Diversity of Oxacillinases and Sequence Types in Carbapenem-Resistant Acinetobacter baumannii from Austria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Bacterial Isolates
2.2. Antimicrobial Susceptibility Testing
2.3. Molecular Identification of β-Lactamases
2.4. Multi-Locus Sequence Analysis
3. Results
3.1. Antimicrobial Susceptibility Testing
3.2. Detection of β-lactamases Genes
3.3. Genotyping/MLST
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garnacho-Montero, J.; Timsit, J.-F. Managing Acinetobacter baumannii infections. Curr. Opin. Infect. Dis. 2019, 32, 69–76. [Google Scholar] [CrossRef]
- Litwin, A.; Fedorowicz, O.; Duszynska, W. Characteristics of Microbial Factors of Healthcare-Associated Infections Including Multidrug-Resistant Pathogens and Antibiotic Consumption at the University Intensive Care Unit in Poland in the Years 2011–2018. Int. J. Environ. Res. Public Health 2020, 17, 6943. [Google Scholar] [CrossRef]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 25 July 2020).
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, B.A.; Hamouda, A.; Amyes, S.G.B. The rise of carbapenem-resistant Acinetobacter baumannii. Curr. Pharm. Des. 2013, 19, 223–238. [Google Scholar] [CrossRef]
- Brown, S.; Amyes, S. OXA β-lactamases in Acinetobacter: The story so far. J. Antimicrob. Chemother. 2005, 57, 1–3. [Google Scholar] [CrossRef]
- Cornaglia, G.; Riccio, M.; Mazzariol, A.; Lauretti, L.; Fontana, R.; Rossolini, G.M. Appearance of IMP-1 metallo-β-lactamase in Europe. Lancet 1999, 353, 899–900. [Google Scholar] [CrossRef]
- Perilli, M.C.; Pelegrini, G.; Calenza, B. Segatore, and G. Amicosante. First report from Italy of blaVIM-1 and blaTEM-1 genes in Pseudomonas putida and Acinetobacter baumannii isolated from wastewater. J. Chemother. 2011, 23, 181–182. [Google Scholar] [CrossRef]
- Lee, K.; Jum, J.H.; Yong, D.; Lee, H.M.; Kim, H.D.; Docquier, J.-D.; Rossolini, G.M.; Chong, Y. Novel acquired metal-lo-β-lactamase gene, bla(SIM-1) in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob. Agents. Chemother 2005, 49, 4485–4491. [Google Scholar] [CrossRef] [Green Version]
- Robledo, I.E.; Aquino, E.E.; Sante, M.I.; Santana, J.L.; Otero, D.M.; Leon, C.F.; Vazqueiz, G.J. Detection of KPC in Acinetobacter spp in Puerto Rico. Antimicrob. Agents. Chemother 2010, 43, 1354–1357. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M.; Woodford, N. The β-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 2006, 14, 413–420. [Google Scholar] [CrossRef]
- Higgins, P.G.; Poirel, L.; Lehmann, M.; Nordmann, P.; Seifert, H. OXA-143, a Novel Carbapenem-Hydrolyzing Class D β-Lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 5035–5038. [Google Scholar] [CrossRef] [Green Version]
