Exposure to Atmospheric Particulate Matter-Bound Polycyclic Aromatic Hydrocarbons and Their Health Effects: A Review
Abstract
1. Introduction
2. Concentrations of Atmospheric PM-Bound PAHs
2.1. Personal Exposure to PM-Bound PAHs
2.2. Indoor Concentrations of PM-Bound PAHs
2.3. Outdoor Concentrations of PM-Bound PAHs
3. Health Effects and Assessments of PAHs
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Shah, A.S.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013, 382, 1039–1048. [Google Scholar] [CrossRef]
- US EPA. National Ambient Air Quality Standards for Particulate Matter; United States Environmental Protection Agency: Washington, DC, USA, 2013.
- Esworthy, R. Air Quality: EPA’s 2013 Changes to the Particulate Matter (PM) Standard; Library of Congress, Congressional Research Service: Washington, DC, USA, 2013.
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- WHO. Health Effects of Particulate Matter. Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2013. [Google Scholar]
- Grivas, G.; Cheristanidis, S.; Chaloulakou, A.; Koutrakis, P.; Mihalopoulos, N. Elemental composition and source apportionment of fine and coarse particles at traffic and urban background locations in Athens, Greece. Aerosol Air Qual. Res. 2018, 18, 1642–1659. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, X.; Xing, W.L.; Zhou, Q.Y.; Yang, L.; Nakatsubo, R.; Wei, Y.J.; Bi, J.R.; Shima, M.; Toriba, A.; et al. Natural aeolian dust particles have no substantial effect on atmospheric polycyclic aromatic hydrocarbons (PAHs): A laboratory study based on naphthalene. Environ. Pollut. 2020, 263, 114454. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Zhang, L.L.; Yang, L.; Zhang, X.; Xing, W.L.; Hu, M.; Chen, B.; Han, C.; Toriba, A.; Hayakawa, K.; et al. Long-term variability of inorganic ions in TSP at a remote background site in Japan (Wajima) from 2005 to 2015. Chemosphere 2021, 264, 128427. [Google Scholar] [CrossRef]
- Jiang, N.; Li, Q.; Su, F.; Wang, Q.; Yu, X.; Kang, P.; Zhang, R.; Tang, X. Chemical characteristics and source apportionment of PM2.5 between heavily polluted days and other days in Zhengzhou, China. J. Environ. Sci. 2018, 66, 188–198. [Google Scholar] [CrossRef]
- Zhang, L.L.; Morisaki, H.; Wei, Y.J.; Li, Z.G.; Yang, L.; Zhou, Q.Y.; Zhang, X.; Xing, W.L.; Hu, M.; Shima, M.; et al. Characteristics of air pollutants inside and outside a primary school classroom in Beijing and respiratory health impact on children. Environ. Pollut. 2019, 255, 113147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.L.; Yang, L.; Zhou, Q.Y.; Xing, W.L.; Toriba, A.; Hayakawa, K.; Wei, Y.J.; Tang, N. Characteristics of polycyclic aromatic hydrocarbons (PAHs) and common air pollutants at Wajima, a remote background site in Japan. Int. J. Environ. Res. Public Health 2020, 17, 957. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.L.; Yang, L.; Zhou, Q.Y.; Zhang, X.; Xing, W.L.; Kazuichi, H.; Toriba, A.; Tang, N. Impact of COVID-19 outbreak on the long-range transport of common air pollutants in KUWAMS. Chem. Pharm. Bull. 2020, in press. [Google Scholar]
- Lee, H.; Honda, Y.; Hashizume, M.; Guo, Y.L.; Wu, C.-F.; Kan, H.; Jung, K.; Lim, Y.-H.; Yi, S.; Kim, H. Short-term exposure to fine and coarse particles and mortality: A multicity time-series study in East Asia. Environ. Pollut. 2015, 207, 43–51. [Google Scholar] [CrossRef]
- Karageorgou, K.; Manoli, E.; Kouras, A.; Samara, C. Commuter exposure to particle-bound polycyclic aromatic hydrocarbons in Thessaloniki, Greece. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Wang, X.; Banks, A.P.; He, C.; Drage, D.S.; Gallen, C.L.; Li, Y.; Li, Q.; Thai, P.K.; Mueller, J.F. Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and legacy and current pesticides in indoor environment in Australia–occurrence, sources and exposure risks. Sci. Total Environ. 2019, 693, 133588. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W.; Kozielska, B.; Majewski, G.; Rogula-Kopiec, P.; Mucha, W.; Kociszewska, K. Submicron particle-bound polycyclic aromatic hydrocarbons in the Polish teaching rooms: Concentrations, origin and health hazard. J. Environ. Sci. 2018, 64, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, X.; Xing, W.L.; Zhou, Q.Y.; Zhang, L.L.; Wu, Q.; Zhou, Z.J.; Chen, R.J.; Toriba, A.; Hayakawa, K.; et al. Yearly variation in characteristics and health risk of polycyclic aromatic hydrocarbons and nitro-PAHs in urban shanghai from 2010 to 2018. J. Environ. Sci. 2021, 99, 72–79. [Google Scholar] [CrossRef]
- Ai, S.; Qian, Z.M.; Guo, Y.; Yang, Y.; Rolling, C.A.; Liu, E.; Wu, F.; Lin, H. Long-term exposure to ambient fine particles associated with asthma: A cross-sectional study among older adults in six low-and middle-income countries. Environ. Res. 2019, 168, 141–145. [Google Scholar] [CrossRef]
- Beelen, R.; Hoek, G.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.H. Natural-cause mortality and long-term exposure to particle components: An analysis of 19 European cohorts within the multi-center escape project. Environ. Health Perspect. 2015, 123, 525–533. [Google Scholar] [CrossRef]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Akhavan, A. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 2012, 33, 8017–8025. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]
- Hashemi, E.; Akhavan, O.; Shamsara, M.; Rahighi, R.; Esfandiar, A.; Tayefeh, A.R. Cyto and genotoxicities of graphene oxide and reduced graphene oxide sheets on spermatozoa. RSC Adv. 2014, 4, 27213–27223. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Hashemi, E.; Akbari, E. Dose-dependent effects of nanoscale graphene oxide on reproduction capability of mammals. Carbon 2015, 95, 309–317. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, F.; Yu, H.; Wu, S.; Xiang, H. Association between exposure to ambient air pollution and hospital admission, incidence, and mortality of stroke: An updated systematic review and meta-analysis of more than 23 million participants. Environ Health Prev. Med. 2021, 26, 1–14. [Google Scholar] [CrossRef]
- IARC. Outdoor Air Pollution; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2015; Volume 109. [Google Scholar]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- WHO. Who Guidelines for Indoor Air Quality: Household Fuel Combustion; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- WHO. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide; World Health Organization: Copenhagen, Denmark, 2006. [Google Scholar]
- INECC. Mexico-Ambient Air Quality Standard; Mexico’s Ecology and Climate Change Institute, Instituto Nacional de Ecologia y Cambio Climatico (INECC): Mexico City, Mexico, 2014.
- CAAQS. Canadian Ambient Air Quality Standards (CAAQS) for Fine Particulate Matter (PM2.5) and Ozone. 2020. Available online: https://www.ccme.ca/files/current_priorities/aqms_elements/caaqs_and_azmf.pdf (accessed on 2 July 2020).
- Siciliano, B.; Dantas, G.; Silva, C.M.D.; Arbilla, G. The updated Brazilian national air quality standards: A critical review. J. Braz. Chem. Soc. 2020, 31, 523–535. [Google Scholar] [CrossRef]
- Díaz-Robles, L.; Saavedra, H.; Schiappacasse, L.; Cereceda-Balic, F. The Air Quality in Chile; Air, Waste Management Association: Pittsburgh, PA, USA, 2011. [Google Scholar]
- NEPM Australia. Australia-National Air Quality Standards; National Environment Protection Measure for Ambient Air Quality: Canberra, Australia, 2016.
- Sato, K.; Ohara, T. The status of PM2.5 pollution in Asia and direction toward solving the issue. Glob. Environ. Res. 2018, 22, 39–45. [Google Scholar]
- EU. Directive 2008/50/EC of the European Parliament and of the council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off. J. Eur. Union 2008. Available online: http://news.cleartheair.org.hk/wp-content/uploads/2013/02/LexUriServ.pdf (accessed on 12 February 2021).
- Lisetskii, F.; Borovlev, A. Monitoring of emission of particulate matter and air pollution using lidar in Belgorod, Russia. Aerosol Air Qual. Res. 2019. [Google Scholar] [CrossRef]
- SANS. South African National Standard (SANS). 2005. Available online: https://www.environment.gov.za/sites/default/files/docs/stateofair_executive_iaiquality_standardsonjectives.pdf (accessed on 2 July 2020).
- Zhang, Y.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Baek, S.; Field, R.; Goldstone, M.; Kirk, P.; Lester, J.; Perry, R. A review of atmospheric polycyclic aromatic hydrocarbons: Sources, fate and behavior. Water. Air Soil. Pollut. 1991, 60, 279–300. [Google Scholar] [CrossRef]
- Ambrose, D.; Ellender, J.; Sprake, C.; Townsend, R. Thermodynamic properties of fluorine compounds. Part 15—Vapour pressures of the three tetrafluorobenzenes and 1,3,5-trichloro-2,4,6-trifluorobenzene. Faraday Transactions 1: Physical Chemistry in Condensed Phases. J. Chem. Soc. 1975, 71, 35–41. [Google Scholar]
- Coover, M.P.; Sims, R.C. The effect of temperature on polycyclic aromatic hydrocarbon persistence in an unacclimated agricultural soil. Hazard. Waste Hazard. Mater. 1987, 4, 69–82. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Suuberg, E.M. Vapor pressures and enthalpies of sublimation of ten polycyclic aromatic hydrocarbons determined via the Knudsen effusion method. J. Chem. Eng. Data 2008, 53, 670–676. [Google Scholar] [CrossRef]
- Lee, W.M.G.; Tong, H.C.; Yeh, S.Y. Partitioning model of PAHs between gaseous and particulate phases with consideration of reactivity of PAHs in an urban atmosphere. J. Environ. Sci. Health A 1993, 28, 563–583. [Google Scholar] [CrossRef]
- Lei, Y.D.; Chankalal, R.; Chan, A.; Wania, F. Supercooled liquid vapor pressures of the polycyclic aromatic hydrocarbons. J. Chem. Eng. Data 2002, 47, 801–806. [Google Scholar] [CrossRef]
- PubChem. National Library of Medicine, National Center of Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 3 July 2020).
