.92) | 70 (23.32) | 61 (18.43) | |
| 48 to <58 | 131 (14.56) | 85 (24.72) | 45 (12.04) | |
| 58 to <68 | 147 (12.51) | 105 (19.08) | 26 (5.54) | |
| 68+ | 113 (15.08) | 12 (4.74) | 46 (18.26) | |
| Ethnicity (N, %) | 0.01 | |||
| Mexican American | 175 (9.82) | 49 (5.24) | 47 (7.86) | |
| Other Hispanic | 95 (5.44) | 36 (4.29) | 22 (3.37) | |
| Non-Hispanic Black | 120 (8.97) | 32 (4.89) | 61 (12.90) | |
| Other Race/Multiracial | 42 (6.12) | 15 (5.83) | 11 (5.50) | |
| Non-Hispanic White | 380 (69.65) | 216 (79.75) | 164 (70.37) | |
| Serum cotinine, ng/mL, (N, %) | <0.0001 | |||
| ≤1.7 × 10−6 | 269 (34.86) | 90 (25.50) | 1 (0.21) | |
| 1.7 × 10−6 > to 5.20 × 10−6 | 290 (36.08) | 110 (32.83) | 1 (0.15) | |
| 5.2 × 10-6 > to 113.00 × 10−6 | 198 (22.27) | 98 (26.81) | 15 (3.43) | |
| >113.0 × 10−6 | 55 (6.80) | 50 (14.86) | 288 (96.21) | |
| Smoking intensity, total lifetime cigarettes, (N, %) | NA | |||
| 0 | 812 (100.00) | NA | NA | |
| 0 > to 50,400 | NA | 152 (45.29) | 122 (38.90) | |
| 50,400+ | NA | 196 (54.72) | 183 (61.11) | |
| Body Mass Index, kg/m2, (N, %) | 0.04 | |||
| <18.5 | 8 (1.13) | 3 (1.20) | 7 (3.98) | |
| 18.5–25 | 222 (30.00) | 73 (25.04) | 92 (29.99) | |
| 25–30 | 251 (30.42) | 139 (35.70) | 100 (34.94) | |
| 30+ | 331 (38.44) | 133 (38.06) | 106 (31.09) | |
| Blood pressure status (N, %) | 0.02 | |||
| Hypotensive | 6 (0.85) | 2 (0.30) | 3 (1.02) | |
| Normotensive | 384 (52.39) | 124 (38.29) | 151 (52.47) | |
| Pre-Hypertensive | 278 (33.46) | 148 (45.19) | 104 (34.83) | |
| Hypertensive | 144 (13.29) | 74 (16.23) | 47 (11.68) | |
| Diabetes (N, %) | <0.0001 | |||
| Yes | 79 (5.99) | 55 (13.67) | 20 (5.88) | |
| Borderline | 11 (1.00) | 11 (4.08) | 11 (2.63) | |
| No | 722 (93.01) | 282 (82.25) | 274 (91.49) | |
| Fasting Status, hours (N, %) | 0.56 | |||
| 0–8 | 415 (53.63) | 180 (49.91) | 159 (53.47) | |
| 8–12 | 187 (23.33) | 76 (24.13) | 86 (26.82) | |
| 12+ | 210 (23.05) | 92 (25.96) | 60 (19.71) | |
| Liver Condition (N, %) | 0.20 | |||
| Former | 14 (1.26) | 6 (1.37) | 5 (3.12) | |
| Current | 6 (0.43) | 5 (1.37) | 4 (0.84) | |
| No Liver Condition | 792 (98.31) | 337 (97.26) | 296 (96.04) | |
| Estimated Glomerular Filtration Rate, mL/min/1.73 cm2 (N, %) | 0.0002 | |||
| Less than 60 | 528 (65.51) | 179 (59.20) | 223 (74.93) | |
| 60–90 | 237 (30.65) | 126 (32.46) | 75 (23.16) | |
| Greater than 90 | 47 (3.84) | 43 (8.34) | 7 (1.91) | |
| Physical activity, metabolic equivalent-hours/week, (N, %) | 0.02 | |||
| 0 | 220 (21.57) | 89 (22.13) | 89 (24.39) | |
| 0>−4 | 48 (4.97) | 30 (8.66) | 15 (3.68) | |
| 4>−40 | 323 (42.91) | 115 (32.89) | 92 (33.18) | |
| 40+ | 221 (30.56) | 114 (36.32) | 109 (38.75) | |
