Chronic Exposure to Lead and Cadmium in Residents Living near a Zinc Smelter
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area and Participants
2.2. Questionnaire
2.3. Sampling and Analytical Methods
2.4. Statistical Analysis
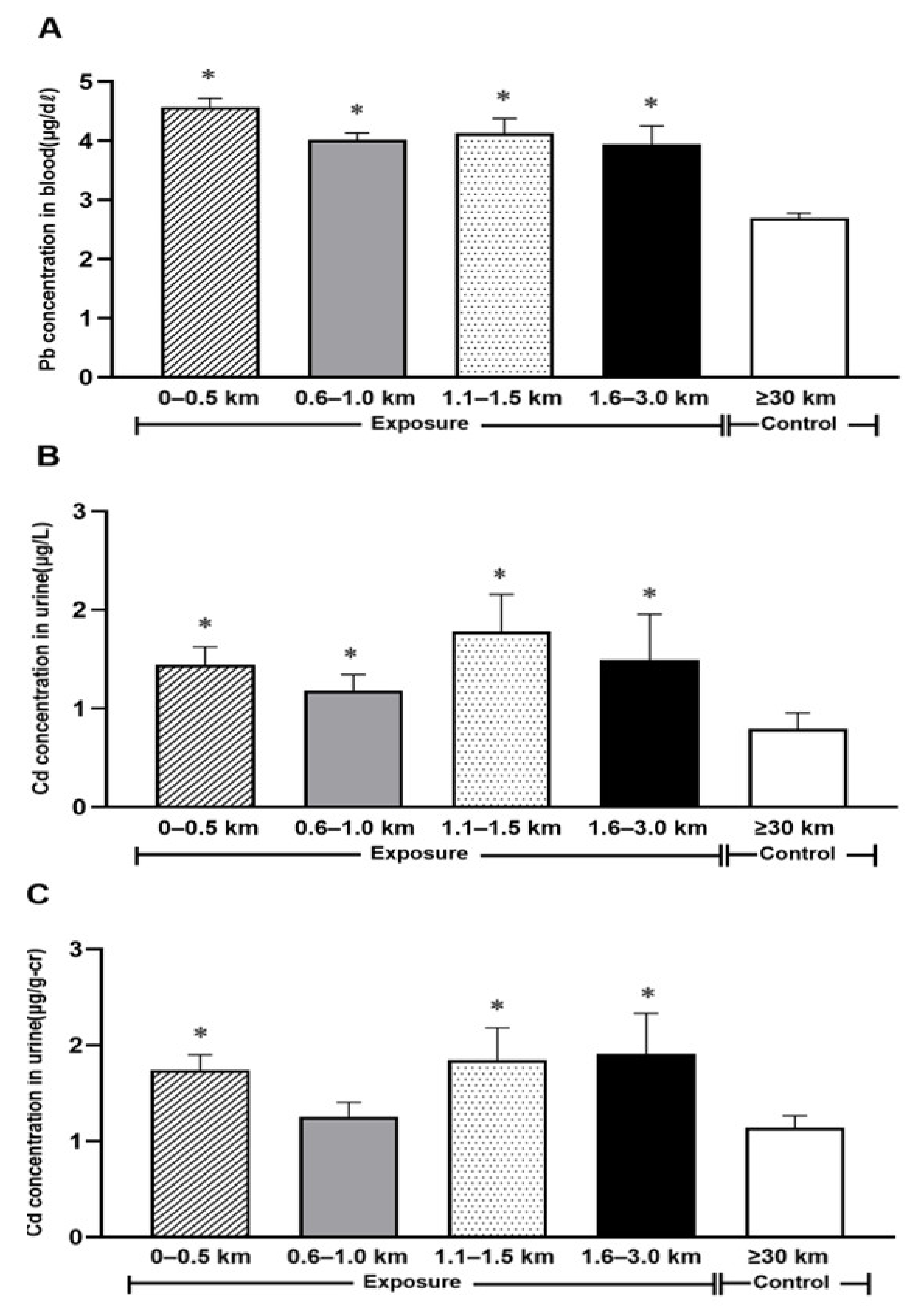
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kříbek, B.; Majer, V.; Knésl, I.; Keder, J.; Mapani, B.; Kamona, F.; Mihaljevič, M.; Ettler, V.; Penížek, V.; Vaněk, A.; et al. Contamination of soil and grass in the Tsumeb smelter area, Namibia: Modeling of contaminants dispersion and ground geochemical verification. Appl. Geochem. 2016, 64, 75–97. [Google Scholar] [CrossRef]
- Soto-Jiménez, M.F.; Flegal, A.R. Childhood lead poisoning from the smelter in Torreón, México. Environ. Res. 2011, 111, 590–596. [Google Scholar] [CrossRef]
- Benin, A.L.; Sargent, J.D.; Dalton, M.; Roda, S. High concentrations of heavy metals in neighborhoods near ore smelters in northern Mexico. Environ. Health Perspect. 1999, 107, 279–284. [Google Scholar] [CrossRef]
- Yang, Y.-G.; Jin, Z.-S.; Bi, X.-Y.; Li, F.-L.; Li, S.; Liu, J.; Fu, Z.-Y. Atmospheric Deposition-Carried Pb, Zn, and Cd from a Zinc Smelter and Their Effect on Soil Microorganisms. Pedosphere 2009, 19, 422–433. [Google Scholar] [CrossRef]
- ChosunBiz(Online Newspaper). Available online: https://biz.chosun.com/site/data/html_dir/2018/09/21/2018092102108.html (accessed on 26 September 2018).
