A Neurophysiological Pattern as a Precursor of Work-Related Musculoskeletal Disorders Using EEG Combined with EMG
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Conditions
2.3. Data Collection
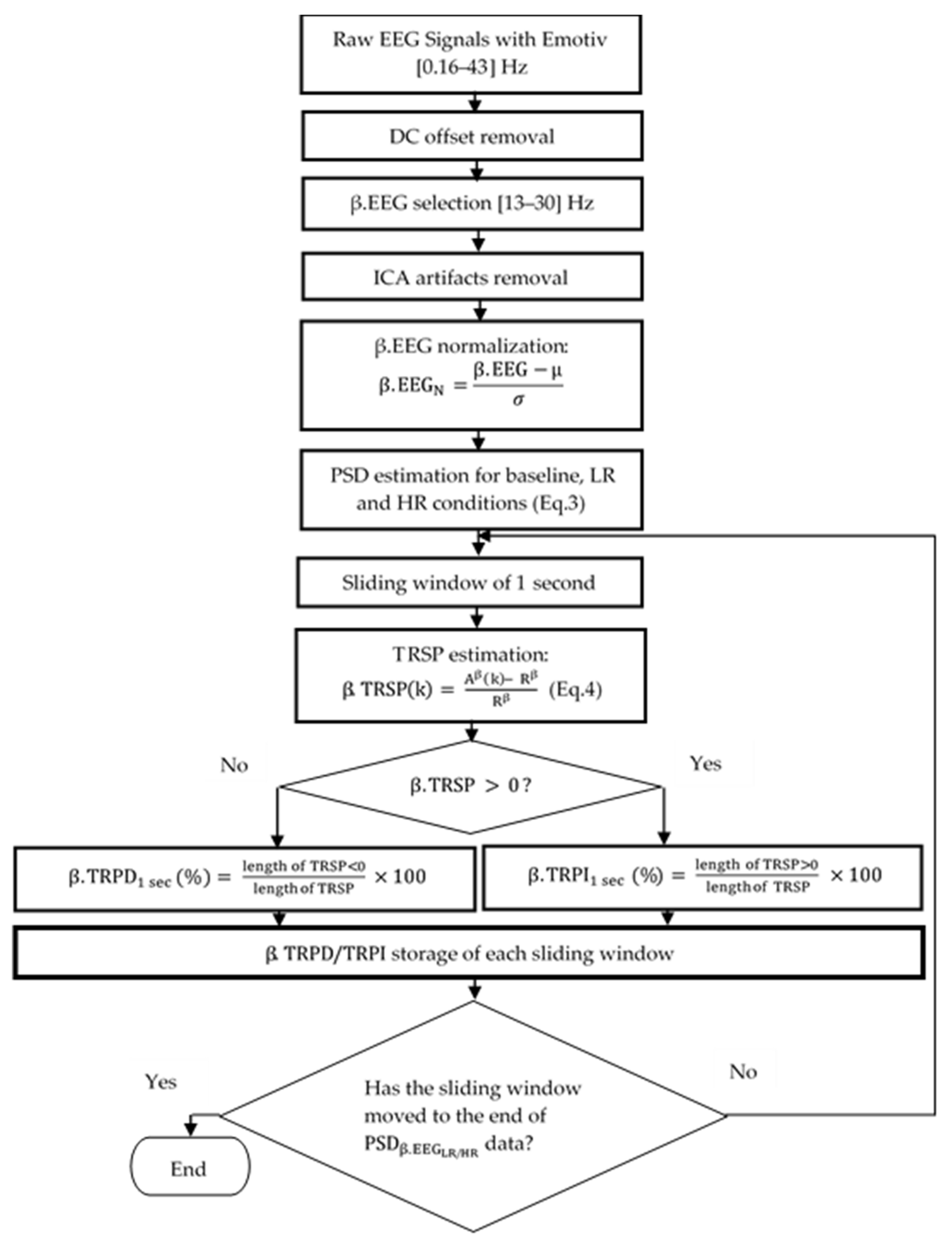
2.3.1. EEG Measurement and Processing
- The Power Spectral Density (PSD) in the beta EEG frequency band was computed as a correlate of the degree of beta activity of the targeted neuronal population [37]. When the PSD of the signal is high, it means that there is an increase in the neuronal activity. On the contrary, a decrease in neuronal activity is translated by a decrease in PSD. One must first calculate the autocorrelation function of the signal, followed by the application of the Fast Fourier Transform (FFT) on that autocorrelation function in order to obtain the PSD of a non-stationary signal such as an EEG signal [38]. Autocorrelation function ( of the normalized beta EEG signal ().where is the signal and N the length of .
- PSD estimation from normalized EEG signal ().