- Yamasaki, H.; Kuwata, K.; Miyamoto, H. Effects of ambient temperature on aspects of airborne polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 1982, 16, 189–194. [Google Scholar] [CrossRef]
- Hoyer, H.; Peperle, W. Vapor pressure measurements on organic compounds and their sublimation heats. Z. Electrochem. 1958, 62, 61–66. [Google Scholar]
- Sonnefeld, W.; Zoller, W.; May, W. Dynamic coupled-column liquid-chromatographic determination of ambient-temperature vapor pressures of polynuclear aromatic hydrocarbons. Anal. Chem. 1983, 55, 275–280. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Zhang, L.; Yang, F.; Peng, C.; Chen, Y.; Shi, G.; Yao, X.; Jiang, C.; Wang, J. Sources and gas-particle partitioning of atmospheric parent, oxygenated, and nitrated polycyclic aromatic hydrocarbons in a humid city in southwest China. Atmos. Environ. 2019, 206, 1–10. [Google Scholar] [CrossRef]
- Ray, D.; Ghosh, S.K.; Raha, S. Impacts of photochemical ageing on the half-lives and diagnostic ratio of polycyclic aromatic hydrocarbons intrinsic to PM2.5 collected from ‘real-world’ like combustion events of wood and rice straw burning. J. Hazard. Mater. 2019, 366, 10–15. [Google Scholar] [CrossRef]
- Yang, L.; Tang, N.; Matsuki, A.; Takami, A.; Hatakeyama, S.; Kaneyasu, N.; Nagato, E.G.; Sato, K.; Yoshino, A.; Hayakawa, K. A comparison of particulate-bound polycyclic aromatic hydrocarbons long-range transported from the Asian continent to the Noto peninsula and Fukue island, Japan. Asian J. Atmos. Environ. 2018, 12, 369–376. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.L.; Zhang, H.; Zhou, Q.Y.; Zhang, X.; Xing, W.L.; Takami, A.; Sato, K.; Shimizu, A.; Yoshino, A.; et al. Comparative analysis of PM2.5-bound polycyclic aromatic hydrocarbons (PAHs), nitro-NPAHs (NPAHs) and water-soluble inorganic ions (WSIIs) at two background sites in japan. Int. J. Environ. Res. Public Health 2020, 17, 8224. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Kawamura, K.; Yanase, A.; Barrie, L.A. Distributions of polycyclic aromatic hydrocarbons, aromatic ketones, carboxylic acids, and trace metals in arctic aerosols: Long-range atmospheric transport, photochemical degradation/production at polar sunrise. Environ. Sci. Technol. 2017, 51, 8992–9004. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Hakamata, M.; Sato, K.; Okada, Y.; Yang, X.; Tatematsu, M.; Toriba, A.; Kameda, T.; Hayakawa, K. Atmospheric behaviors of polycyclic aromatic hydrocarbons at a Japanese remote background site, Noto Peninsula, from 2004 to 2014. Atmos. Environ. 2015, 120, 144–151. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yang, L.; Zhang, H.; Zhou, Q.Y.; Zhang, X.; Xing, W.L.; Toriba, A.; Hayakawa, K.; Tang, N. Impact of the COVID-19 outbreak on the long-range transport of particulate PAHs in East Asia. Aerosol Air Qual. Res. 2020, 20, 2035–2046. [Google Scholar] [CrossRef]
- WHO. Part II Evaluation of Risks to Human Health, Chapter 5 Organic Pollutants. In Air Quality Guidelines for Europe; WHO Regional Publications, European Series, No. 91; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: A review and meta-analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, F.; Feng, S.; Cai, Z.; Shen, Y.; Ying, C.; Wang, X.; Liu, Q. Long-range transport of ozone across the eastern china seas: A case study in coastal cities in southeastern China. Sci. Total Environ. 2021, 768, 144520. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment—A review. Sci. Total Environ. 2017, 581–582, 237–257. [Google Scholar] [CrossRef]
- Taga, R.; Tang, N.; Hattori, T.; Tamura, K.; Sakai, S.; Toriba, A.; Kizu, R.; Hayakawa, K. Direct-acting mutagenicity of extracts of coal burning-derived particulates and contribution of nitropolycyclic aromatic hydrocarbons. Mutat. Res. 2005, 581, 91–95. [Google Scholar] [CrossRef]
- IARC. Bitumens and Bitumen Emissions, and Some N- and S-Heterocyclic Polycyclic Aromatic Hydrocarbons; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2013; Volume 103. [Google Scholar]
- IARC. Diesel and Gasoline Engine Exhausts and Some Nitroarenes; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2013; Volume 105. [Google Scholar]
- Li, Y.J.; Sun, Y.; Zhang, Q.; Li, X.; Li, M.; Zhou, Z.; Chan, C.K. Real-time chemical characterization of atmospheric particulate matter in China: A review. Atmos. Environ. 2017, 158, 270–304. [Google Scholar] [CrossRef]
- Amador-Muñoz, O.; Martínez-Domínguez, Y.; Gómez-Arroyo, S.; Peralta, O. Current situation of polycyclic aromatic hydrocarbons (PAH) in PM2.5 in a receptor site in Mexico City and estimation of carcinogenic PAH by combining non-real-time and real-time measurement techniques. Sci. Total Environ. 2020, 703, 134526. [Google Scholar] [CrossRef] [PubMed]
- Cropper, P.M.; Overson, D.K.; Cary, R.A.; Eatough, D.J.; Chow, J.C.; Hansen, J.C. Development of the GC-MS organic aerosol monitor (GC-MS OAM) for in-field detection of particulate organic compounds. Atmos. Environ. 2017, 169, 258–266. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yang, L.; Zhou, Q.Y.; Zhang, X.; Xing, W.L.; Wei, Y.J.; Hu, M.; Zhao, L.X.; Toriba, A.; Hayakawa, K.; et al. Size distribution of particulate polycyclic aromatic hydrocarbons in fresh combustion smoke and ambient air: A review. J. Environ. Sci. 2020, 88, 370–384. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Zhang, J.; Liu, W.; Xu, Y.; Cai, C.; Liu, Y.; Tao, S.; Liu, W. Distribution characteristics of and personal exposure with polycyclic aromatic hydrocarbons and particulate matter in indoor and outdoor air of rural households in northern China. Environ. Pollut. 2019, 255, 113176. [Google Scholar] [CrossRef]
- Orakij, W.; Chetiyanukornkul, T.; Chuesaard, T.; Kaganoi, Y.; Uozaki, W.; Homma, C.; Boongla, Y.; Tang, N.; Hayakawa, K.; Toriba, A. Personal inhalation exposure to polycyclic aromatic hydrocarbons and their nitro-derivatives in rural residents in northern Thailand. Environ. Monit. Assess. 2017, 189, 510. [Google Scholar] [CrossRef]
- Chen, X.-C.; Chuang, H.-C.; Ward, T.J.; Tian, L.; Cao, J.-J.; Ho, S.S.-H.; Lau, N.-C.; Hsiao, T.-C.; Yim, S.H.; Ho, K.-F. Indoor, outdoor, and personal exposure to PM2.5 and their bioreactivity among healthy residents of Hong Kong. Environ. Res. 2020, 188, 109780. [Google Scholar] [CrossRef]
- Mu, G.; Fan, L.; Zhou, Y.; Liu, Y.; Ma, J.; Yang, S.; Wang, B.; Xiao, L.; Ye, Z.; Shi, T. Personal exposure to PM2.5-bound polycyclic aromatic hydrocarbons and lung function alteration: Results of a panel study in china. Sci. Total Environ. 2019, 684, 458–465. [Google Scholar] [CrossRef]
- Gatto, M.P.; Gariazzo, C.; Gordiani, A.; L’Episcopo, N.; Gherardi, M. Children and elders exposure assessment to particle-bound polycyclic aromatic hydrocarbons (PAHs) in the city of Rome, Italy. Environ. Sci. Pollut. Res. 2014, 21, 13152–13159. [Google Scholar] [CrossRef]
- Han, J.; Zhang, N.; Niu, C.; Han, B.; Bai, Z. Personal exposure of children to particle-associated polycyclic aromatic hydrocarbons in Tianjin, China. Polycycl. Aromat. Compd. 2014, 34, 320–342. [Google Scholar] [CrossRef]
- Che, C.; Li, J.; Dong, F.; Zhang, C.; Liu, L.; Sun, X.; Ma, L.; Qi, H.; Wang, K. Seasonal characteristic composition of inorganic elements and polycyclic aromatic hydrocarbons in atmospheric fine particulate matter and bronchoalveolar lavage fluid of COPD patients in northeast China. Respir. Med. 2020, 171, 106082. [Google Scholar] [CrossRef]
- Svecova, V.; Topinka, J.; Solansky, I.; Rossner, P.; Sram, R.J. Personal exposure to carcinogenic polycyclic aromatic hydrocarbons in the Czech Republic. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Wang, J.; Ho, S.S.H.; He, K.; Shen, Z.; Ning, Z.; Sun, J.; Li, L.; Lei, R. Personal exposure to PM2.5-bound organic species from domestic solid fuel combustion in rural Guanzhong basin, China: Characteristics and health implication. Chemosphere 2019, 227, 53–62. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xu, H.; Feng, R.; Shen, Z.; Li, Y.; Zhang, Y.; Sun, J.; Zhang, Q.; Zhang, T.; Yang, L. Characteristics of indoor and personal exposure to particulate organic compounds emitted from domestic solid fuel combustion in rural areas of northwest China. Atmos. Res. 2021, 248, 105181. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Shou, Y.-P.; Mao, T.-Y.; Guo, L.-Q.; Li, P.-H.; Yi, X.; Li, Q.-Q.; Shen, L.-Z.; Zuo, H.-R.; Wang, J. PAHs exposure assessment for highway toll station workers through personal particulate sampling and urinary biomonitoring in Tianjin, China. Polycycl. Aromat. Compd. 2018, 38, 379–388. [Google Scholar] [CrossRef]