| (B) | ||||
| Alkaline Phosphatase, U/L, (N, %) | 0.03 | |||
| <30.00 (Abnormal) | 6 (0.96) | 1 (0.07) | 2 (0.28) | |
| 30.00 to <55.29 | 234 (33.06) | 85 (29.26) | 61 (22.03) | |
| 55.29 to < 68.64 | 209 (25.29) | 101 (26.25) | 87 (29.29) | |
| 68.64 to <86.05 | 207 (24.25) | 93 (27.47) | 94 (32.01) | |
| ≥86.05 | 156 (16.44) | 68 (16.96) | 61 (16.39) | |
| Alanine Aminotransferase, U/L, (N, %) | 0.06 | |||
| <15.34 | 155 (17.87) | 47 (10.88) | 69 (21.36) | |
| 15.34 to <19.70 | 204 (24.84) | 70 (20.58) | 71 (21.43) | |
| 19.73 to <27.09 | 247 (30.29) | 125 (36.50) | 87 (28.48) | |
| 27.09 to <40.00 | 126 (17.02) | 74 (21.40) | 49 (17.15) | |
| ≥40.00 (Abnormal) | 80 (9.98) | 32 (10.64) | 29 (11.58) | |
| Aspartate Aminotransferase, U/L, (N, %) | ||||
| <19.46 | 176 (19.90) | 60 (16.48) | 94 (29.74) | 0.0003 |
| 19.46 to <22.85 | 165 (21.47) | 63 (17.18) | 64 (22.11) | |
| 22.8 to <27.43 | 240 (30.24) | 116 (34.02) | 66 (21.02) | |
| 27.43 to <40.00 | 190 (24.14) | 94 (29.34) | 62 (20.44) | |
| ≥40.00 (Abnormal) | 41 (4.25) | 15 (2.97) | 19 (6.68) | |
| Aspartate Aminotransferase/Alanine Aminotransferase (N, %) | 0.09 | |||
| <0.92 | 212 (26.11) | 101 (29.02) | 90 (29.51) | |
| 0.92 to <1.14 | 211 (25.99) | 100 (28.74) | 77 (25.25) | |
| 1.14 to <1.37 | 232 (28.57) | 83 (23.85) | 72 (23.61) | |
| ≥1.37 | 157 (19.33) | 64 (18.39) | 66 (21.64) | |
| Bilirubin, U/L, (N, %) | <0.0001 | |||
| <0.52 | 165 (18.30) | 66 (16.73) | 88 (26.19) | |
| 0.52 to <0.60 | 139 (17.36) | 61 (17.20) | 80 (27.91) | |
| 0.650 to <0.82 | 273 (32.85) | 137 (39.17) | 91 (30.59) | |
| 0.82 to <1.00 | 134 (17.55) | 45 (14.46) | 26 (6.85) | |
| ≥1.00 (abnormal) | 101 (13.94) | 39 (12.44) | 20 (8.47) | |
| Gamma-glutamyl transpeptidase, U/L | ||||
| Female (N, %)) | 0.17 | |||
| <12.55 | 133 (33.88) | 26 (23.32) | 31 (22.44) | |
| 12.55 to <17.94 | 140 (27.61) | 39 (33.05) | 36 (26.35) | |
| 17.94 to <27.71 | 118 (23.49) | 34 (22.27) | 42 (28.71) | |
| 27.71–40.00 | 67 (9.98) | 17 (11.13) | 20 (13.94) | |
| ≥40.00 (Abnormal) | 31 (5.04) | 20 (10.23) | 17 (8.56) | |
| Male (N, %)) | 0.73 | |||
| <12.55 | 33 (10.04) | 18 (7.81) | 13 (7.51) | |
| 12.55 to <17.94 | 65 (20.00) | 51 (25.13) | 23 (16.33) | |
| 17.94 to <27.71 | 103 (33.36) | 71 (32.62) | 53 (37.14) | |
| 27.71–50.00 | 82 (25.82) | 55 (25.82) | 44 (26.85) | |
| ≥50.00 (Abnormal) | 40 (10.78) | 17 (8.62) | 26 (12.18) | |
| Never Smoker | Former Smoker | Current Smoker | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log (Theophylline)/Caffeine | Log (Paraxanthine)/Caffeine | Log (Theobromine)/Caffeine | Log (1-methyluric acid**)/Caffeine | Log(URXAMU)/Caffeine | Log (Theophylline)/Caffeine | Log (Paraxanthine)/Caffeine | Log (Theobromine)/Caffeine | Log (1-methyluric acid)/Caffeine | Log (URXAMU)/Caffeine | Log (Theophylline)/Caffeine | Log (Paraxanthine)/Caffeine | Log (Theobromine)/Caffeine | Log (1-methyluric acid**)/Caffeine | Log (URXAMU)/Caffeine | ||
| Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | ||
| Alkaline Phosphatase (U/L) | ||||||||||||||||
| Abnormal | <30 | −0.16 (0.70) | −0.12 (0.77) | −0.67 (0.25) | 0.86 (0.22) | 0.9 (0.14) | 1.24 (0.04) | 0.95 (0.12) | 0.03 (0.98) | 2.22 (0.04) | 1.51 (0.18) | −1.77 (0.07) | −1.56 (0.11) | −2.04 (0.08) | −0.95 (0.40) | −1.13 (0.26) |
| Q1 | 30 to <55.29 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 55.29 to < 68.64 | 0.02 (0.83) | −0.01 (0.91) | 0.33 (0.04) | 0.48 (0.04) | 0.37 (0.15) | −0.02 (0.91) | 0.09 (0.70) | 0.17 (0.52) | 0.37 (0.15) | 0.4 (0.17) | 0.13 (0.44) | 0.06 (0.74) | −0.07 (0.79) | 0.10 (0.80) | 0.11 (0.80) |
| Q3 | 68.64 to <86.05 | −0.18 (0.31) | −0.19 (0.32) | 0.11 (0.60) | 0.05 (0.84) | 0.004 (0.99) | −0.09 (0.66) | −0.05 (0.83) | −0.01 (0.98) | 0.91 (<0.0001) | 0.84 (<0.0001) | 0.23 (0.14) | 0.21 (0.27) | −0.04 (0.84) | 0.41 (0.11) | 0.50 (0.09) |
| Q4 | ≥86.05 | −0.21 (0.15) | −0.23 (0.10) | 0.10 (0.52) | 0.07 (0.79) | −0.03 (0.91) | −0.1 (0.67) | −0.06 (0.82) | −0.20 (0.51) | 0.41 (0.17) | 0.30 (0.25) | 0.40 (0.06) | 0.59 (0.01) | 0.47 (0.055) | 0.30 (0.39) | 0.40 (0.35) |
| P for trend | 0.12 | 0.08 | 0.58 | 0.84 | 0.64 | 0.47 | 0.58 | 0.44 | 0.01 | 0.03 | 0.03 | 0.007 | 0.06 | 0.07 | 0.07 | |
| Aspartate Aminotransferase (U/L) | ||||||||||||||||
| Q1 | <19.46 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 19.46 to <22.85 | −0.32 (0.06) | −0.31 (0.11) | −0.58 (0.01) | −0.12 (0.68) | −0.31 (0.26) | 0.26 (0.24) | 0.13 (0.56) | −0.04 (0.89) | 0.28 (0.39) | 0.21 (0.42) | −0.17 (0.42) | −0.21 (0.43) | −0.33 (0.16) | −0.96 (0.01) | −1.00 (0.01) |
| Q3 | 22.8 to <27.43 | −0.24 (0.11) | −0.26 (0.11) | −0.67 (0.004) | −0.28 (0.12) | −0.33 (0.14) | 0.39 (0.04) | 0.38 (0.03) | 0.03 (0.90) | −0.20 (0.38) | −0.19 (0.51) | 0.45 (0.12) | 0.33 (0.32) | 0.43 (0.26) | −0.15 (0.63) | −0.34 (0.29) |
| Q4 | 27.43 to <40 | −0.33 (0.07) | −0.33 (0.13) | −0.57 (0.02) | −0.50 (0.004) | −0.65 (0.001) | −0.03 (0.78) | −0.01 (0.95) | −0.07 (0.80) | −0.22 (0.42) | −0.06 (0.82) | 0.28 (0.33) | 0.32 (0.30) | 0.27 (0.50) | −0.44 (0.41) | −0.52 (0.38) |
| Abnormal | ≥40 | −0.25 (0.32) | −0.47 (0.09) | −0.53 (0.07) | −0.46 (0.19) | −0.54 (0.14) | −0.67 (0.10) | −0.8 (0.06) | −1.16 (0.01) | −0.14 (0.71) | −0.29 (0.45) | −0.6 (0.07) | −0.49 (0.18) | −0.59 (0.10) | −0.23 (0.62) | −0.42 (0.33) |
| P for trend | 0.12 | 0.09 | 0.02 | 0.008 | 0.005 | 0.32 | 0.31 | 0.42 | 0.29 | 0.55 | 0.44 | 0.41 | 0.57 | 0.60 | 0.41 | |