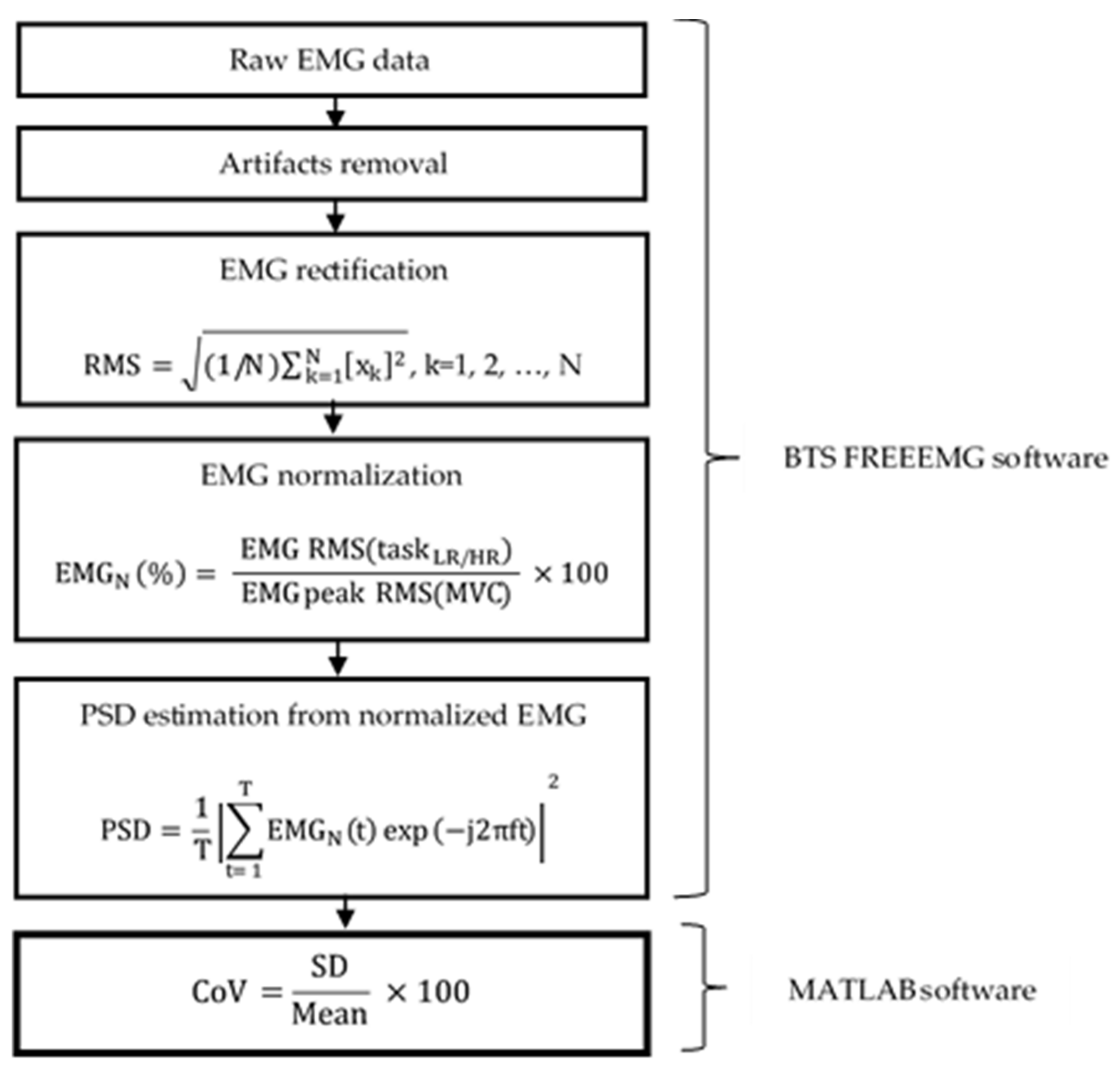
2.3.2. EMG Measurement and Processing
2.4. Statistical Analysis
3. Results
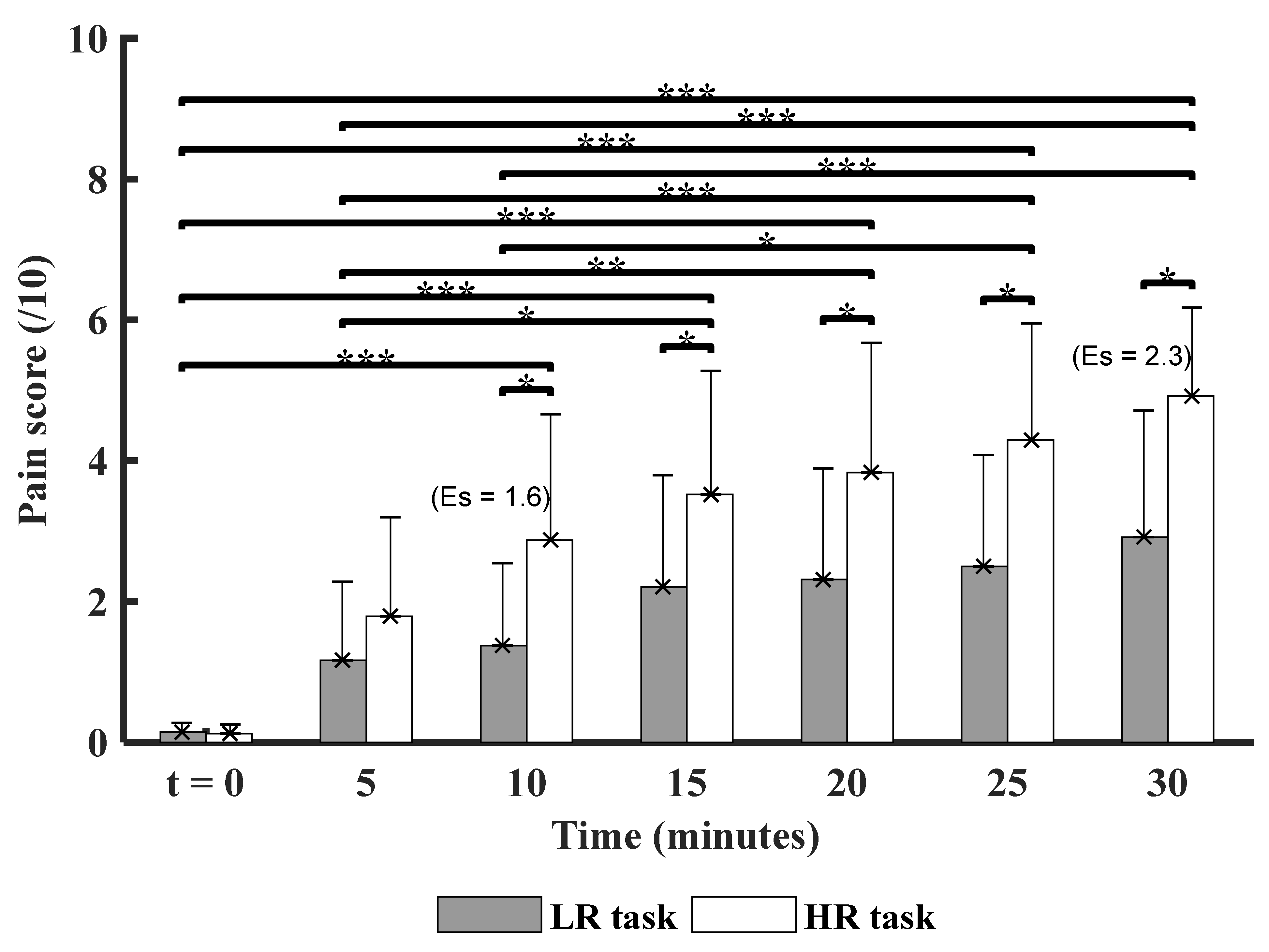
3.1. Time and Experimental Conditions Factor on the Pain Score
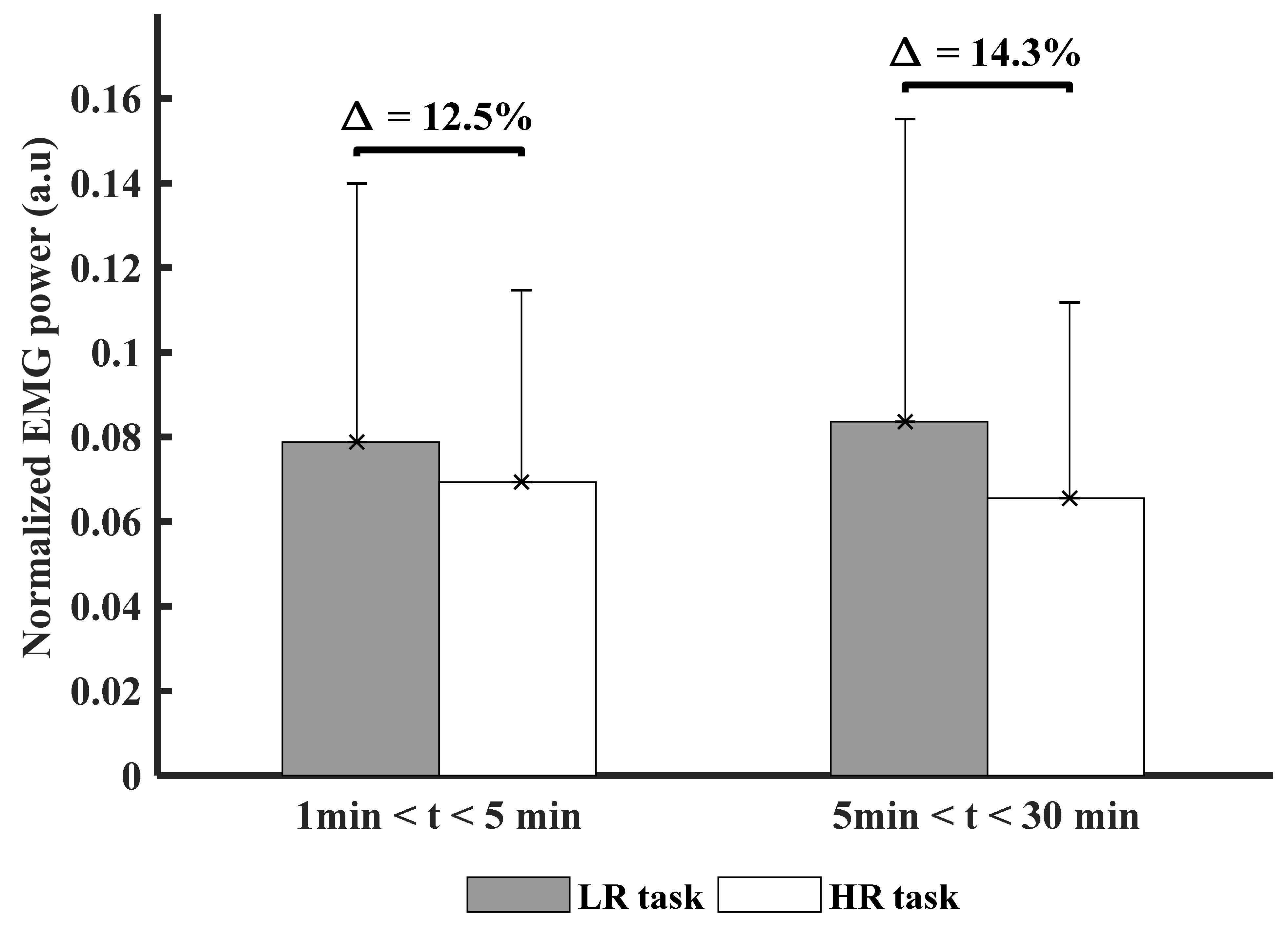
3.2. Time and Experimental Conditions Factor on Muscle Activity (PSD)
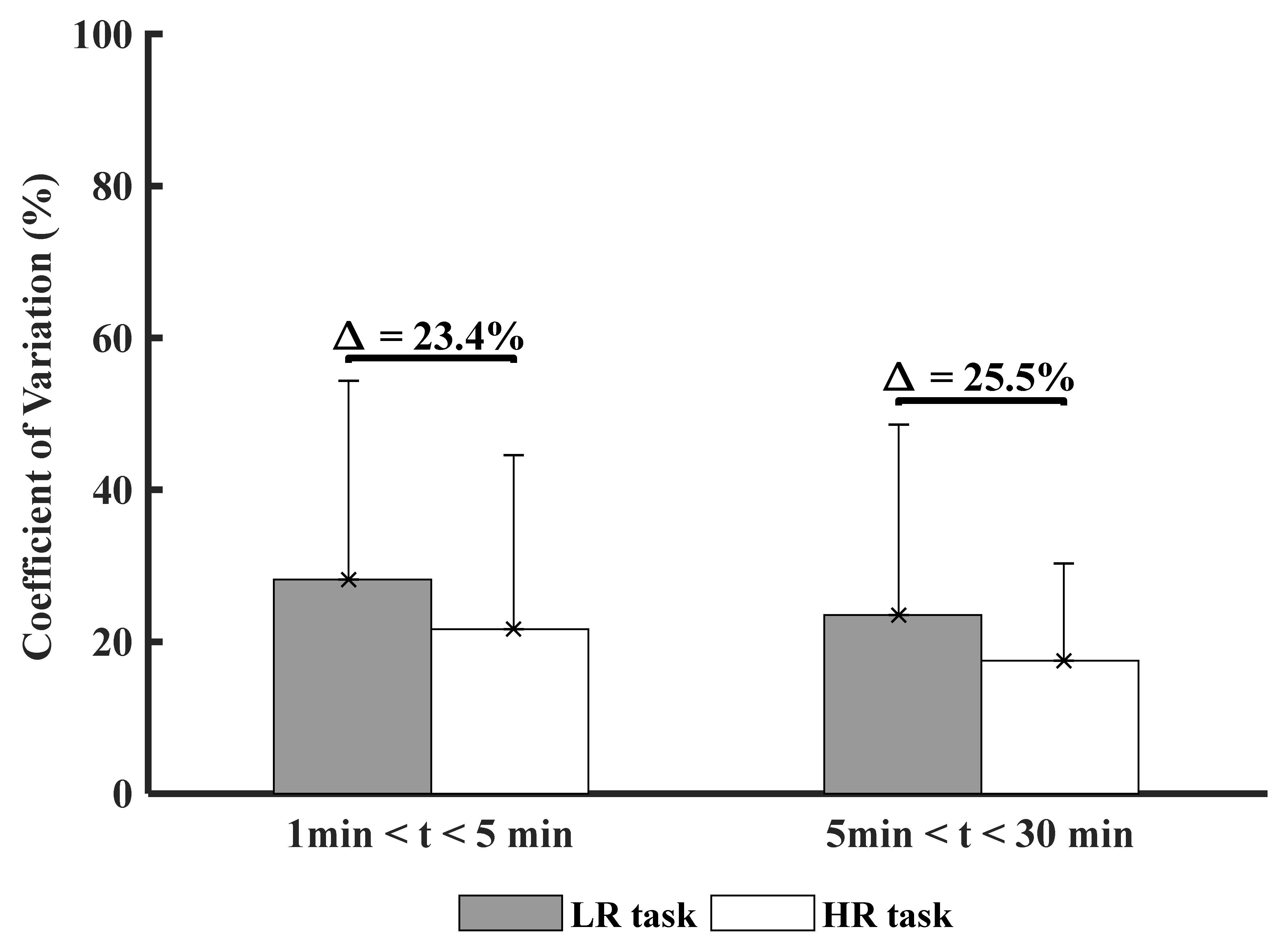
3.3. Time and Experimental Conditions Factor on Muscle Variability (CoV)
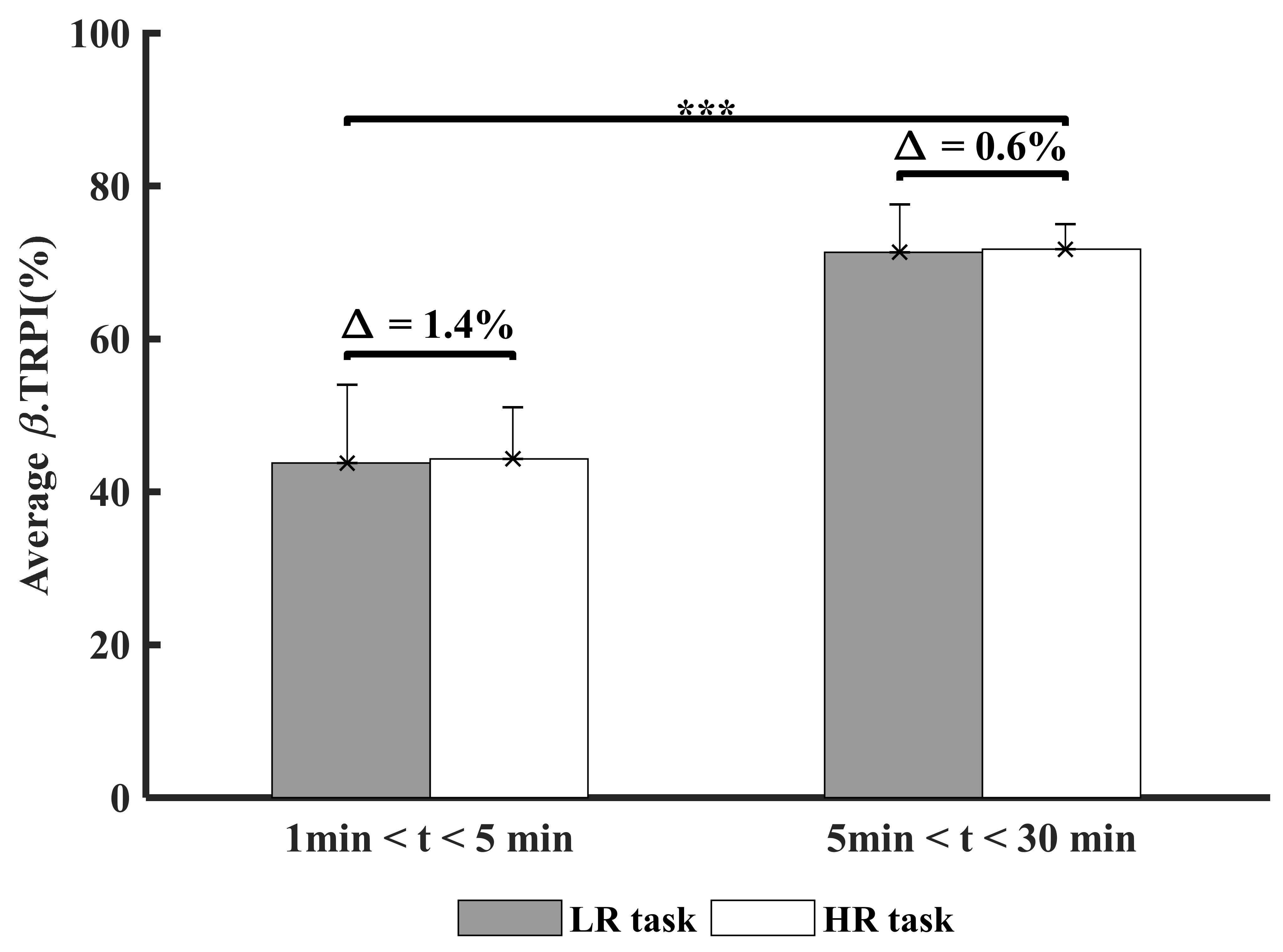
3.4. Time and Experimental Conditions Factor Cortical Inhibition (β-TRPI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stock, S.F.; Delisle, A.A.; St-Vincent, M.; Turcot, A.; Messing, K. Troubles musculo-squelettique. In Enquête Québécoise sur des Conditions de Travail, D’Emploi, de Santé et de Sécurité du Travail (EQCOTESST); Québec—Institut de Recherche Robert-Sauvé en Santé et Sécurité du Travail: Montreal, QC, Canada, 2011; Chapter 7. [Google Scholar]
- Woolf, A.D. Global burden of osteoarthritis and musculoskeletal diseases. BMC Musculoskelet. Disord. 2015, 16, S3. (In English) [Google Scholar] [CrossRef][Green Version]
- Nordander, C.; Ohlsson, K.; Åkesson, I.; Arvidsson, I.; Balogh, I.; Hansson, G.Å.; Strömberg, U.; Rittner, R.; Skerfving, S. Risk of musculoskeletal disorders among females and males in repetitive/constrained work. Ergonomics 2009, 52, 1226–1239. (In English) [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, N.; Motamedzade, M.; Jamshidi, A.A.; Darvishi, E.; Moghimbeygi, A.; Moghadam, R.H. Upper trapezius fatigue in carpet weaving: The impact of a repetitive task cycle. Int. J. Occup. Saf. Ergon. JOSE 2018, 24, 41–51. (In English) [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, M.; Erb, M.D.; Nichols, J.M.; Joiner, K.L.; Weeks, J.B. Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve 2001, 24, 98–106. (In English) [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M.; The, D.J.; Doldo, N.A.; Ploutz-Snyder, L.L. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J. Appl. Physiol. 2003, 94, 2263–2272. (In English) [Google Scholar] [CrossRef]
- Da Silva, R.A.; Lariviere, C.; Arsenault, A.B.; Nadeau, S.; Plamondon, A. The comparison of wavelet- and Fourier-based electromyographic indices of back muscle fatigue during dynamic contractions: Validity and reliability results. Electromyography Clin. Neurophysiol. 2008, 48, 147–162. (In English) [Google Scholar]
- Da Silva, R.A.; Vieira, E.R.; Cabrera, M.; Altimari, L.R.; Aguiar, A.F.; Nowotny, A.H.; Carvalho, A.F.; Oliveira, M.R. Back muscle fatigue of younger and older adults with and without chronic low back pain using two protocols: A case-control study. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2015, 25, 928–936. (In English) [Google Scholar] [CrossRef]