- Rezaei, F.; Kakooei, H.; Ahmadkhaniha, R.; Azam, K.; Omidi, L.; Shahtaheri, S.J. Inhalation exposure and health risks for newsagents exposed to atmospheric polycyclic aromatic hydrocarbons in Tehran, Iran. Urban Clim. 2018, 24, 796–802. [Google Scholar] [CrossRef]
- Strandberg, B.; Österman, C.; Koca Akdeva, H.; Moldanová, J.; Langer, S. The use of polyurethane foam (PUF) passive air samplers in exposure studies to PAHs in Swedish seafarers. Polycycl. Aromat. Compd. 2020, 1–12. [Google Scholar] [CrossRef]
- Wu, M.-T.; Lin, P.-C.; Pan, C.-H.; Peng, C.-Y. Risk assessment of personal exposure to polycyclic aromatic hydrocarbons and aldehydes in three commercial cooking workplaces. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Saleh, H.N.; Barjasteh-Askari, F.; Nasseri, S.; Davoudi, M. The effect of gas versus charcoal open flames on the induction of polycyclic aromatic hydrocarbons in cooked meat: A systematic review and meta-analysis. J. Environ. Health Eng. 2020, 18, 345–354. [Google Scholar] [CrossRef]
- Afé, O.H.I.; Douny, C.; Kpoclou, Y.E.; Igout, A.; Mahillon, J.; Anihouvi, V.; Hounhouigan, D.J.; Scippo, M.-L. Insight about methods used for polycyclic aromatic hydrocarbons reduction in smoked or grilled fishery and meat products for future re-engineering: A systematic review. Food Chem. Toxicol. 2020, 141, 111372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Morisaki, H.; Wei, Y.J.; Li, Z.G.; Yang, L.; Zhou, Q.Y.; Zhang, X.; Xing, W.L.; Hu, M.; Shima, M.; et al. PM2.5-bound polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons inside and outside a primary school classroom in Beijing: Concentration, composition, and inhalation cancer risk. Sci. Total Environ. 2020, 705, 135840. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhu, T.; Han, Y.; Lv, D. Pm2. 5-bound pahs in three indoor and one outdoor air in Beijing: Concentration, source and health risk assessment. Sci. Total Environ. 2017, 586, 255–264. [Google Scholar] [CrossRef]
- Ali, N. Polycyclic aromatic hydrocarbons (PAHs) in indoor air and dust samples of different saudi microenvironments; health and carcinogenic risk assessment for the general population. Sci. Total Environ. 2019, 696, 133995. [Google Scholar] [CrossRef]
- Esen, F.; Kayikci, G. Polycyclic aromatic hydrocarbons in indoor and outdoor air in turkey: Estimations of sources and exposure. Environ. Forensics 2018, 19, 39–49. [Google Scholar] [CrossRef]
- Madruga, D.G.; Ubeda, R.M.; Terroba, J.M.; dos Santos, S.G.; García-Cambero, J.P. Particle-associated polycyclic aromatic hydrocarbons in a representative urban location (indoor-outdoor) from south Europe: Assessment of potential sources and cancer risk to humans. Indoor Air 2019, 29, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Liu, L.-Y.; Zhang, Z.-F.; Ma, W.-L.; Sverko, E.; Zhang, Z.; Song, W.-W.; Sun, Y.; Li, Y.-F. Semi-volatile organic compounds in infant homes: Levels, influence factors, partitioning, and implications for human exposure. Environ. Pollut. 2019, 251, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Yin, W.; Xu, T.; Hu, C.; Cheng, J.; Hou, J.; He, Z.; Yuan, J. Seasonal exposure to PM2.5-bound polycyclic aromatic hydrocarbons and estimated lifetime risk of cancer: A pilot study. Sci. Total Environ. 2020, 702, 135056. [Google Scholar] [CrossRef] [PubMed]
- Yury, B.; Zhang, Z.; Ding, Y.; Zheng, Z.; Wu, B.; Gao, P.; Jia, J.; Lin, N.; Feng, Y. Distribution, inhalation and health risk of PM2.5 related pahs in indoor environments. Ecotoxicol. Environ. Saf. 2018, 164, 409–415. [Google Scholar] [CrossRef]
- Ouyang, R.; Yang, S.; Xu, L. Analysis and risk assessment of PM2.5-bound pahs in a comparison of indoor and outdoor environments in a middle school: A case study in Beijing, China. Atmosphere 2020, 11, 904. [Google Scholar] [CrossRef]
- Pereira, D.C.A.; Custódio, D.; de Andrade, M.d.F.; Alves, C.; de Castro Vasconcellos, P. Air quality of an urban school in são Paulo city. Environ. Monit. Assess. 2019, 191, 659. [Google Scholar] [CrossRef]
- Slezakova, K.; Oliveira, M.; Madureira, J.; Fernandes, E.d.O.; Delerue-Matos, C.; Morais, S.; Pereira, M.d.C. Polycyclic aromatic hydrocarbons (PAH) in Portuguese educational settings: A comparison between preschools and elementary schools. J. Toxicol. Environ. A 2017, 80, 630–640. [Google Scholar] [CrossRef]
- Hamid, N.; Syed, J.H.; Junaid, M.; Mahmood, A.; Li, J.; Zhang, G.; Malik, R.N. Elucidating the urban levels, sources and health risks of polycyclic aromatic hydrocarbons (PAHs) in Pakistan: Implications for changing energy demand. Sci. Total Environ. 2018, 619, 165–175. [Google Scholar] [CrossRef]
- Ielpo, P.; Taurino, M.R.; Buccolieri, R.; Placentino, C.M.; Gallone, F.; Ancona, V.; Di Sabatino, S. Polycyclic aromatic hydrocarbons in a bakery indoor air: Trends, dynamics, and dispersion. Environ. Sci. Pollut. Res. 2018, 25, 28760–28771. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Chen, X.; Gao, Y.; Jiang, P.; Zhang, J.; Yu, H.; Wang, W. PM2. 5-bound pahs in indoor and outdoor of hotels in urban and suburban of Jinan, China: Concentrations, sources, and health risk impacts. Aerosol Air Qual. Res. 2017, 17, 2463–2473. [Google Scholar] [CrossRef]
- Adesina, O.A.; Nwogu, A.S.; Sonibare, J.A. Indoor levels of polycyclic aromatic hydrocarbons (PAHs) from environment tobacco smoke of public bars. Ecotoxicol. Environ. Saf. 2021, 208, 111604. [Google Scholar] [CrossRef]
- Bai, L.; Chen, W.; He, Z.; Sun, S.; Qin, J. Pollution characteristics, sources and health risk assessment of polycyclic aromatic hydrocarbons in pm2. 5 in an office building in northern areas, China. Sustain. Cities Soc. 2020, 53, 101891. [Google Scholar] [CrossRef]
- Arı, A. A comprehensive study on gas and particle emissions from laser printers: Chemical composition and health risk assessment. Atmos. Pollut. Res. 2020, 11, 269–282. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Bralewska, K.; Rogula-Kopiec, P.; Makowski, R.; Majder-Łopatka, M.; Łukawski, A.; Brandyk, A.; Majewski, G. Respirable particles and polycyclic aromatic hydrocarbons at two polish fire stations. Build. Environ. 2020, 184, 107255. [Google Scholar] [CrossRef]
- Yang, L.; Suzuki, G.; Zhang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Shima, M.; Yoda, Y.; Nakatsubo, R.; Hiraki, T.; et al. The characteristics of polycyclic aromatic hydrocarbons in different emission source areas in Shenyang, China. Int. J. Environ. Res. Public Health 2019, 16, 2817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Tokuda, T.; Yang, L.; Zhou, Q.Y.; Zhang, X.; Xing, W.L.; Wu, Q.; Zhou, Z.J.; Chen, R.J.; Kameda, T.; et al. Characteristics and health risks of particulate polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons at urban and suburban elementary schools in Shanghai, China. Asian J. Atmos. Environ. 2019, 13, 266–275. [Google Scholar] [CrossRef]
- Amarillo, A.C.; Carreras, H. Quantifying the influence of meteorological variables on particle-bound PAHs in urban environments. Atmos. Pollut. Res. 2016, 7, 597–602. [Google Scholar] [CrossRef]
- Kalisa, E.; Nagato, E.; Bizuru, E.; Lee, K.; Tang, N.; Pointing, S.; Hayakawa, K.; Archer, S.; Lacap-Bugler, D. Pollution characteristics and risk assessment of ambient PM2.5-bound PAHs and NPAHs in typical Japanese and New Zealand cities and rural sites. Atmos. Pollut. Res. 2019, 10, 1396–1403. [Google Scholar] [CrossRef]
- Valdivia, A.E.L.; Larico, J.A.R.; Peña, J.S.; Wannaz, E.D. Health risk assessment of polycyclic aromatic hydrocarbons (PAHs) adsorbed in PM2.5 and PM10 in a region of Arequipa, Peru. Environ. Sci. Pollut. Res. 2020, 27, 3065–3075. [Google Scholar] [CrossRef]