| Alanine Aminotransferase (U/L) | ||||||||||||||||
| Q1 | <15.34 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 15.34 to <19.73 | −0.14 (0.32) | −0.09 (0.52) | −0.22 (0.30) | −0.17 (0.28) | −0.15 (0.42) | −0.65 (0.04) | −0.47 (0.07) | −0.58 (0.11) | −0.46 (0.31) | −0.26 (0.52) | 0.32 (0.06) | 0.30 (0.12) | 0.35 (0.20) | −0.18 (0.62) | −0.33 (0.39) |
| Q3 | 19.73 to <27.09 | −0.09 (0.49) | −0.08 (0.56) | −0.32 (0.13) | −0.26 (0.19) | −0.19 (0.41) | −0.39 (0.02) | −0.26 (0.20) | −0.54 (0.19) | −1.04 (0.004) | −0.85 (0.02) | 0.24 (0.33) | 0.19 (0.44) | 0.28 (0.47) | 0.46 (0.19) | 0.44 (0.20) |
| Q4 | 27.09 to <40 | −0.35 (0.052) | −0.30 (0.13) | −0.53 (0.03) | −0.33 (0.14) | −0.27 (0.25) | −0.60 (0.04) | −0.41 (0.19) | −0.63 (0.19) | −0.39 (0.33) | 0.01 (0.97) | 0.16 (0.68) | 0.06 (0.89) | 0.58 (0.28) | 0.36 (0.37) | 0.34 (0.42) |
| Abnormal | ≥40 | −0.17 (0.55) | −0.08 (0.78) | −0.07 (0.83) | 0.10 (0.56) | −0.15 (0.42) | −0.73 (0.07) | −0.59 (0.18) | −0.60 (0.15) | −0.32 (0.37) | −0.03 (0.93) | −0.24 (0.57) | −0.07 (0.87) | 0.07 (0.87) | 0.005 (0.99) | 0.05 (0.90) |
| P for trend | 0.33 | 0.48 | 0.30 | 0.55 | 0.54 | 0.25 | 0.37 | 0.28 | 0.67 | 0.50 | 0.69 | 0.75 | 0.56 | 0.42 | 0.32 | |
| Aspartate/Alanine Aminotransferase | ||||||||||||||||
| Q1 | <0.92 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 0.92 to <1.14 | 0.02 (0.88) | −0.06 (0.64) | −0.16 (0.40) | −0.23 (0.32) | −0.32 (0.17) | −0.22 (0.15) | −0.18 (0.24) | −0.24 (0.10) | −0.89 (0.02) | −0.81 (0.03) | −0.09 (0.64) | −0.05 (0.82) | −0.51 (0.03) | −0.17 (0.47) | −0.32 (0.26) |
| Q3 | 1.14 to <1.37 | 0.06 (0.69) | −0.06 (0.68) | 0.12 (0.63) | −0.37 (0.09) | −0.50 (0.03) | 0.12 (0.70) | 0.17 (0.61) | 0.24 (0.36) | −0.50 (0.03) | −0.58 (0.005) | 0.49 (0.06) | 0.53 (0.06) | 0.24 (0.30) | −0.24 (0.36) | −0.45 (0.13) |
| Q4 | ≥1.37 | 0.08 (0.59) | −0.06 (0.70) | −0.11 (0.61) | −0.28 (0.19) | −0.38 (0.11) | 0.22 (0.39) | 0.18 (0.50) | 0.10 (0.73) | −0.28 (0.25) | −0.42 (0.10) | 0.45 (0.08) | 0.47 (0.14) | −0.09 (0.74) | −0.53 (0.18) | −0.85 (0.04) |
| P for trend | 0.56 | 0.71 | 0.33 | 0.20 | 0.07 | 0.36 | 0.41 | 0.21 | 0.045 | 0.0015 | 0.02 | 0.06 | 0.99 | 0.16 | 0.02 | |
| Bilirubin (U/L) | ||||||||||||||||
| Q1 | <0.52 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 0.52 to <0.650 | −0.03 (0.78) | −0.02 (0.87) | −0.24 (0.15) | −0.01 (0.98) | 0.02 (0.95) | −0.05 (0.84) | −0.19 (0.45) | -0.18 (0.51) | 0.20 (0.53) | 0.30 (0.45) | -0.13 (0.61) | -0.08 (0.74) | 0.10 (0.77) | -0.51 (0.049) | -0.37 (0.13) |