- Lariviere, C.; Arsenault, A.B.; Gravel, D.; Gagnon, D.; Loisel, P. Evaluation of measurement strategies to increase the reliability of EMG indices to assess back muscle fatigue and recovery. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2002, 12, 91–102. (In English) [Google Scholar] [CrossRef]
- Nasseroleslami, B.; Lakany, H.; Conway, B.A. EEG signatures of arm isometric exertions in preparation, planning and execution. Neuroimage 2014, 90, 1–14. (In English) [Google Scholar] [CrossRef]
- Tecchio, F.; Zappasodi, F.; Porcaro, C.; Barbati, G.; Assenza, G.; Salustri, C.; Rossini, P.M. High-gamma band activity of primary hand cortical areas: A sensorimotor feedback efficiency index. Neuroimage 2008, 40, 256–264. (In English) [Google Scholar] [CrossRef] [PubMed]
- Borghuis, J.; Hof, A.L.; Lemmink, K.A. The importance of sensory-motor control in providing core stability: Implications for measurement and training. Sports Med. 2008, 38, 893–916. (In English) [Google Scholar] [CrossRef]
- Neuper, C.; Wortz, M.; Pfurtscheller, G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 2006, 159, 211–222. (In English) [Google Scholar]
- Pfurtscheller, G. Quantification of ERD and ERS in the time domain. In Event-Related Desynchronization. Handbook of Electroencephalography and Clinical Neurophysiology; Elsevier BV: Amsterdam, The Netherlands, 1999; pp. 89–105. [Google Scholar]
- Hummel, F.; Andres, F.; Altenmüller, E.; Dichgans, J.; Gerloff, C. Inhibitory control of acquired motor programmes in the human brain. Brain 2002, 125, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Ushiba, J.; Kimura, A.; Liu, M.; Tomita, Y. Correlation between EEG-EMG coherence during isometric contraction and its imaginary execution. Acta Neurobiol Exp. 2010, 70, 76–85. [Google Scholar]
- Descatha, A.; Roquelaure, Y.; Chastang, J.F.; Evanoff, B.; Melchior, M.; Mariot, C.; Ha, C.; Imbernon, E.; Goldberg, M.; Leclerc, A. Validity of Nordic-style questionnaires in the surveillance of upper-limb work-related musculoskeletal disorders. Scand. J. Work. Environ. Health 2007, 33, 58–65. (In English) [Google Scholar] [CrossRef]
- Descatha, A.; Roquelaure, Y.; Chastang, J.F.; Evanoff, B.; Cyr, D.; Leclerc, A. Work, a prognosis factor for upper extremity musculoskeletal disorders? Occup Environ. Med. 2009, 66, 351–352. (In English) [Google Scholar] [CrossRef]
- Andersen, J.H.; Haahr, J.P.; Frost, P. Risk factors for more severe regional musculoskeletal symptoms: A two-year prospective study of a general working population. Arthritis Rheum. 2007, 56, 1355–1364. (In English) [Google Scholar] [CrossRef] [PubMed]
- Husemann, B.; von Mach, C.Y.; Borsotto, D.; Zepf, K.I.; Scharnbacher, J. Comparisons of musculoskeletal complaints and data entry between a sitting and a sit-stand workstation paradigm. Hum. Factors 2009, 51, 310–320. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sorensen, C.J.; Johnson, M.B.; Callaghan, J.P.; George, S.Z.; van Dillen, L.R. Validity of a paradigm for low back pain symptom development during prolonged standing. Clin. J. Pain 2015, 31, 652–659. (In English) [Google Scholar] [CrossRef]