- Amarillo, A.C.; Mateos, A.C.; Carreras, H. Source apportionment of PM10-bound polycyclic aromatic hydrocarbons by positive matrix factorization in Cordoba city, Argentina. Arch. Environ. Contam. Toxicol. 2017, 72, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.R.; de Lourdes Cardeal, Z.; Menezes, H.C. Phase distribution of polycyclic aromatic hydrocarbons and their oxygenated and nitrated derivatives in the ambient air of a Brazilian urban area. Chemosphere 2020, 250, 126223. [Google Scholar] [CrossRef]
- Jariyasopit, N.; Tung, P.; Su, K.; Halappanavar, S.; Evans, G.J.; Su, Y.; Khoomrung, S.; Harner, T. Polycyclic aromatic compounds in urban air and associated inhalation cancer risks: A case study targeting distinct source sectors. Environ. Pollut. 2019, 252, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.L.; Campbell, L.; Donatuto, J.; Heidt, M.; Kile, M.; Simonich, S.L.M. Impact of local and regional sources of PAHs on tribal reservation air quality in the US pacific northwest. Sci. Total Environ. 2020, 710, 136412. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Piñero, J.; Moreda-Piñeiro, J.; Concha-Graña, E.; Fernández-Amado, M.; Muniategui-Lorenzo, S.; López-Mahía, P. Inhalation bioaccessibility estimation of polycyclic aromatic hydrocarbons from atmospheric particulate matter (PM10): Influence of PM10 composition and health risk assessment. Chemosphere 2020, 263, 127847. [Google Scholar] [CrossRef]
- Pastor, R.P.; Salvador, P.; Alonso, S.G.; Alastuey, A.; dos Santos, S.G.; Querol, X.; Artíñano, B. Characterization of organic aerosol at a rural site influenced by olive waste biomass burning. Chemosphere 2020, 248, 125896. [Google Scholar] [CrossRef]
- Hakimzadeh, M.; Soleimanian, E.; Mousavi, A.; Borgini, A.; De Marco, C.; Ruprecht, A.A.; Sioutas, C. The impact of biomass burning on the oxidative potential of PM2.5 in the metropolitan area of Milan. Atmos. Environ. 2020, 224, 117328. [Google Scholar] [CrossRef]
- Skiba, A.; Styszko, K.; Furman, P.; Dobrowolska, N.; Kistler, M.; Kasper-Giebl, A.; Zięba, D. Polycyclic aromatic hydrocarbons (PAHs) associated with PM10 collected in Wadowice, south Poland. E3S Web Conf. 2019, 108, 02007. [Google Scholar] [CrossRef]
- Iakovides, M.; Iakovides, G.; Stephanou, E.G. Atmospheric particle-bound polycyclic aromatic hydrocarbons, n-alkanes, hopanes, steranes and trace metals: PM2.5 source identification, individual and cumulative multi-pathway lifetime cancer risk assessment in the urban environment. Sci. Total Environ. 2021, 752, 141834. [Google Scholar] [CrossRef]
- Křůmal, K.; Mikuška, P. Mass concentrations and lung cancer risk assessment of PAHs bound to pm1 aerosol in six industrial, urban and rural areas in the Czech Republic, central Europe. Atmos. Pollut. Res. 2020, 11, 401–408. [Google Scholar] [CrossRef]
- Pehnec, G.; Jakovljević, I.; Godec, R.; Štrukil, Z.S.; Žero, S.; Huremović, J.; Džepina, K. Carcinogenic organic content of particulate matter at urban locations with different pollution sources. Sci. Total Environ. 2020, 734, 139414. [Google Scholar] [CrossRef]
- Khalikov, I.; Korunov, A. The content of high-molecular polycyclic aromatic hydrocarbons in urban air during FIFA World Cup 2018. Russ. J. Gen. Chem. 2019, 89, 2821–2826. [Google Scholar] [CrossRef]
- Rabhi, L.; Lemou, A.; Cecinato, A.; Balducci, C.; Cherifi, N.; Ladji, R.; Yassaa, N. Polycyclic aromatic hydrocarbons, phthalates, parabens and other environmental contaminants in dust and suspended particulates of Algiers, Algeria. Environ. Sci. Pollut. Res. 2018, 25, 24253–24265. [Google Scholar] [CrossRef]
- Morakinyo, O.M.; Mukhola, M.S.; Mokgobu, M.I. Concentration levels and carcinogenic and mutagenic risks of PM2.5-bound polycyclic aromatic hydrocarbons in an urban–industrial area in South Africa. Environ. Geochem. Health 2019. [Google Scholar] [CrossRef]
- Kalisa, E.; Nagato, E.G.; Bizuru, E.; Lee, K.C.; Tang, N.; Pointing, S.B.; Hayakawa, K.; Archer, S.D.; Lacap-Bugler, D.C. Characterization and risk assessment of atmospheric PM2.5 and PM10 particulate-bound PAHs and NPAHs in Rwanda, Central-East Africa. Environ. Sci. Technol. 2018, 52, 12179–12187. [Google Scholar] [CrossRef] [PubMed]
- Ofori, S.A.; Cobbina, S.J.; Doke, D.A. The occurrence and levels of polycyclic aromatic hydrocarbons (PAHs) in African environments—A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 32389–32431. [Google Scholar] [CrossRef]
- Yan, D.; Wu, S.; Zhou, S.; Tong, G.; Li, F.; Wang, Y.; Li, B. Characteristics, sources and health risk assessment of airborne particulate PAHs in Chinese cities: A review. Environ. Pollut. 2019, 248, 804–814. [Google Scholar] [CrossRef]
- Wang, L.; Dong, S.; Liu, M.; Tao, W.; Xiao, B.; Zhang, S.; Zhang, P.; Li, X. Polycyclic aromatic hydrocarbons in atmospheric PM2.5 and PM10 in the semi-arid city of Xi’an, northwest china: Seasonal variations, sources, health risks, and relationships with meteorological factors. Atmos. Res. 2019, 229, 60–73. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, M.; Shi, S.; Zhao, Y.; Chen, X.; Li, C.; Li, Y.; Wang, H.; Bao, L.; Cui, X. Size-distribution-based assessment of human inhalation and dermal exposure to airborne parent, oxygenated and chlorinated PAHs during a regional heavy haze episode. Environ. Pollut. 2020, 263, 114661. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Li, R.; Chen, W.; Chung, C.K.A.; Cai, Z. The cellular effects of PM2.5 collected in Chinese Taiyuan and Guangzhou and their associations with polycyclic aromatic hydrocarbons (PAHs), nitro-PAHs and hydroxy-PAHs. Ecotoxicol. Environ. Saf. 2020, 191, 110225. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zhang, X.; Li, J.; Zhao, T.; Gao, Y.; Jiang, P.; Li, Y.; Chen, X.; Wang, W. Characteristics of PM2.5-bound pahs at an urban site and a suburban site in Jinan in north china plain. Aerosol Air Qual. Res. 2019, 19, 871–884. [Google Scholar] [CrossRef]
- Song, W.; Cao, F.; Lin, Y.-C.; Haque, M.M.; Wu, X.; Zhang, Y.; Zhang, C.; Xie, F.; Zhang, Y.-L. Extremely high abundance of polycyclic aromatic hydrocarbons in aerosols from a typical coal-combustion rural site in china: Size distribution, source identification and cancer risk assessment. Atmos. Res. 2019, 248, 105192. [Google Scholar] [CrossRef]
- Wang, W.; Ding, X.; Turap, Y.; Tursun, Y.; Abulizi, A.; Wang, X.; Shao, L.; Talifu, D.; An, J.; Zhang, X. Distribution, sources, risks, and vitro DNA oxidative damage of PM2.5-bound atmospheric polycyclic aromatic hydrocarbons in Urumqi, NW China. Sci. Total Environ. 2020, 139518. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, W.; Cheng, H. Seasonal variations and sources of airborne polycyclic aromatic hydrocarbons (PAHs) in Chengdu, China. Atmosphere 2018, 9, 63. [Google Scholar] [CrossRef]
- Wu, X.; Cao, F.; Haque, M.; Fan, M.-Y.; Zhang, S.-C.; Zhang, Y.-L. Molecular composition and source apportionment of fine organic aerosols in northeast china. Atmos. Environ. 2020, 239, 117722. [Google Scholar] [CrossRef]
- Ma, L.; Li, B.; Liu, Y.; Sun, X.; Fu, D.; Sun, S.; Thapa, S.; Geng, J.; Qi, H.; Zhang, A. Characterization, sources and risk assessment of pm2. 5-bound polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (NPAHs) in Harbin, a cold city in northern China. J. Clean. Prod. 2020, 264, 121673. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yang, L.; Bi, J.R.; Liu, Y.Z.; Toriba, A.; Hayakawa, K.; Nagao, S.; Tang, N. Characteristics and unique sources of polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons inPM2.5 at a highland background site in northwestern China. Environ. Pollut. 2021, 116527. [Google Scholar] [CrossRef]