- Héritier, C.; Poirel, L.; Fournier, P.-E.; Claverie, J.-M.; Raoult, D.; Nordmann, P. Characterization of the Naturally Occurring Oxacillinase of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005, 49, 4174–4179. [Google Scholar] [CrossRef] [Green Version]
- Ayobami, O.; Willrich, N.; Suwono, B.; Eckmanns, T.; Markwart, R. The epidemiology of carbapenem-non-susceptible Acinetobacter species in Europe: Analysis of EARS-Net data from 2013 to 2017. Antimicrob. Resist. Infect. Control. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Lusignani Segagni, L.; Starzengruber, P.; Dosch, V.; Assadian, O.; Presterl, E.; Diab-Elschahawi, M. Molecular epidemiology of mul-tidrug-resistant clinical isolates of Acinetobacter baumannii: A 10-year analysis in a large tertiary care university hospital in central Europe with international admissions. Wien Klin Wochenschr. 2017, 129, 816–822. [Google Scholar] [CrossRef] [Green Version]
- Grisold, A.; Zarfel, G.; Strenger, V.; Feierl, G.; Leitner, E.; Masoud, L.; Hoenigl, M.; Raggam, R.; Dosch, V.; Marth, E. Use of automated repetitive-sequence-based PCR for rapid laboratory confirmation of nosocomial outbreaks. J. Infect. 2010, 60, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement: Wayne, PA, USA, 2018. [Google Scholar]
- Amjad, A.; Mirza, I.; Abbasi, S.; Farwa, U.; Malik, N.; Zia, F. Modified Hodge test: A simple and effective test for detection of car-bapenemase production. Iran. J. Microbiol. 2011, 3, 189–193. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2006, 27, 351–353. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuveiller, V.; Nordman, P. Multiplex PCR for detection of acquired carbapenemases genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–125. [Google Scholar] [CrossRef]
- Higgins, P.G.; Lehmann, M.; Seifert, H. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2010, 35, 305. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Pérez-Llarena, F.J.; Zander, E.; Fernández, A.; Bou, G.; Seifert, H. OXA-235, a Novel Class D β-Lactamase Involved in Resistance to Carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 2121–2126. [Google Scholar] [CrossRef] [Green Version]
- Eckert, C.; Gautier, V.; Saladin-Allard, M.; Hidri, N.; Verdet, C.; Ould-Hocine, Z.; Barnaud, G.; Delisle, F.; Rossier, A.; Lambert, T.; et al. Dissemination of CTX-M-type beta-lactamases among clinical isolates of Enterobacteriaceae in Paris, France. Antimicrob. Agents Chemother. 2004, 48, 1249–1255. [Google Scholar] [CrossRef] [Green Version]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diancourt, L.; Passet, V.; Nemec, A.; Dijkshoorn, L.; Brisse, S. The population structure of Acinetobacter baumannii: Expanding mul-tiresistant clones from an ancestral susceptible genetic pool. PLoS ONE 2010, 5, e10034. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, M.N.L.; De Sales, R.O.; Da Silva, K.E.; Maciel, W.G.; Simionatto, S. Multidrug-resistant Acinetobacter baumannii outbreaks: A global problem in healthcare settings. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200248. [Google Scholar] [CrossRef] [PubMed]
- Rahal, J.J.; Urban, C. Acinetobacter. Semin. Respir. Crit. Care. Med. 2000, 21, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter spp. in food and drinking water—A review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef]
- Kittinger, C.; Kirschner, A.; Lipp, M.; Baumert, R.; Mascher, F.; Farnleitner, A.H.; Zarfel, G.E. Antibiotic Resistance of Acinetobacter spp. Isolates from the River Danube: Susceptibility Stays High. Int. J. Environ. Res. Public Health 2017, 15, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrenovic, J.; Music, M.S.; Durn, G.; Dekic, S.; Hunjak, B.; Kisic, I. Carbapenem-Resistant Acinetobacter baumannii Recovered from Swine Manure. Microb. Drug Resist. 2019, 25, 725–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wareth, G.; Neubauer, H.; Sprague, L.D. Acinetobacter baumannii—A neglected pathogen in veterinary and environmental health in Germany. Vete Res. Commun. 2018, 43, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieland, K.; Chhatwal, P.; Vonberg, R.-P. Nosocomial outbreaks caused by Acinetobacter baumannii and Pseudomonas aeruginosa: Results of a systematic review. Am. J. Infect. Control. 2018, 46, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Paton, R.; Miles, R.; Hood, J.; Amyes, S. ARI 1: β-lactamase-mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 1993, 2, 81–87. [Google Scholar] [CrossRef]
- Towner, K.; Levi, K.; Vlassiadi, M. Genetic diversity of carbapenem-resistant isolates of Acinetobacter baumannii in Europe. Clin. Microbiol. Infect. 2008, 14, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Zong, Z.; Lu, X.; Valenzuela, J.K.; Partridge, S.R.; Iredell, J. An outbreak of carbapenem-resistant Acinetobacter baumannii producing OXA-23 β-lactamase. Int. J. Antimicrob. Agents 2008, 31, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Bell, J.M.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Emergence and widespread disseimnation of OXA-23, OXA-24/40 and OXA-58-carbapenemases among Acinetobacter spp, in Asia-Pacific nations: Report from the SENTRY Surveillance Program. J. Antimicrob. Chemother. 2009, 63, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, K.R.; D’Alincourt, A.P.; Carvalho-Assef, P.; Peirano, G.; Galvão Dos Santos, L.C.; Pereira, M.J.F.; Dutra Asensi, M. Dissemi-nation of multidrug-resistant Acinetobacter baumannii genotypes carrying bla(OXA-23) collected from hospitals in Rio de Janeiro, Brazil. Int. J. Antimicrob. Agents 2009, 34, 25–28. [Google Scholar] [CrossRef]