- Kozak, A.; Wirth, T.; Verhamme, M.; Nienhaus, A. Musculoskeletal health, work-related risk factors and preventive measures in hairdressing: A scoping review. J. Occup. Med. Toxicol. 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Silverstein, B.A.; Fine, L.J.; Armstrong, T.J. Hand wrist cumulative trauma disorders in industry. Br. J. Ind. Med. 1986, 43, 779–784. (In English) [Google Scholar] [CrossRef] [PubMed]
- Spielholz, P.; Silverstein, B.; Morgan, M.; Checkoway, H.; Kaufman, J. Comparison of self-report, video observation and direct measurement methods for upper extremity musculoskeletal disorder physical risk factors. Ergonomics 2001, 44, 588–613. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. (In English) [Google Scholar] [CrossRef] [PubMed]
- Stytsenko, K.; Jablonskis, E.; Prahm, C. Evaluation of consumer EEG device Emotiv EPOC. In Proceedings of the MEi CogSci Conference, Ljubljana, Slovenia, 17–18 June 2011. [Google Scholar]
- Kilavik, B.E.; Zaepffel, M.; Brovelli, A.; MacKay, W.A.; Riehle, A. The ups and downs of beta oscillations in sensorimotor cortex. Exp. Neurol. 2013, 245, 15–26. (In English) [Google Scholar] [CrossRef] [PubMed]
- Bartur, G.; Pratt, H.; Soroker, N. Changes in mu and beta amplitude of the EEG during upper limb movement correlate with motor impairment and structural damage in subacute stroke. Clin. Neurophysiol. 2019, 130, 1644–1651. [Google Scholar] [CrossRef]
- Aboalayon, K.A.; Almuhammadi, W.S.; Faezipour, M.; Aboalayon, K.A.; Almuhammadi, W.S.; Faezipour, M. A comparison of different machine learning algorithms using single channel EEG signal for classifying human sleep stages. In 2015 Long Island Systems, Applications and Technology; IEEE: Piscataway, NJ, USA, 2015; pp. 1–6. [Google Scholar]
- Chen, X.; Liu, A.; Chiang, J.; Wang, Z.J.; McKeown, M.J.; Ward, R.K. Removing muscle artifacts from EEG data: Multichannel or single-channel techniques? IEEE Sens. J. 2015, 16, 1986–1997. [Google Scholar] [CrossRef]
- Joyce, C.A.; Gorodnitsky, I.F.; Kutas, M. Automatic removal of eye movement and blink artifacts from EEG data using blind component separation. Psychophysiology 2004, 41, 313–325. [Google Scholar] [CrossRef]
- Jiang, X.; Bian, G.-B.; Tian, Z. Removal of artifacts from EEG signals: A review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef]
- Molina, V.; Bachiller, A.; Gomez-Pilar, J.; Lubeiro, A.; Hornero, R.; Cea-Cañas, B.; Valcárcel, C.; Haidar, M.K.; Poza, J. Deficit of entropy modulation of the EEG in schizophrenia associated to cognitive performance and symptoms. A replication study. Schizophr. Res. 2018, 195, 334–342. [Google Scholar] [CrossRef]