| Q3 | 0.650 to <0.82 | −0.11 (0.45) | −0.11 (0.54) | −0.47 (0.02) | −0.39 (0.10) | −0.41 (0.10) | −0.22 (0.38) | −0.22 (0.43) | −0.20 (0.50) | −0.19 (0.54) | −0.23 (0.56) | −0.14 (0.42) | −0.26 (0.11) | −0.31 (0.27) | −1.09 (0.0003) | −0.73 (0.01) |
| Q4 | 0.82 to <1 | −0.02 (0.90) | 0.07 (0.69) | −0.30 (0.14) | −0.17 (0.38) | −0.19 (0.43) | 0.05 (0.86) | −0.04 (0.91) | −0.28 (0.39) | 0.13 (0.78) | 0.14 (0.75) | 0.14 (0.61) | 0.001 (0.998) | −0.10 (0.81) | −0.99 (0.02) | −0.82 (0.03) |
| Abnormal | ≥1 | −0.14 (0.42) | −0.12 (0.53) | −0.18 (0.52) | −0.09 (0.64) | −0.18 (0.47) | −0.40 (0.16) | −0.36 (0.27) | −0.60 (0.15) | −0.22 (0.66) | −0.11 (0.86) | −0.25 (0.48) | −0.61 (0.06) | −0.74 (0.03) | −1.07 (0.02) | −0.85 (0.04) |
| P for trend | 0.56 | 0.82 | 0.37 | 0.41 | 0.29 | 0.32 | 0.50 | 0.24 | 0.63 | 0.72 | 0.68 | 0.09 | 0.22 | 0.002 | 0.02 | |
| Gamma-glutamyl transpeptidase (U/L) | ||||||||||||||||
| Q1 | <12.55 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 12.55 to <17.94 | 0.22 (0.25) | 0.15 (0.41) | 0.14 (0.60) | 0.04 (0.90) | 0.06 (0.83) | 0.23 (0.23) | 0.23 (0.31) | 0.79 (0.01) | 0.17 (0.59) | 0.51 (0.15) | −0.32 (0.18) | −0.34 (0.14) | 0.25 (0.44) | −0.26 (0.51) | −0.23 (0.56) |
| Q3 | 17.94 to <27.71 | 0.08 (0.53) | −0.00004 (0.9997) | −0.17 (0.44) | −0.02 (0.94) | −0.01 (0.94) | 0.31 (0.06) | 0.23 (0.29) | 0.95 (0.01) | 0.30 (0.32) | 0.60 (0.11) | −0.30 (0.02) | −0.38 (0.02) | 0.03 (0.89) | −0.23 (0.59) | −0.22 (0.59) |
| Q4 | Female: 27.71–40; Male: 27.71−50 | 0.48 (0.003) | 0.36 (0.02) | 0.26 (0.27) | 0.47 (0.07) | 0.56 (0.04) | 0.48 (0.04) | 0.43 (0.08) | 0.96 (0.01) | 0.83 (0.01) | 0.93 (0.02) | 0.07 (0.78) | −0.08 (0.74) | 0.35 (0.23) | 0.55 (0.33) | 0.44 (0.41) |
| Abnormal | Female: ≥40; Male: ≥50 | 0.45 (0.03) | 0.30 (0.12) | −0.08 (0.78) | 0.26 (0.43) | 0.35 (0.23) | 0.65 (0.01) | 0.43 (0.07) | 0.81 (0.01) | 0.27 (0.38) | 0.91 (0.03) | 0.43 (0.17) | 0.09 (0.79) | −0.13 (0.78) | 0.46 (0.44) | 0.37 (0.47) |
| P for trend | 0.01 | 0.08 | 0.94 | 0.10 | 0.04 | 0.02 | 0.07 | 0.01 | 0.07 | 0.04 | 0.24 | 0.99 | 0.95 | 0.21 | 0.245 | |
| Never Smoker | Former Smoker | Current Smoker | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log (Theophylline)/Caffeine | Log (Paraxanthine)/Caffeine | Log (Theobromine)/Caffeine | Log (1-methyluric acid)/Caffeine | Log(URXAMU)/Caffeine | Log (Theophylline)/Caffeine | Log (Paraxanthine)/Caffeine | Log (Theobromine)/Caffeine | Log (1-methyluric acid**)/Caffeine | Log(URXAMU)/Caffeine | Log (Theophylline)/Caffeine | Log (Paraxanthine)/Caffeine | Log (Theobromine)/Caffeine | Log (1-methyluric acid)/Caffeine | Log(URXAMU)/Caffeine | ||
| Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | Beta (P) | ||
| Cotinine (ng/mL) | ||||||||||||||||