- Stoeva, T.; Higgins, P.; Bojkova, K.; Seifert, H. Clonal spread of carbapenem-resistant OXA-23-positive Acinetobacter baumannii in a Bulgarian university hospital. Clin. Microbiol. Infect. 2008, 14, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, X.; Higgins, P.G.; Wisplinghoff, H.; Korber-Irrgang, B.; Kresken, M.; Seifert, H. Molecular epidemiology of Acinetobacter baumannii and Acinetobacter nosomialis in Germny over a 5-year period (2005–2009). Clin. Microbiol. Infect. 2012, 19, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Minadri, F.; D’Arezzo, S.; Antuneus, L.C.; Pourcel, C.; Principe, L.; Petrosillo, N.; Visca, P. Evidence of diversity among epidemiolog-ically related carbapenemase producing Acinetobacter baumannii strains belonging to International Clonal Lineage II. J. Clin. Microbiol. 2012, 50, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Vranić-Ladavac, M.; Bedenić, B.; Minandri, F.; Ištok, M.; Frančula-Zaninović, S.; Ladavac, R.; Visca, P. Carbapenem-resistance and acquired class D carbapenemases in Acinetobacter baumannii from Croatia 2009–2010. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 471–478. [Google Scholar] [CrossRef]
- Ladavac, R.; Bedenić, B.; Vranić-Ladavac, M.; Barišić, N.; Karčić, N.; Pompe, K.; Ferenčić, A.; Stojanović, A.; Seifert, H.; Katić, S.; et al. Emergence of different Acinetobacter baumannii clones in a Croatian hospital and correlation with antibiotic susceptibility. J. Glob. Antimicrob. Resist. 2017, 10, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedenić, B.; Beader, N.; Godič-Torkar, K.; Vranić-Ladavac, M.; Luxner, J.; Veir, Z.; Grisold, A.J.; Zarfel, G. Nursing Home as a Reservoir of Carbapenem-ResistantAcinetobacter baumannii. Microb. Drug Resist. 2015, 21, 270–278. [Google Scholar] [CrossRef]
- Marque, S.; Poirel, L.; Heritier, C.; Brisse, S.; Blasco, M.D.; Filip, R.; Coman, G.; Naas, T.; Nordmann, P. Regional occurrence of plas-mid-mediated carbapenem-hydrolyzing oxacillinase OXA-58 in Acinetobacter spp in Europe. J. Clin. Microbiol. 2005, 43, 4885–4888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Marqué, S.; Héritier, C.; Segonds, C.; Chabanon, G.; Nordmann, P. OXA-58, a Novel Class D β-Lactamase Involved in Resistance to Carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005, 49, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakris, A.; Ikonomidis, A.; Poulou, A.; Spanakis, N.; Vrizas, D.; Diomidous, M.; Pournaras, S.; Markou, F. Clusters of imipenem-resistant Acinetobacter baumannii clones producing different carbapenemases in an intensive care unit. Clin. Microbiol. Infect. 