- Xing, W.L.; Zhang, L.L.; Yang, L.; Zhou, Q.Y.; Zhang, X.; Toriba, A.; Hayakawa, K.; Tang, N. Characteristics of PM2.5-bound polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons at a roadside air pollution monitoring station in Kanazawa, Japan. Int. J. Environ. Res. Public Health 2020, 17, 805. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Watanabe, T.; Horimoto, Y.; Ishii, K.; Naito, S. Measurements of 50 non-polar organic compounds including polycyclic aromatic hydrocarbons, n-alkanes and phthalate esters in fine particulate matter (PM2.5) in an industrial area of Chiba prefecture, Japan. Asian J. Atmos. Environ. 2018, 12, 274–288. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Q.Y.; Zhang, H.; Zhang, X.; Xing, W.L.; Wang, Y.; Bai, P.; Yamauchi, M.; Chohji, T.; Zhang, L.L.; et al. Atmospheric behaviour of polycyclic and nitro-polycyclic aromatic hydrocarbons and water-soluble inorganic ions in winter in kirishima, a typical japanese commercial city. Int. J. Environ. Res. Public Health 2021, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, K.; Choi, N.; Kim, Y.P.; Lee, J.Y. Recent occurrence of pahs and n-alkanes in PM2.5 in Seoul, Korea and characteristics of their sources and toxicity. Int. J. Environ. Res. Public Health 2020, 17, 1397. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, K.; Lee, S.; Kim, S.D. Characteristics and health effects of PM2.5 emissions from various sources in Gwangju, South Korea. Sci. Total Environ. 2019, 696, 133890. [Google Scholar] [CrossRef]
- Pham, C.-T.; Boongla, Y.; Nghiem, T.-D.; Le, H.-T.; Tang, N.; Toriba, A.; Hayakawa, K. Emission characteristics of polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons from open burning of rice straw in the north of Vietnam. Int. J. Environ. Res. Public Health 2019, 16, 2343. [Google Scholar] [CrossRef] [PubMed]
- Urbančok, D.; Payne, A.J.; Webster, R.D. Regional transport, source apportionment and health impact of PM10 bound polycyclic aromatic hydrocarbons in Singapore’s atmosphere. Environ. Pollut. 2017, 229, 984–993. [Google Scholar] [CrossRef]
- Sulong, N.A.; Latif, M.T.; Sahani, M.; Khan, M.F.; Fadzil, M.F.; Tahir, N.M.; Mohamad, N.; Sakai, N.; Fujii, Y.; Othman, M. Distribution, sources and potential health risks of polycyclic aromatic hydrocarbons (PAHs) in PM2.5 collected during different monsoon seasons and haze episode in Kuala Lumpur. Chemosphere 2019, 219, 1–14. [Google Scholar] [CrossRef]
- Thepnuan, D.; Yabueng, N.; Chantara, S.; Prapamontol, T.; Tsai, Y.I. Simultaneous determination of carcinogenic PAHs and levoglucosan bound to PM2.5 for assessment of health risk and pollution sources during a smoke haze period. Chemosphere 2020, 257, 127154. [Google Scholar] [CrossRef]
- Javed, W.; Iakovides, M.; Stephanou, E.G.; Wolfson, J.M.; Koutrakis, P.; Guo, B. Concentrations of aliphatic and polycyclic aromatic hydrocarbons in ambient PM2.5 and PM10 particulates in Doha, Qatar. J. Air Waste Manag. 2019, 69, 162–177. [Google Scholar] [CrossRef]
- Fadel, M.; Ledoux, F.; Farhat, M.; Kfoury, A.; Courcot, D.; Afif, C. PM2. 5 characterization of primary and secondary organic aerosols in two urban-industrial areas in the East Mediterranean. J. Environ. Sci. 2021, 101, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Byambaa, B.; Yang, L.; Matsuki, A.; Nagato, E.G.; Gankhuyag, K.; Chuluunpurev, B.; Banzragch, L.; Chonokhuu, S.; Tang, N.; Hayakawa, K. Sources and characteristics of polycyclic aromatic hydrocarbons in ambient total suspended particles in Ulaanbaatar city, Mongolia. Int. J. Environ. Res. Public Health 2019, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Tianle, Z.; Ahmad, I.; Li, X. Ambient PM2.5 and PM10 bound PAHs in Islamabad, Pakistan: Concentration, source and health risk assessment. Chemosphere 2020, 257, 127187. [Google Scholar] [CrossRef]
- Kumar, A.; Ambade, B.; Sankar, T.K.; Sethi, S.S.; Kurwadkar, S. Source identification and health risk assessment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons in Jamshedpur, India. Sustain. Cities Soc. 2020, 52, 101801. [Google Scholar] [CrossRef]
- Gadi, R.; Sharma, S.K.; Mandal, T.K. Seasonal variation, source apportionment and source attributed health risk of fine carbonaceous aerosols over national capital region, India. Chemosphere 2019, 237, 124500. [Google Scholar]
- Roy, R.; Jan, R.; Gunjal, G.; Bhor, R.; Pai, K.; Satsangi, P.G. Particulate matter bound polycyclic aromatic hydrocarbons: Toxicity and health risk assessment of exposed inhabitants. Atmos. Environ. 2019, 210, 47–57. [Google Scholar] [CrossRef]
- Norouzian Baghani, A.; Bahmani, Z.; Sorooshian, A.; Farzadkia, M.; Nabizadeh, R.; Delikhoon, M.; Barkhordari, A.; Rezaei Kalantary, R.; Golbaz, S.; Kermani, M. Characterization of polycyclic aromatic hydrocarbons associated with PM10 emitted from the largest composting facility in the Middle East. Toxin Rev. 2020, 1–15. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Torkmahalleh, M.A.; Saeedi, R.; Aibaghi, R.; Ghasemi, F.F. Suspended fine particulate matter (PM2.5), microplastics (MPS), and polycyclic aromatic hydrocarbons (PAHs) in air: Their possible relationships and health implications. Environ. Res. 2020, 192, 110339. [Google Scholar] [CrossRef] [PubMed]
- Farahani, V.J.; Arhami, M. Contribution of Iraqi and Syrian dust storms on particulate matter concentration during a dust storm episode in receptor cities: Case study of Tehran. Atmos. Environ. 2020, 222, 117163. [Google Scholar] [CrossRef]
- Tsiouri, V.; Kakosimos, K.E.; Kumar, P. Concentrations, sources and exposure risks associated with particulate matter in the Middle East area—A review. Air Qual. Atmos. Health 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review on human health perspective of air pollution with respect to allergies and asthma. Environ. Int. 2013, 59, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-F.; Fang, G.-C.; Chen, J.-C.; Wu, Y.-S. Atmospheric polycyclic aromatic hydrocarbons (PAHs) in Asia: A review from 1999 to 2004. Environ. Pollut. 2006, 142, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Unwin, J.; Cocker, J.; Scobbie, E.; Chambers, H. An assessment of occupational exposure to polycyclic aromatic hydrocarbons in the UK. Ann. Occup. Hyg. 2006, 50, 395–403. [Google Scholar]
- US EPA. Development of a Relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures. External Review Draft; Environmental Protection Agency; Integrated Risk Information System: Washington, DC, USA, 2010.
- Rota, M.; Bosetti, C.; Boccia, S.; Boffetta, P.; La Vecchia, C. Occupational exposures to polycyclic aromatic hydrocarbons and respiratory and urinary tract cancers: An updated systematic review and a meta-analysis to 2014. Arch. Toxicol. 2014, 88, 1479–1490. [Google Scholar] [CrossRef]
- Petit, P.; Maître, A.; Persoons, R.; Bicout, D.J. Lung cancer risk assessment for workers exposed to polycyclic aromatic hydrocarbons in various industries. Environ. Int. 2019, 124, 109–120. [Google Scholar] [CrossRef]
- Poursafa, P.; Moosazadeh, M.; Abedini, E.; Hajizadeh, Y.; Mansourian, M.; Pourzamani, H.; Amin, M.-M. A systematic review on the effects of polycyclic aromatic hydrocarbons on cardiometabolic impairment. Int. J. Prev. Med. 2017, 8, 19. [Google Scholar]
- Kalantary, R.R.; Jaffarzadeh, N.; Rezapour, M.; Arani, M.H. Association between exposure to polycyclic aromatic hydrocarbons and attention deficit hyperactivity disorder in children: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2020, 27, 11531–11540. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.M. Evaluation and estimation of potential carcinogenic risks of polynuclear aromatic hydrocarbons. In Proceedings of the Symposium of Polycyclic Aromatic Hydrocarbons in the Workplace, Pacific Rim Risk Conference, Honolulu, HI, USA, 4–7 December 1984. [Google Scholar]
- Clement Associates. Comparative Potency Approach for Estimating the Cancer Risk Associated with Exposure to Mixtures of Polycyclic Aromatic Hydrocarbons; Prepared for the US EPA under contract 68-02-4403; U.S. Environmental Protection Agency: Fairfax, VA, USA, 1988.
- Nisbet, I.C.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharm. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- US EPA. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons; U.S. Environmental Protection Agency: Cincinnati, OH, USA; Office of Health and Environmental Assessment; Environmental Criteria and Assessment Office: Cincinnati, OH, USA, 1993.
- Malcolm, H.; Dobson, S. The Calculation of an Environmental Assessment Level (EAL) for Atmospheric PAHs Using Relative Potencies; Department of the Environment: London, UK, 1994.
- Muller, P.; Leece, B.; Raha, D. Scientific Criteria Document for Multimedia Standards Development, Polycyclic Aromatic Hydrocarbons (PAH), Part 1: Hazard Identification and Dose-Response Assessment; Ministry of Environment & Energy, Standards Development Branch: Toronto, ON, Canada, 1997.