- Power, J.D.; Lynch, C.J.; Dubin, M.J.; Silver, B.M.; Martin, A.; Jones, R.M. Characteristics of respiratory measures in young adults scanned at rest, including systematic changes and “missed” deep breaths. Neuroimage 2020, 204, 116234. [Google Scholar] [CrossRef]
- Wang, D.; Miao, D.; Blohm, G. Multi-class motor imagery EEG decoding for brain-computer interfaces. Front. Neurosci. 2012, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Huang, G.; Tu, Y.; Tan, A.; Hung, Y.S.; Zhang, Z. Normalization of pain-evoked neural responses using spontaneous EEG improves the performance of EEG-based cross-individual pain prediction. Front. Comput. Neurosci. 2016, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Mangia, A.L.; Ursino, M.; Lannocca, M.; Cappello, A. Transcallosal inhibition during motor imagery: Analysis of a neural mass model. Front. Comput. Neurosci. 2017, 11, 57. [Google Scholar] [CrossRef]
- Al-Nashash, H.; Tong, S.; Thakor, N.V. Single-channel EEG analysis. In Quantitative EEG Analysis Methods and Clinical Applications; Artech House: New York, NY, USA, 2009; pp. 70–126. [Google Scholar]
- Makeig, S.; Debener, S.; Onton, J.; Delorme, A. Mining event-related brain dynamics. Trends Cogn. Sci. 2004, 8, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hatta, T.; Giambini, H.; Sukegawa, K.; Yamanaka, Y.; Sperling, J.W.; Steinmann, S.P.; Itoi, E.; An, K.N. Quantified mechanical properties of the deltoid muscle using the shear wave elastography: Potential implications for reverse shoulder arthroplasty. PLoS ONE 2016, 11, e0155102. [Google Scholar] [CrossRef]
- Boettcher, C.E.; Ginn, K.A.; Cathers, I. Standard maximum isometric voluntary contraction tests for normalizing shoulder muscle EMG. J. Orthop. Res. Off. Publ. Orthop. Res. 2008, 26, 1591–1597. (In English) [Google Scholar] [CrossRef]
- Kumar, D.K.; Pah, N.D.; Bradley, A. Wavelet analysis of surface electromyography. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 400–406. [Google Scholar] [CrossRef]
- Czaprowski, D.; Afeltowicz, A.; Gębicka, A.; Pawłowska, P.; Kędra, A.; Barrios, C.; Hadała, M. Abdominal muscle EMG-activity during bridge exercises on stable and unstable surfaces. Phys. Ther. Sport 2014, 15, 162–168. [Google Scholar] [CrossRef]
- Burden, A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2010, 20, 1023–1035. (In English) [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, H.; Chan, S.; Luk, K.; Hu, Y. Time-dependent power spectral density estimation of surface electromyography during isometric muscle contraction: Methods and comparisons. J. Electromyogr. Kinesiol. 2010, 20, 89–101. [Google Scholar] [CrossRef]
- Terrier, P.; Schutz, Y. Variability of gait patterns during unconstrained walking assessed by satellite positioning (GPS). Eur. J. Appl. Physiol. 2003, 90, 554–561. [Google Scholar] [CrossRef]