| Q1 | ≤0.015 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 | 0.015–0.044 | −0.04 (0.66) | 0.06 (0.61) | 0.002 (0.99) | 0.27 (0.17) | 0.31 (0.06) | −0.13 (0.58) | −0.05 (0.84) | −0.11 (0.61) | 0.35 (0.30) | 0.41 (0.13) | 0.99 (0.22) | 0.37 (0.68) | −0.53 (0.48) | 1.39 (0.23) | 2.50 (0.05) |
| Q3 | 0.044−0.988 | 0.16 (0.18) | 0.25 (0.053) | −0.17 (0.31) | 0.61 (0.02) | 0.59 (0.03) | −0.12 (0.55) | 0.004 (0.98) | −0.28 (0.25) | 0.03 (0.93) | 0.06 (0.82) | 0.75 (0.37) | 0.25 (0.77) | 1.26 (0.28) | 2.62 (0.05) | 3.13 (0.01) |
| Q4 | >0.988 | −0.32 (0.17) | −0.22 (0.34) | −0.72 (0.04) | 0.28 (0.39) | 0.42 (0.19) | −0.0002 (1.00) | 0.15 (0.56) | −0.34 (0.32) | −0.26 (0.20) | −0.3 (0.13) | −0.68 (0.27) | −1.04 (0.12) | −0.60 (0.43) | 1.83 (0.03) | 2.26 (0.01) |
| P for trend | 0.86 | 0.54 | 0.03 | 0.05 | 0.03 | 0.92 | 0.54 | 0.28 | 0.22 | 0.16 | 0.04 | 0.03 | 0.02 | 0.51 | 0.47 | |
| Total Smoking Intensity (cigs) | ||||||||||||||||
| Q1 | Former: ≤15,059; Current: ≤26,068 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||||
| Q2 | Former: 15,059−60,878; Current: 26,068−72,238 | −0.03 (0.87) | −0.07 (0.66) | 0.07 (0.78) | 0.48 (0.28) | 0.17 (0.68) | −0.33 (0.25) | −0.42 (0.18) | −0.48 (0.16) | −0.35 (0.24) | −0.37 (0.21) | |||||
| Q3 | Former: 60,878−175,09; Former: 72,238−212,420 | 0.36 (0.06) | 0.35 (0.07) | 0.36 (0.26) | 0.46 (0.22) | 0.46 (0.22) | −0.6 (0.02) | −0.6 (0.03) | −0.77 (0.02) | −0.67 (0.03) | −0.45 (0.12) | |||||
| Q4 | Current: >175,091; Current: >212,420 | 0.35 (0.13) | 0.32 (0.08) | 0.13 (0.66) | 0.17 (0.49) | 0.06 (0.81) | −0.92 (0.01) | −1.01 (0.01) | −0.87 (0.03) | −0.8 (0.04) | −0.68 (0.10) | |||||
| P for trend | 0.09 | 0.048 | 0.52 | 0.49 | 0.59 | 0.006 | 0.004 | 0.03 | 0.04 | 0.13 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garduno, A.; Wu, T. Tobacco Smoke and CYP1A2 Activity in a US Population with Normal Liver Enzyme Levels. Int. J. Environ. Res. Public Health 2021, 18, 2225. https://doi.org/10.3390/ijerph18052225
Garduno A, Wu T. Tobacco Smoke and CYP1A2 Activity in a US Population with Normal Liver Enzyme Levels. International Journal of Environmental Research and Public Health. 2021; 18(5):2225. https://doi.org/10.3390/ijerph18052225
Chicago/Turabian StyleGarduno, Alexis, and Tianying Wu. 2021. "Tobacco Smoke and CYP1A2 Activity in a US Population with Normal Liver Enzyme Levels" International Journal of Environmental Research and Public Health 18, no. 5: 2225. https://doi.org/10.3390/ijerph18052225
APA StyleGarduno, A., & Wu, T. (2021). Tobacco Smoke and CYP1A2 Activity in a US Population with Normal Liver Enzyme Levels. International Journal of Environmental Research and Public Health, 18(5), 2225. https://doi.org/10.3390/ijerph18052225