- Larsen, J.; Larsen, P. Chemical carcinogens. In Air Pollution and Health; Royal Society of Chemistry: Cambridge, UK, 1998; pp. 33–56. [Google Scholar]
- Collins, J.; Brown, J.; Alexeeff, G.; Salmon, A. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul. Toxicol. Pharmacol. 1998, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- CEPA. No Significant Risk Levels (NSRLs) for the Proposition 65 Carcinogens Benzo[b]fluoranthene, Benzo[j]fluoranthene, Chrysene, Dibenzo[a,h]pyrene, Dibenzo[a,i]pyrene, and 5-Methylchrysene by the Oral Route; Office of Environmental Health Hazard Assessment, Reproductive and Cancer Hazard Assessment Section: Oakland, CA, USA; California Environmental Protection Agency: Oakland, CA, USA, 2004.
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Roy, P.D.; Elizalde-Martínez, I.; Shruti, V. Impacts of the COVID-19 lockdown on air quality and its association with human mortality trends in megapolis Mexico City. Air Qual. Atmos. Health 2020, 1–10. [Google Scholar] [CrossRef]
- Venter, Z.S.; Aunan, K.; Chowdhury, S.; Lelieveld, J. Air pollution declines during COVID-19 lockdowns mitigate the global health burden. Environ. Res. 2021, 192, 110403. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, M.; Zheng, M. Effects of COVID-19 lockdown on global air quality and health. Sci. Total Environ. 2021, 755, 142533. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Shen, F.; Wang, J.; Ma, X.; Li, Z.; Ge, P.; Ou, Y.; Jiang, Y.; Chen, M.; Chen, M. Changes of air quality and its associated health and economic burden in 31 provincial capital cities in China during COVID-19 pandemic. Atmos. Res. 2021, 249, 105328. [Google Scholar] [CrossRef]
- Durant, J.L.; Busby, W.F., Jr.; Lafleur, A.L.; Penman, B.W.; Crespi, C.L. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat. Res. 1996, 371, 123–157. [Google Scholar] [CrossRef]
| Continent | Country | PM2.5 (µg/m3) | PM10 (µg/m3) | References | ||
|---|---|---|---|---|---|---|
| 24-h | Annual | 24-h | Annual | |||
| North America | USA | 35 | 12 | 150 | None | [3] |
| Mexico | 45 | 12 | 75 | 40 | [31] | |
| Canada | 27 | 8.8 | None | None | [32] | |
| South America | Brazil | 25 | 10 | 50 | 20 | [33] |
| Chile | 50 | 20 | 150 | 50 | [34] | |
| Australia | Australia | 25 | 8 | 50 | 25 | [35] |
| Africa | South Africa | None | None | 75 | 40 | [39] |
| Europe | EU | None | 25 | 50 | 40 | [37] |
| Russia | 35 | 25 | 60 | 40 | [38] | |
| Asia | China | 75 | 35 | 150 | 70 | [36] |
| Japan | 35 | 15 | None | 100 | [36] | |
| South Korea | 25 | 25 | 100 | 50 | [36] | |
| Mongolia | 50 | 25 | 150 | 50 | [36] | |
| India | 60 | 40 | 100 | 60 | [36] | |
| Species (Abbreviation) | CAS Number | MW a Category | Vapor Pressure b | Structure |
|---|---|---|---|---|
| US EPA 16 PAHs | ||||
| Naphthalene (Nap) | 91-20-3 | 128.17 LMW | 11.3 | 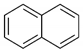 |
| Acenaphthylene (Acy) | 208-96-8 | 152.19 LMW | 0.64 | 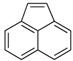 |
| Acenaphthene (Ace) | 83-32-9 | 154.21 LMW | 0.29 | 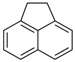 |
| Fluorene (Flu) | 86-73-7 | 166.22 LMW | 0.08 | 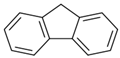 |
| Anthracene (Ant) | 120-12-7 | 178.23 LMW | 0.08 | 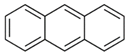 |
| Phenanthrene (Phe) | 85-01-8 | 178.23 LMW | 1.61 × 10−2 | 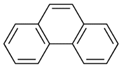 |
| Fluoranthene (FR) | 206-44-0 | 202.25 MMW | 1.23 × 10−3 | 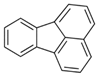 |
| Pyrene (Pyr) | 129-00-0 | 202.25 MMW | 6.00 × 10−4 | 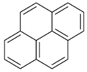 |
| Benz[a]anthracene (BaA) | 56-55-3 | 228.30 MMW | 2.80 × 10−4 | 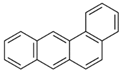 |
| Chrysene (Chr) | 218-01-9 | 228.30 MMW | 8.31 × 10−4 | 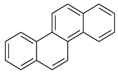 |
| Benzo[b]fluoranthene (BbF) | 205-99-2 | 252.30 HMW | 6.67 × 10−5 | 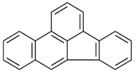 |
| Benzo[k]fluoranthene (BkF) | 207-08-9 | 252.30 HMW | 1.29 × 10−7 |  |
| Benzo[a]pyrene (BaP) | 50-32-8 | 252.30 HMW | 7.32 × 10−7 | 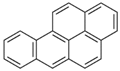 |
| Dibenz[a,h]anthracene (DBA) | 53-70-3 | 278.30 HMW | 1.27 × 10−7 |  |
| Indeno[1,2,3-cd]pyrene (IDP) | 193-39-5 | 276.30 HMW | 1.67 × 10−8 | 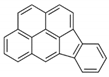 |
| Benzo[ghi]perylene (BgPe) | 191-24-2 | 276.30 HMW | 1.33 × 10−8 | 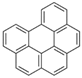 |
| Non-priority PAHs | ||||
| Cyclopenta[def]phenanthrene (CdefP) | 203-64-5 | 190.24 LMW | - c | 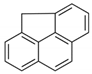 |
| Benzo[c]fluorene (BcF) | 205-12-9 | 216.28 MMW | - c | 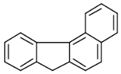 |
| Cyclopenta[c,d]pyrene (CcdP) | 27208-37-3 | 226.30 MMW | - c | 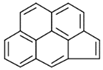 |
| Benzo[ghi]fluoranthene (BghiF) | 203-12-3 | 226.30, MMW | 2.56 × 10−5 | 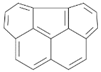 |
| Triphenylene (Tri) | 217-59-4 | 228.30 MMW | 2.8× 10−6 | 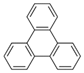 |
| Benzo[c]phenanthrene (BcP) | 195-19-7 | 228.30 MMW | - c | 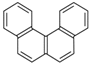 |
| Retene (Ret) | 483-65-8 | 234.30, MMW | - c | 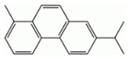 |
| 11H-Benz[bc]aceanthrylene (11H-BbcA) | 202-94-8 | 240.30 MMW | - c | 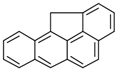 |
| 4H-Cyclopenta[def]chrysene (4H-CdefC) | 202-98-2 | 240.30 MMW | - c | 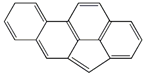 |
| Benz[j]aceanthrylene (BjA) | 202-33-5 | 252.30 HMW | - c | 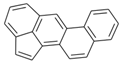 |
| Benz[e]aceanthrylene (BeA) | 199-54-2 | 252.30 HMW | - c | 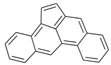 |
| Benz[l]aceanthrylene (BlA) | 211-91-6 | 252.30 HMW | - c | 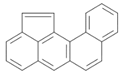 |
| Perylene (Per) | 198-55-0 | 252.30 HMW | 6.67 × 10−8 |  |
| Benzo[a]fluoranthene (BaF) | 203-33-8 | 252.30 HMW | - c | 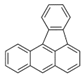 |
| Benzo[j]fluoranthene (BjF) | 205-82-3 | 252.30 HMW | 3.60 × 10−6 | 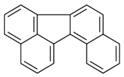 |
| Benzo[e]pyrene (BeP) | 192-97-2 | 252.30 HMW | 7.60 × 10−7 | 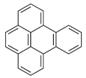 |
| 13H-Dibenzo[a,h]fluorene (13H-DahF) | 239-85-0 | 266.3 HMW | - c | 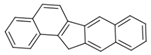 |
| Anthanthrene (Anth) | 191-26-4 | 276.30 HMW | - c |  |
| Indeno[1,2,3-cd]fluoranthene (IDF) | 193-43-1 | 276.30 HMW | - c | 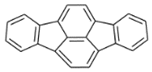 |
| Benzo[b]chrysene (BbC) | 214-17-5 | 278.30 HMW | - c | 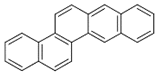 |
| Benzo[g]chrysene (BgC) | 196-78-1 | 278.30 HMW | 3.07 × 10−6 | 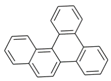 |
| Benzo[c]chrysene (BcC) | 194-69-4 | 278.30 HMW | 1.20 × 10−7 | 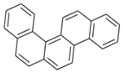 |
| Dibenz[a,c]anthracene (DBacA) | 215-58-7 | 278.30 HMW | 1.33 × 10−8 | 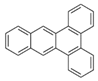 |