- Bosch, T.; Mathiassen, S.E.; Visser, B.; Looze, M.D.; Dieën, J.V. The effect of work pace on workload, motor variability and fatigue during simulated light assembly work. Ergonomics 2011, 54, 154–168. [Google Scholar] [CrossRef]
- Van Dieën, J.H.; der Putten, E.P.W.; Kingma, I.; de Looze, M.P. Low-level activity of the trunk extensor muscles causes electromyographic manifestations of fatigue in absence of decreased oxygenation. J. Electromyogr. Kinesiol. 2009, 19, 398–406. [Google Scholar] [CrossRef]
- Madeleine, P.; Mathiassen, S.E.; Arendt-Nielsen, L. Changes in the degree of motor variability associated with experimental and chronic neck–shoulder pain during a standardised repetitive arm movement. Exp. Brain Res. 2008, 185, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Mathiass, S.; Aminoff, T. Motor control and cardiovascular responses during isoelectric contractions of the upper trapezius muscle: Evidence for individual adaptation strategies. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 434–444. [Google Scholar] [CrossRef]
- Cohen, J. Some issues in power analysis. In Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013; pp. 542–553. [Google Scholar]
- Punnett, L.; Wegman, D.H. Work-related musculoskeletal disorders: The epidemiologic evidence and the debate. J. Electromyogr. Kinesiol. 2004, 14, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int J. Sports Phys. Ther. 2014, 9, 726–736. (In English) [Google Scholar] [PubMed]
- Srinivasan, D.; Mathiassen, S.E. Motor variability in occupational health and performance. Clin. Biomech. 2012, 27, 979–993. (In English) [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-R.; He, L.-H.; Wu, S.-S.; Li, J.-Y.; Ye, K.-P.; Sheng, W. Quantify work load and muscle functional activation patterns in neck-shoulder muscles of female sewing machine operators using surface electromyogram. Chin. Med. J. 2011, 124, 3731–3737. [Google Scholar]
- Bohannon, R.W. Differentiation of maximal from submaximal static elbow flexor efforts by measurement variability. Am. J. Phys. Med. Rehabil. 1987, 66, 213–218. [Google Scholar] [CrossRef]
- Simonsen, J.C. Coefficient of variation as a measure of subject effort. Arch. Phys. Med. Rehabil. 1995, 76, 516–520. [Google Scholar] [CrossRef]
- Harber, P.; SooHoo, K. Static ergonomic strength testing in evaluating occupational back pain. J. Occup. Med. Off. Publ. Ind. Med. Assoc. 1984, 26, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Lin, J.H.; Buchholz, B.; Xu, X. Shoulder muscle fatigue development in young and older female adults during a repetitive manual task. Ergonomics 2014, 57, 1201–1212. (In English) [Google Scholar] [CrossRef]
- Cowley, J.C.; Gates, D.H. Influence of remote pain on movement control and muscle endurance during repetitive movements. Exp. Brain Res. 2018, 236, 2309–2319. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kaiser, V.; Bauernfeind, G.; Kreilinger, A.; Kaufmann, T.; Kübler, A.; Neuper, C.; Müller-Putz, G.R. Cortical effects of user training in a motor imagery based brain–computer interface measured by fNIRS and EEG. NeuroImage 2014, 85, 432–444. [Google Scholar] [CrossRef]