2008, 14, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pournas, S.; Markogiannakis, A.; Ikonomidis, A.; Kondyli, L.; Behtiomouti, K.; Maniatis, A.N.; Legakis, N.J.; Tsakris, A. Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit. J. Antimicrob. Chemother. 2006, 57, 557–561. [Google Scholar] [CrossRef]
- Franolić-Kukina, I.; Bedenić, B.; Budimir, A.; Herljević, Z.; Vraneš, J.; Higgins, P.G. Clonal spread of carbapenem-resistant OXA-72-positive Acinetobacter baumannii in a Croatian university hospital. Int. J. Infect. Dis. 2011, 15, e706–e709. [Google Scholar] [CrossRef] [Green Version]
- Goić-Barišić, I.; Towner, K.J.; Kovačić, A.; Šiško-Kraljević, K.; Tonkić, M.; Novak, A.; Punda-Polić, V. Outbreak in Croatia caused by a new carbapenem-resistant clone of Acinetobacter baumannii producing OXA-72 carbapenemase. J. Hosp. Infect. 2011, 77, 368–369. [Google Scholar] [CrossRef]
- Petrović, T.; Uzunović, S.; Barišić, I.; Luxner, J.; Grisold, A.; Zarfel, G.; Ibrahimagić, A.; Jakovac, S.; Slaćanac, D.; Bedenić, B. 2018. Arrival of carbapenem-hydrolyzing-oxacillinases in Acinetobacter baumannii in Bosnia and Herzegovina. Infect. Genet. Evol. 2019, 58, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Dortet, L.; Bonnin, R.A.; Girlich, D.; Imanci, D.; Bernabeu, S.; Fortineau, N.; Naas, T. Whole-Genome Sequence of a European Clone II and OXA-72-Producing Acinetobacter baumannii Strain from Serbia. Genome Announc. 2015, 3, e01390-15. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, S.M.; Farshadzadeh, Z.; Janabadi, S.; Musavi, M.; Shahi, F.; Moradi, M.; Khoshnood, S. Evaluating the frequency of car-bapenem and aminoglycoside resistance genes among clinical isolates of Acinetobacter baumannii from Ahvaz, south-west Iran. New Microbes New Infect. 2020, 38, 100779. [Google Scholar] [CrossRef]
- Angles-Yanqui, E.; Huaringa-Marcelo, J.; Sacsaquispe-Contreras, R.; Pampa-Espinoza, L. Panorama de las carbapenemasas en Perú. Rev. Panam. Salud. Publ. 2020, 44, e61. [Google Scholar] [CrossRef] [PubMed]
- Zander, E.; Bonnin, R.A.; Seifert, H.; Higgins, P.G. Characterization of blaOXA-143 Variants in Acinetobacter baumannii and Acinetobacter pittii. Antimicrob. Agents Chemother. 2014, 58, 2704–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pormohammad, A.; Mehdinejadiani, K.; Gholizadeh, P.; Nasiri, M.J.; Mohtavinejad, N.; Dadashi, M.; Karimaei, S.; Safari, H.; Azimi, T. Global prevalence of colistin resistance in clinical isolates of Acinetobacter baumannii: A systematic review and meta-analysis. Microb. Pathog. 2020, 139, 103887. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.; Ergönül, Ö.; Azap, A.; Bilgin, H.; Aydın, G.; Çavuş, S.A.; Demiroğlu, Y.Z.; Alışkan, H.E.; Memikoğlu, O.; Menekşe, Ş.; et al. Rapid emergence of colistin resistance and its impact on fatality among healthcare-associated infections. J. Hosp. Infect. 2018, 98, 260–263. [Google Scholar] [CrossRef]