| Dibenz[a,j]anthracene (DBajA) | 224-41-9 | 278.30 HMW | - c | 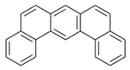 |
| Picene (Pic) | 213-46-7 | 278.30 HMW | - c |  |
| Coronene (Cor) | 191-07-1 | 300.40 HMW | 2.89 × 10−10 |  |
| Benzo[b]perylene (BbPer) | 197-70-6 | 302.40 HMW | - c | 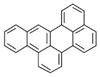 |
| Naphtho[2,3-e]pyrene (NeP) | 193-09-9 | 302.40 HMW | - c | 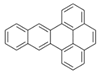 |
| Dibenzo[a,e]fluoranthene (DBaeF) | 5385-75-1 | 302.40 HMW | 9.77 × 10−9 |  |
| Dibenzo[a,l]pyrene (DBalF) | 191-30-0 | 302.40 HMW | 6.40 × 10−8 | 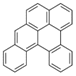 |
| Dibenzo[a,e]pyrene (DBaeP) | 192-65-4 | 302.40 HMW | 6.93 × 10−9 |  |
| Dibenzo[a,i]pyrene (DBaiP) | 189-55-9 | 302.40 HMW | 2.40 × 10−9 |  |
| Dibenzo[a,h]pyrene (DBahP) | 189-64-0 | 302.40 HMW | 8.53 × 10−10 | 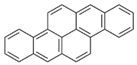 |
| Evaluation | Species |
|---|---|
| Group 1 a | Benzo[a]pyrene |
| Group 2A b | Dibenz[a,h]anthracene, Cyclopenta[cd]pyrene, Dibenzo[a,l]pyrene, Dibenzo[c,h]acridine, 1-Nitropyrene, 6-Nitrochrysene, 2-Nitrotoluene, |
| Group 2B c | Naphthalene, Benz[a]anthracene, Chrysene, Benzo[b]fluoranthene, Benzo[k]fluoranthene, Benzo[j]fluoranthene, Indeno[1,2,3-cd]pyrene, Benzo[c]phenanthrene, Dibenzo[a,e]pyrene, Dibenzo[a,h]pyrene, Dibenzo[a,i]pyrene, Dibenzo[a,h]acridine, Dibenzo[a,j]acridine, Dibenzo[c,g]carbazole, 5-Methylchrysene, 2-Nitrofluorene, 4-Nitropyrene, 3,7-Dinitrofluoranthene, 3,9-Dinitrofluoranthene, 1,3-Dinitropyrene, 1,6-Dinitropyrene, 1,8-Dinitropyrene, 2,6-Dinitrotoluene, 3-Nitrobenzanthrone, 5-Nitroacenaphthene |
| Participants | PM | PAHs | Period | Concentration | Country, City |
|---|---|---|---|---|---|
| Rural residents | PM | 28 | July | 655 ± 250 | China, Laiyang [71] |
| Rural residents | PM2.5 | 10 | 9–12 March 2013 | 4.2–224 | Thailand, Lampang [72] |
| Health residents | PM2.5 | 26 | 2014–2016 | 1.7 (0.4–5.2) | China, Hongkong [73] |
| Residents | PM2.5 | 16 | 2015–2018 | 8.27 | China, Zhuhai [74] |
| Residents | PM2.5 | 16 | 2014–2017 | 11.9 | China, Wuhan [74] |
| Children | PM2.5 | 8 | 11 April–9 May 2012 15 July–3 November 2012 | 0.65 0.63 | Italy, Rome [75] |
| Children | PM2.5 | 16 | 17 May–23 June 2010 8 November–13 December 2010 | 27.31 58.18 | China, Tianjin [76] |
| COPD patients | PM2.5 | 16 | June 2017–October 2018 | 186.85 | China, Harbin [77] |
| Drivers | PM4 | 12 | 2015–2018 | 9.97 | Greece, Thessaloniki [15] |
| Office workers | PM2 | 13 | 2015 | 4.0 ± 2.3 | Australia, Canberra [16] |
| Office workers | PM2.5 | 8 | 6–13 March 2009 10–19 June 2009 | 15.19 ± 15.15 3.04 ± 1.38 | Czech Republic, Ostrava [78] |
| Policemen | PM2.5 | 8 | 8–20 February 2009 17–27 May 2009 | 4.27 ± 2.95 1.03 ± 0.61 | Czech Republic, Prague [78] |
| Policemen | PM2.5 | 8 | 2–6 March 2009 6–10 June 2009 | 39.08 ± 17.33 4.27 ± 1.99 | Czech Republic, Karvina [78] |
| Housewife | PM2.5 | 19 | 4–21 November 2016 | 310 ± 443 | China, Xingping [79] |
| Housewife | PM2.5 | 19 | January 2018 | 116 (32–224) | China, Xi’an, [80] |
| Highway toll station workers | PM2.5 | 16 | March–May 2014 | 319.90 | China, Tianjin [81] |
| Newsagent | PM | 16 | 2013 | 5570 | Iran, Tehran [82] |
| Seafarers | PM | 32 | July 2016 | 760–8400 | Sweden [83] |
| Chinese kitchen worker | PM | 16 | 4 September–1 November 2014 | 1794–12,108 | China, Taiwan [84] |
| Place | Country, City | PM | PAHs | Periods | Concentration |
|---|---|---|---|---|---|
| Rural households | China, Laiyang [71] | PM | 28 | July | 738 ± 321 |
| Households | China, Hongkong [73] | PM2.5 | 26 | 2014–2016 | 3.0 (1.0–7.3) |
| China, Xingping [79] | PM2.5 | 19 | 4–21 November 2016 | 211 ± 120 | |
| China, Xi’an [80] | PM2.5 | 19 | January 2018 | 92 (15–276) | |
| China, Beijing [88] | PM2.5 | 16 | December 2014–February 2016 | 39.8 | |
| Saudi Arabia, Jeddah [89] | PM10 | 13 | - | 18.5 ± 11.2 | |
| Turkey, Bursa [90] | PM | 16 | July 2014– January 2015 | 22 | |
| Spain, Madrid [91] | PM10 | 14 | May 2017–April 2018 | 0.186 | |
| Infant room | China, Harbin [92] | PM | 16 | December 2013–March 2014 | 318 ± 314 |
| University (dormitory) | China, Beijing [88] | PM2.5 | 16 | December 2014–February 2016 | 34.1 |
| China, Wuhan [93] | PM2.5 | 16 | December 2014–June 2015 | 31.3 | |
| University (laboratory) | China, Wuhan [93] | PM2.5 | 16 | December 2014–June 2015 | 27.0 |
| China, Harbin [94] | PM2.5 | 16 | January 2015 | 115 | |
| University (office) | China, Beijing [88] | PM2.5 | 16 | December 2014–February 2016 | 32.1 |
| Saudi Arabia, Jeddah [89] | PM10 | 13 | - | 12.7 ± 5.1 | |
| China, Wuhan [93] | PM2.5 | 16 | December 2014–June 2015 | 32.4 | |
| China, Harbin [94] | PM2.5 | 16 | January 2015 | 96.6 | |
| Classroom | China, Beijing [95] | PM2.5 | 12 | October 2016–March 2017 | 29.83 |
| Brazil, São Paulo [96] | PM | 15 | 7–11 November 2016 | 0.45 | |
| Poland, Warsaw [17] | PM1 | 16 | April–June 2015 | 10.9 | |
| Poland, Gliwice [17] | PM1 | 16 | April–June 2015 | 21.6 | |
| Portugal, Porto [97] | PM2.5 | 18 | March–May 2014 | 5.03–23.6 | |
| Shopping malls | Pakistan, Islamabad [98] | PM2.5 | 16 | February–April 2014 | 2.39 ± 1.45 |
| Bakery | Italy, Bari [99] | PM2.5 | 7 | 7–19 April 2013 | 7.4 |
| Hotels | Saudi Arabia, Jeddah [89] | PM10 | 13 | - | 6.3 ± 1.3 |
| China, Jinan [100] | PM2.5 | 19 | January 2016 | 39.58–115.63 | |
| Public bars | Nigeria, Warri [101] | PM | 16 | - | 43.43–155.11 |
| Office building | China, Changchun [102] | PM2.5 | 16 | April–October 2018 December 2017–April 2018 | 48.6 67.9 |
| Fire station | Poland, North Poland [104] | PM4 | 15 | September 2018 | 1882–5924 |
| Ship | Sweden [83] | PM | 32 | July 2016 | 550–39,000 |
| Chinese kitchen | China, Taiwan [84] | PM | 16 | 4 September–1 November 2014 | 1648–5342 |
| Country, City | PM | PAHs | Periods | Concentration |
|---|---|---|---|---|
| Oceania | ||||
| New Zealand, Auckland [108] | PM2.5 | 15 | 2016–2017 | 0.31 ± 0.19 |
| Americas | ||||
| Mexico, South Mexico [67] | PM2.5 | 24 | November 2016–March 2017 | 4.82 ± 1.97 |
| Peru, Arequipa [109] | PM2.5 PM10 | 14 | January–December 2018 | 7.4 ± 2.3 9.6 ± 3.9 |
| Argentina, Cordoba [110] | PM10 | 14 | August 2011–August 2013 | 4.5 ± 4.34 |
| Brazil, Belo Horizonte [111] | PM2.5 | 16 | May 2017–April 2018 | 1.68–6.24 |
| Canada, Toronto [112] | PM10 | 17 | August 2016–August 2017 | 10.2 ± 2.5 |
| US, Washington [113] | PM10 | 19 | April 2016–September 2018 | 0.84 |
| Europe | ||||
| Spain, Coruña [114] | PM10 | 12 | Januray–December 2017 | 7.56 |
| Spain, South Spain [115] | PM2.5 PM10 | 16 | July 2014–June 2015 | 23.0 26.2 |
| Italy, Milan [116] | PM2.5 | - | December 2018–February 2019 May–July 2019 | 72.8 ± 16.6 0.40 ± 0.07 |