- Huysmans, M.; Hoozemans, M.; van der Beek, A.; de Looze, M.; van Dieën, J. Fatigue effects on tracking performance and muscle activity. J. Electromyogr. Kinesiol. 2008, 18, 410–419. [Google Scholar] [CrossRef]
- Moseley, G.L.; Hodges, P.W. Reduced variability of postural strategy prevents normalization of motor changes induced by back pain: A risk factor for chronic trouble? Behav. Neurosci. 2006, 120, 474. [Google Scholar] [CrossRef]
- Parker, R.S.; Lewis, G.N.; Rice, D.A.; McNair, P.J. Is motor cortical excitability altered in people with chronic pain? A systematic review and meta-analysis. Brain Stimul. 2016, 9, 488–500. [Google Scholar] [CrossRef]
- Ngomo, S.; Mercier, C.; Bouyer, L.J.; Savoie, A.; Roy, J.-S. Alterations in central motor representation increase over time in individuals with rotator cuff tendinopathy. Clin. Neurophysiol. 2015, 126, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Schwenkreis, P.; Scherens, A.; Rönnau, A.K.; Höffken, O.; Tegenthoff, M.; Maier, C. Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC Neurosci. 2010, 11, 73. (In English) [Google Scholar] [CrossRef]
- Jodoin, M.; Rouleau, D.M.; Bellemare, A.; Provost, C.; Larson-Dupuis, C.; Sandman, É.; Laflamme, G.Y.; Benoit, B.; Leduc, S.; Levesque, M.; et al. Moderate to severe acute pain disturbs motor cortex intracortical inhibition and facilitation in orthopedic trauma patients: A TMS study. PLoS ONE 2020, 15, e0226452. (In English) [Google Scholar] [CrossRef] [PubMed]
- Le Pera, D.; Graven-Nielsen, T.; Valeriani, M.; Oliviero, A.; Di Lazzaro, V.; Tonali, P.A.; Arendt-Nielsen, L. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin. Neurophysiol. 2001, 112, 1633–1641. [Google Scholar] [CrossRef]
- Thunberg, J.; Ljubisavljevic, M.; Djupsjöbacka, M.; Johansson, H. Effects on the fusimotor-muscle spindle system induced by intramuscular injections of hypertonic saline. Exp. Brain Res. 2002, 142, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Graven-Nielsen, T.; Svensson, P.; Arendt-Nielsen, L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control 1997, 105, 156–164. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Lund, H.; Arendt-Nielsen, L.; Danneskiold-Samsøe, B.; Bliddal, H. Inhibition of maximal voluntary contraction force by experimental muscle pain: A centrally mediated mechanism. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2002, 26, 708–712. [Google Scholar] [CrossRef]
- Greenberg, D.L. Evaluation and treatment of shoulder pain. Med. Clin. 2014, 98, 487–504. [Google Scholar] [CrossRef]
- Luime, J.; Koes, B.W.; Hendriksen, I.J.M.; Burdorf, A.; Verhagen, A.P.; Miedema, H.S.; Verhaar, J.A.N. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand. J. Rheumatol. 2004, 33, 73–381. [Google Scholar] [CrossRef]