- D’Onofrio, V.; Conzemius, R.; Varda-Brkić, D.; Bogdan, M.; Grisold, A.; Gyssens, I.C.; Bedenić, B.; Barišić, I. Epidemiology of colistin-resistant, carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Croatia. Infect. Genet. Evol. 2020, 81, 104263. [Google Scholar] [CrossRef]
- Hoenigl, M.; Drescher, M.; Feierl, G.; Valentin, T.; Zarfel, G.; Seeber, K.; Krause, R.; Grisold, A. Successful management of nosocomial ventriculitis and meningitis caused by extensively drug-resistant Acinetobacter baumannii in Austria. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, e88–e90. [Google Scholar] [CrossRef] [Green Version]
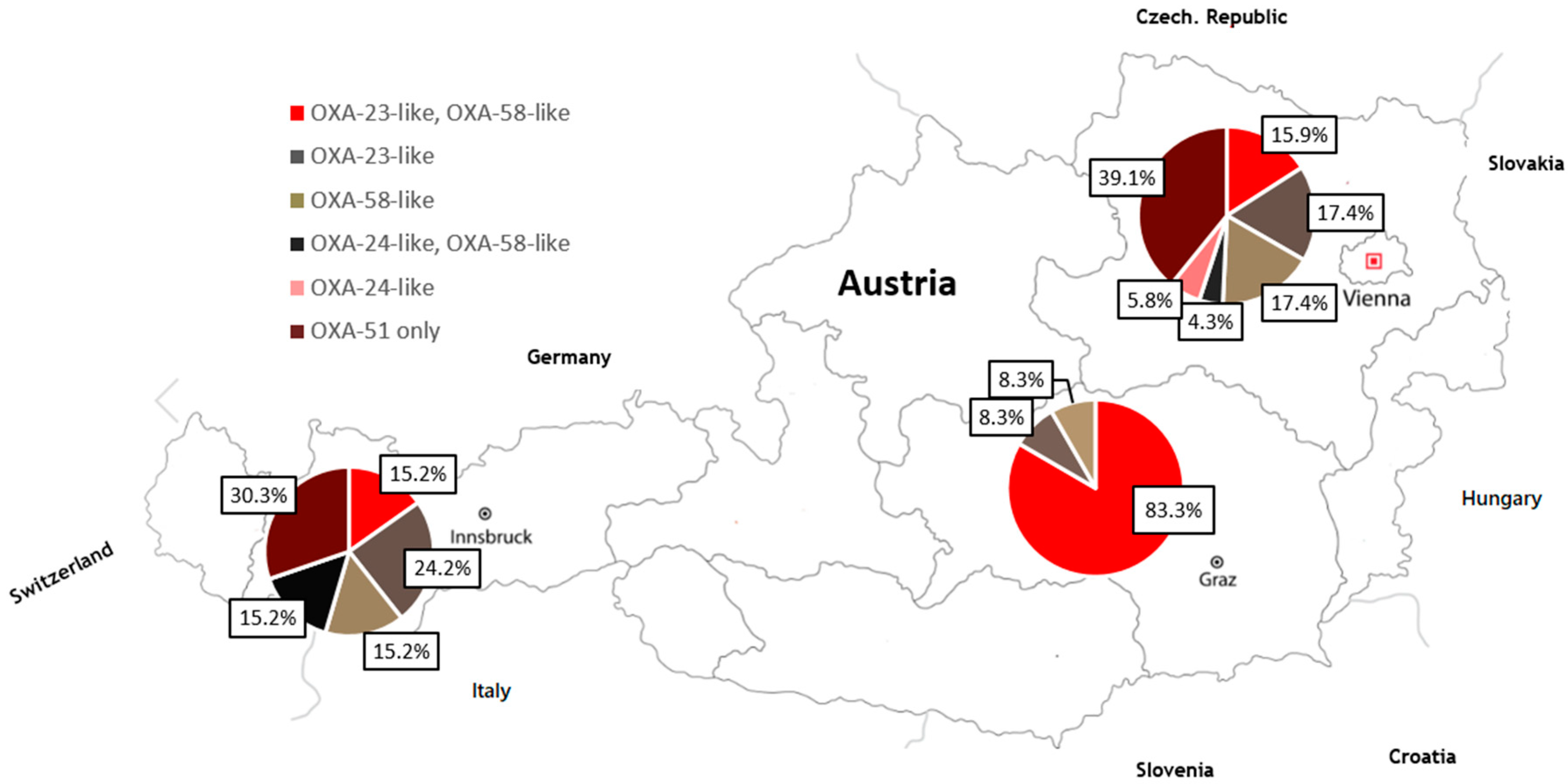
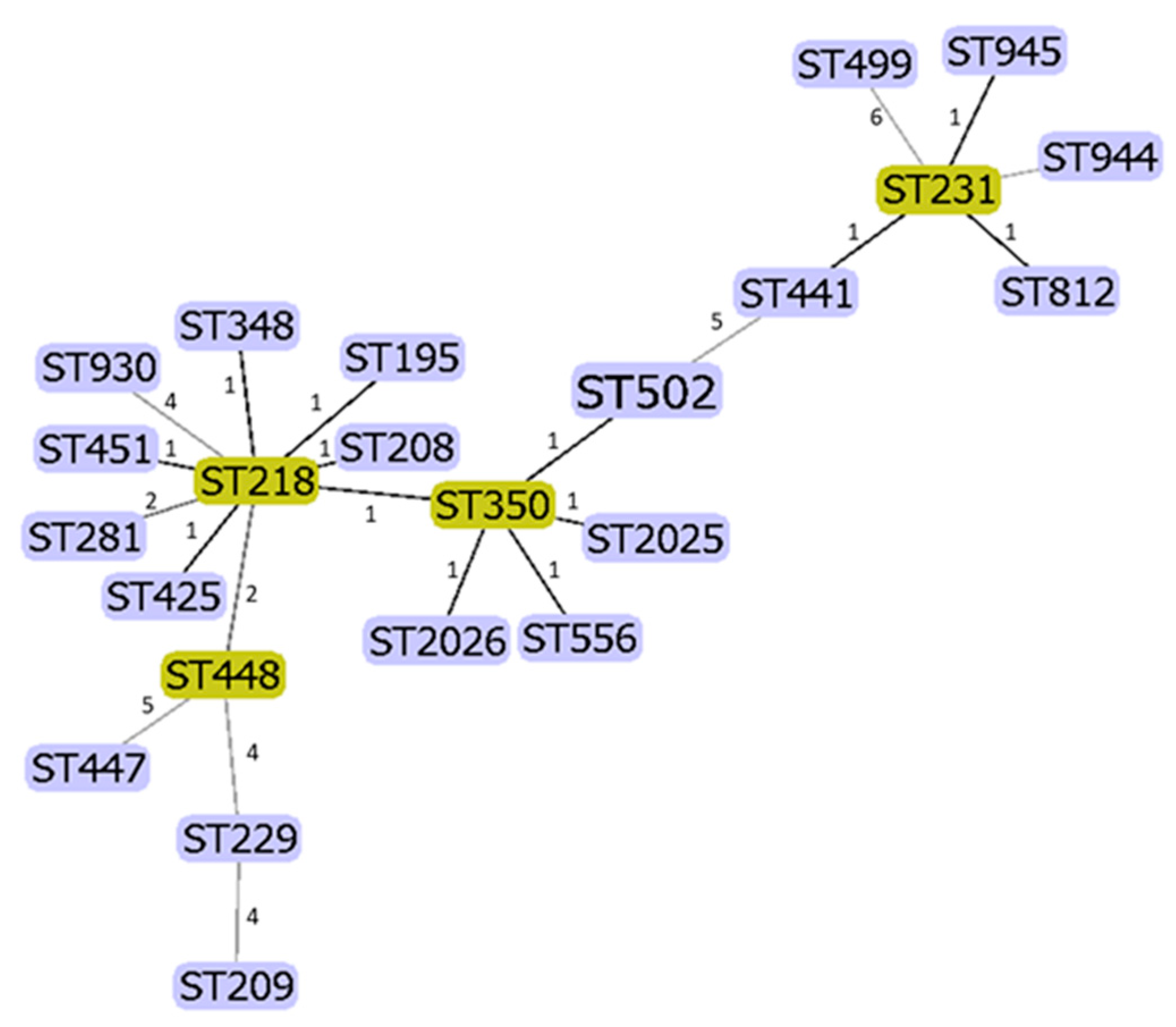
| REGION/AUSTRIA | SEQUENCE TYPE | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 195 | 218 | 231 | 208 | 425 | 451 | 502 | 348 | 350 | 281 | 2026 | 441 | 448 | 812 | 945 | Others 1 | |
| South (n = 12) | 3 | 6 | 1 | - | - | - | - | - | - | - | 1 | - | - | - | - | 1 |
| West (n = 33) | 2 | 5 | 3 | 1 | 1 | 7 | 1 | 7 | 1 | 3 | - | - | - | - | - | 2 |
| North (n = 69) | 13 | 2 | 9 | 10 | 7 | 1 | 7 | - | 5 | 1 | 1 | 2 | 2 | 2 | 2 | 5 |
| Total (n = 114/%) | 18 15.8% | 13 11.4% | 13 11.4% | 11 9.7% | 8 7.0% | 8 7.0% | 8 7.0% | 7 6.1% | 6 5.3% | 4 3.5% | 2 1.8% | 2 1.8% | 2 1.8% | 2 1.8% | 2 1.8% | 8 7.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grisold, A.J.; Luxner, J.; Bedenić, B.; Diab-Elschahawi, M.; Berktold, M.; Wechsler-Fördös, A.; Zarfel, G.E. Diversity of Oxacillinases and Sequence Types in Carbapenem-Resistant Acinetobacter baumannii from Austria. Int. J. Environ. Res. Public Health 2021, 18, 2171. https://doi.org/10.3390/ijerph18042171
Grisold AJ, Luxner J, Bedenić B, Diab-Elschahawi M, Berktold M, Wechsler-Fördös A, Zarfel GE. Diversity of Oxacillinases and Sequence Types in Carbapenem-Resistant Acinetobacter baumannii from Austria. International Journal of Environmental Research and Public Health. 2021; 18(4):2171. https://doi.org/10.3390/ijerph18042171
Chicago/Turabian StyleGrisold, Andrea J., Josefa Luxner, Branka Bedenić, Magda Diab-Elschahawi, Michael Berktold, Agnes Wechsler-Fördös, and Gernot E. Zarfel. 2021. "Diversity of Oxacillinases and Sequence Types in Carbapenem-Resistant Acinetobacter baumannii from Austria" International Journal of Environmental Research and Public Health 18, no. 4: 2171. https://doi.org/10.3390/ijerph18042171