| Poland, Wadowice [117] | PM10 | 9 | March 2017 August 2017 | 80.6 10.5 |
| Cyprus, Nicosia [118] | PM2.5 | 50 | January–March 2018 | 1.62 |
| Czech Republic, Brno [119] | PM1 | 15 | January–February 2017 | 20.7 |
| Croatia, Zagreb [120] | PM10 | 10 | December 2017–February 2018 | 25.4 |
| Bosnia and Herzegovina, Sarajevo [120] | PM10 | 10 | December 2017–February 2018 | 64.8 |
| Russia, Moscow [121] | PM10 | 9 | June–July 2018 | 1.32–7.68 |
| Russia, St. Petersburg [121] | PM10 | 9 | June–July 2018 | 1.71–6.30 |
| Russia, Kazan [121] | PM10 | 9 | June–July 2018 | 2.95–9.61 |
| Africa | ||||
| Algeria, Algiers [122] | PM10 | 22 | June–September 2016 | 7.47 ± 1.21 |
| South Africa, Pretoria [123] | PM2.5 | 16 | June–July 2016 | 4.11 |
| Rwanda, Kigali [124] | PM2.5 | 15 | May–June 2017 | 52.7 |
| Asia | ||||
| China, Hongkong [73] | PM2.5 | 26 | 2014–2016 | 3. 9 (1.5–9.6) |
| China, Xi’an [127] | PM2.5 PM10 | 16 | December 2016–December 2017 | 63.1 (14.3–266) 66.8 (9.69–349) |
| China, Shanghai [18] | PM2.1 | 9 | July 2017 January 2018 | 1.36 ± 0.20 7.72 ± 3.33 |
| China, Beijing [128] | PM10 | 15 | January 2017 | 98.1 ± 48.2 |
| China, Zhengzhou [128] | PM10 | 15 | January 2017 | 77.9 ± 29.6 |
| China, Guangzhou [129] | PM2.5 | 16 | June–July 2016 November–December 2016 | 5.49 10.5 |
| China, Taiyuan [129] | PM2.5 | 16 | June–July 2016November–December 2016 | 29.5 197 |
| China, Jinan [130] | PM2.5 | 18 | March–December 2016 | 39.8 (8.18–246) |
| China, Shanxi [131] | PM10 PM2.1 | 17 | January–February 2017 | 1056 ± 315 937 ± 294 |
| China, Urumqi [132] | PM2.5 | 16 | September 2017–September 2018 | 448 |
| China, Chengdu [133] | PM10 | 16 | March 2015–February 2016 | 82.0 ± 64.8 |
| China, Changchun [134] | PM2.5 | 15 | October–November 2016 | 81.4 ± 46.0 |
| China, Harbin [135] | PM2.5 | 16 | June 2017–May 2018 | 86.9 |
| China, Lanzhou [136] | PM2.5 | 9 | July 2017–October 2018 | 9.86 |
| Japan, Kanazawa [137] | PM2.5 | 9 | April 2017–February 2018 | 0.69 |
| Japan, Chiba [138] | PM2.5 | 21 | June 2016–October 2017 | 2.9 |
| Japan, Kirishima [139] | PM2.5 | 9 | November–December 2016 | 1.32 (0.36–2.90) |
| South Korea, Seoul [140] | PM2.5 | 14 | January–December 2018 | 5.6 ± 7.9 |
| South Korea, Gwangju [141] | PM2.5 | 17 | October 2016–April 2017 | 1.04–29.5 |
| Vietnam, Hanoi [142] | PM10 | 9 | 2016–2018 | 8.51 |
| Singapore, Singapore [143] | PM10 | 16 | May 2015–June 2016 | 0.68–5.97 |
| Malaysia, Lumpur [144] | PM2.5 | 16 | June 2015–May 2016 | 2.04 ± 0.28 |
| Thailand, Chiang Mai [145] | PM2.5 | 8 | February–April 2016 | 5.88 ± 1.97 |
| Qatar, Doha [146] | PM2.5 PM10 | 36 | May–December 2015 | 0.56 0.72 |
| Lebanon, Beirut [147] | PM2.5 | 15 | December 2018–October 2019 | 0.95 |
| Mongolia, Ulaanbaatar [148] | PM10 | 15 | January 2017 March 2017 September 2017 | 131–773 22.2–531 1.4–54.6 |
| Pakistan, Islamabad [149] | PM2.5 PM10 | 16 | January–September 2017 | 25.7 ± 12.0 40.1 ± 16.8 |
| India, Jamshedpur [150] | PM2.5 | 16 | December 2016–February 2017 March–May 2017 | 109 ± 18.2 81.1 ± 13.3 |
| India, Delhi [151] | PM2.5 | 14 | December 2016–December 2017 | 753 ± 252 |
| India, Haryana [151] | PM2.5 | 14 | December 2016–December 2017 | 259 ± 64.6 |
| India, Uttar Pradesh [151] | PM2.5 | 14 | December 2016–December 2017 | 535 ± 143 |
| India, Pune [152] | PM2.5 PM10 | 16 | March 2015–March 2016 | 342.4 ± 14.3 446.1 ± 25.6 |
| Iran, Tehran [153] | PM10 | 16 | February–March 2018 | 213 ± 145 |
| Iran, Bushehr [154] | PM2.5 | 16 | December 2016–September 2017 | 0.66–142.3 |
| Species a | 1984 b | 1988 c | 1992 d | 1993 e | 1994 f | 1997 g | 1998 h | 1998 i | 2004 j | 2010 k |
|---|---|---|---|---|---|---|---|---|---|---|
| US EPA 16 PAHs | ||||||||||
| Acy | 0.001 | 0.001 | ||||||||
| Ace | 0.001 | 0.001 | ||||||||
| Flu | 0.001 | 0.001 | ||||||||
| Ant | 0.01 | 0.01 | 0.0005 | 0 | ||||||
| Phe | 0.001 | 0.00064 | 0.005 | 0 | ||||||
| FR | 0.001 | 0.001 | 0.05 | 0.08 | ||||||
| Pyr | 0.081 | 0.001 | 0 | 0.001 | 0 | |||||
| BaA | 0.013 | 0.145 | 0.1 | 0.1 | 0.1 | 0.014 | 0.005 | 0.1 | 0.2 | |
| Chr | 0.001 | 0.0044 | 0.01 | 0.001 | 0.01 | 0.026 | 0.03 | 0.01 | 0.1 | |
| BbF | 0.08 | 0.14 | 0.1 | 0.1 | 0.1 | 0.11 | 0.1 | 0.1 | 0.62 | 0.8 |
| BkF | 0.004 | 0.066 | 0.1 | 0.01 | 0.1 | 0.037 | 0.05 | 0.1 | 0.17 | 0.03 |
| BaP | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| DBA | 0.69 | 1.11 | 5 | 1 | 1 | 0.89 | 1.1 | 10 | ||
| IDP | 0.017 | 0.232 | 0.1 | 0.1 | 0.1 | 0.067 | 0.1 | 0.1 | 0.07 | |
| BgPe | 0.022 | 0.01 | 0.01 | 0.012 | 0.02 | 0.009 | ||||
| Non-priority PAHs | ||||||||||
| BcF | 20 | |||||||||
| CcdP | 0.023 | 0.1 | 0.012 | 0.02 | 0.4 | |||||
| BcP | 0.023 | 0.023 | ||||||||
| 11H-BbcA | 0.05 | |||||||||
| 4H-CdefC | 0.3 | |||||||||
| BjA | 60 | |||||||||
| BeA | 0.8 | |||||||||
| BlA | 5 | |||||||||
| Per | 0.001 | |||||||||
| BjF | 0.061 | 0.1 | 0.045 | 0.05 | 0.1 | 0.52 | 0.3 | |||
| BeP | 0.004 | 0.01 | 0 | 0.002 | ||||||
| Anth | 0.32 | 0.28 | 0.3 | 0.4 | ||||||
| DBacA | 0.1 | 4 | ||||||||
| Cor | 0.001 | |||||||||
| NeP | 0.3 | |||||||||
| DBaeF | 0.9 | |||||||||
| DBalP | 1 | 10 | 30 | |||||||
| DBaeP | 0.2 | 1 | 0.4 | |||||||
| DBaiP | 1.1 | 0.1 | 10 | 12 | 0.6 | |||||
| DBahP | 1.2 | 1 | 10 | 11 | 0.9 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, H.; Zhang, X.; Xing, W.; Wang, Y.; Bai, P.; Zhang, L.; Hayakawa, K.; Toriba, A.; Tang, N. Exposure to Atmospheric Particulate Matter-Bound Polycyclic Aromatic Hydrocarbons and Their Health Effects: A Review. Int. J. Environ. Res. Public Health 2021, 18, 2177. https://doi.org/10.3390/ijerph18042177
Yang L, Zhang H, Zhang X, Xing W, Wang Y, Bai P, Zhang L, Hayakawa K, Toriba A, Tang N. Exposure to Atmospheric Particulate Matter-Bound Polycyclic Aromatic Hydrocarbons and Their Health Effects: A Review. International Journal of Environmental Research and Public Health. 2021; 18(4):2177. https://doi.org/10.3390/ijerph18042177
Chicago/Turabian StyleYang, Lu, Hao Zhang, Xuan Zhang, Wanli Xing, Yan Wang, Pengchu Bai, Lulu Zhang, Kazuichi Hayakawa, Akira Toriba, and Ning Tang. 2021. "Exposure to Atmospheric Particulate Matter-Bound Polycyclic Aromatic Hydrocarbons and Their Health Effects: A Review" International Journal of Environmental Research and Public Health 18, no. 4: 2177. https://doi.org/10.3390/ijerph18042177
APA StyleYang, L., Zhang, H., Zhang, X., Xing, W., Wang, Y., Bai, P., Zhang, L., Hayakawa, K., Toriba, A., & Tang, N. (2021). Exposure to Atmospheric Particulate Matter-Bound Polycyclic Aromatic Hydrocarbons and Their Health Effects: A Review. International Journal of Environmental Research and Public Health, 18(4), 2177. https://doi.org/10.3390/ijerph18042177