- Crippa, M.; Torri, D.; Fogliata, L.; Belleri, L.; Alessio, L. Implementation of a health education programme in a sample of hairdressing trainees. Med. Lav. 2007, 98, 48. [Google Scholar]
- Hanvold, T.N.; Wærsted, M.; Mengshoel, A.M.; Bjertness, E.; Twisk, J.; Veiersted, K.B. A longitudinal study on risk factors for neck and shoulder pain among young adults in the transition from technical school to working life. Scand. J. Work Environ. Health 2014, 40, 597–609. [Google Scholar] [CrossRef]
- Aweto, H.A.; Tella, B.A.; Johnson, O.Y. Prevalence of work-related musculoskeletal disorders among hairdressers. Int. J. Occup. Med. Environ. Health 2015, 28, 545. [Google Scholar] [CrossRef]
| Characteristics | Men (n = 10) | Women (n = 2) | Mean |
|---|---|---|---|
| Age (years) | 26.3 ± 4.3 | 29.0 ± 2.8 | 27.6 ± 3.5 |
| Height (cm) | 178.2 ± 6.7 | 165 ± 8.5 | 171.6 ± 7.6 |
| Weight (kg) | 75.8 ± 9.1 | 69.5 ± 14.8 | 72.6 ± 11.9 |
| Body mass index | 24.0 ± 2.9 | 25.3 ± 2.9 | 24.2 ± 2.7 |
| Baseline | 1 min < t ≤ 5 min | 5 min < t ≤ 30 min | |||||
|---|---|---|---|---|---|---|---|
| LR | HR | LR | HR | LR | HR | ||
| Pain scores (/10) | - | - | 1.2 ±1.1 | 1.8 ± 1.4 | 2.3 ± 1.5 | 3.9 ± 1.6 | |
| EMG | PSDEMG (V2/Hz) | - | 0.08 ± 0.06 | 0.07 ± 0.04 | 0.07 ± 0.07 | 0.06 ± 0.05 | |
| CoVEMG (%) | - | 28.2 ± 26.2 | 21.6 ± 22.9 | 23.5 ± 25.1 | 17.5 ± 12.8 | ||
| EEG | β.TRPD (%) | 39 ± 1.8 | 56.2 ± 10.3 | 55.7 ± 6.7 | 28.6 ± 6.3 | 28.3 ± 3.3 | |
| β.TRPI (%) | 61 ± 1.8 | 43.7 ± 10.3 | 44.3 ± 6.7 | 71.3 ± 6.3 | 71.7 ± 3.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segning, C.M.; Ezzaidi, H.; da Silva, R.A.; Ngomo, S. A Neurophysiological Pattern as a Precursor of Work-Related Musculoskeletal Disorders Using EEG Combined with EMG. Int. J. Environ. Res. Public Health 2021, 18, 2001. https://doi.org/10.3390/ijerph18042001
Segning CM, Ezzaidi H, da Silva RA, Ngomo S. A Neurophysiological Pattern as a Precursor of Work-Related Musculoskeletal Disorders Using EEG Combined with EMG. International Journal of Environmental Research and Public Health. 2021; 18(4):2001. https://doi.org/10.3390/ijerph18042001
Chicago/Turabian StyleSegning, Colince Meli, Hassan Ezzaidi, Rubens A. da Silva, and Suzy Ngomo. 2021. "A Neurophysiological Pattern as a Precursor of Work-Related Musculoskeletal Disorders Using EEG Combined with EMG" International Journal of Environmental Research and Public Health 18, no. 4: 2001. https://doi.org/10.3390/ijerph18042001
APA StyleSegning, C. M., Ezzaidi, H., da Silva, R. A., & Ngomo, S. (2021). A Neurophysiological Pattern as a Precursor of Work-Related Musculoskeletal Disorders Using EEG Combined with EMG. International Journal of Environmental Research and Public Health, 18(4), 2001. https://doi.org/10.3390/ijerph18042001