- Korea Ministry of Environment. Survey of Environmental Effects in the Region Surrounding Seokpo Zinc Smelter; Korean Ministry of Environment: Sejong, Korea, 2016.
- Environment and Labor Committee. State of Heavy Metal Pollution by Young Poong Seokpo Smelter; Environment and Labor Committee: Seoul, Korea, 2014.
- World Health Organization Regional Office for Europe Copenhagen. Air Quality Guidelines for Europe, 2nd ed.; European Series, No. 91; WHO Regional Publications: Geneva, Switzerland, 2000. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Draft Toxicological Profile for Cadmium Agency for Toxic Substances and Disease Registry; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2008. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf (accessed on 15 September 2012).
- Järup, L.; Berglund, M.; Elinder, C.G.; Nordberg, G.; Vahter, M. Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health 1998, 24, 1–52. [Google Scholar]
- Thomas, L.D.K.; Hodgson, S.; Nieuwenhuijsen, M.; Jarup, L. Early kidney damage in a population exposed to cadmium and other heavy metals. Environ. Health Perspect. 2009, 117, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-D.; Eom, S.-Y.; Yim, D.-H.; Kim, I.-S.; Won, H.-K.; Park, C.-H.; Kim, G.-B.; Yu, S.-D.; Choi, B.-S.; Park, J.-D.; et al. Environmental exposure to arsenic, lead, and cadmium in people living near Janghang copper smelter in Korea. J. Korean Med. Sci. 2016, 31, 489–496. [Google Scholar] [CrossRef]
- Skerfving, S.; Gerhardsson, L.; Schütz, A.; Strömberg, U. Lead-biological monitoring of exposure and effects. J. Trace Elem. Exp. Med. 1998, 11, 289–301. [Google Scholar] [CrossRef]
- Bertke, S.J.; Lehman, E.J.; Wurzelbacher, S.J.; Hein, M.J. Mortality of lead smelter workers: A follow-up study with exposure assessment. Am. J. Ind. Med. 2016, 59, 979–986. [Google Scholar] [CrossRef]
- Reilly, R.; Spalding, S.; Walsh, B.; Wainer, J.; Pickens, S.; Royster, M.; Villanacci, J.; Little, B.B. Chronic environmental and occupational lead exposure and kidney function among African Americans: Dallas lead project II. Int. J. Environ. Res. Public Health 2018, 15, 2875. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Research Institute. Study on Biological Exposure Evaluation Criteria and Analysis Methods III–10 Heavy Metals; Occupational Safety and Health Research Institute: Incheon, Korea, October 2010; Available online: http://www.kosha.or.kr/oshri/publication/researchReportSearch.do?mode=download&articleNo=63144&attachNo=56829.
- World Health Organization. Biological Monitoring of Chemical Exposure in the Workplace Guidelines Volume 1; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Korea National Institute of Environmental Research. Korean National Environmental Health Survey (KoNEHS)—Annual Report on cycle 3, 3rd year (2017); Korea National Institute of Environmental Research: Incheon, Korea, 2017. [Google Scholar]
- Korean Statistical Information Service. Available online: http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01 (accessed on 7 July 2020).
- Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Health Canada. Results of the Canadian Health Measures Survey Cycle 5 (2016–2017); Health Canada: Ottawa, ON, Canada, 2019.
- Betts, K.S. CDC updates guidelines for children’s lead exposure. Environ. Health Perspect. 2012, 120, A268. [Google Scholar] [CrossRef]
- National Toxicology Program. Health Effects of Low-Level Lead; National Toxicology Program: Research Triangle Park, NC, USA, 2012.
- Ettinger, A.S.; Leonard, M.L.; Mason, J. CDC’s lead poisoning prevention program: A long-standing responsibility and commitment to protect children from lead exposure. J. Public Health Manag. Pract. 2019, 25, S5. [Google Scholar] [CrossRef] [PubMed]
- Human-Biomonitoring (HBM) Values, Derived by the Human Biomonitoring Commission of the German Environment Agency. Available online: https://www.umweltbundesamt.de/en/image/current-human-biomonitoring-hbm-values-for-blood (accessed on 15 March 2020).
- Eom, S.-Y.; Yim, D.-H.; Moon, S.-I.; Ochirpurev, B.; Choi, Y.-S.; Park, Y.-S.; Kim, Y.-S.; Yu, S.-D.; Choi, B.-S.; Park, J.-D.; et al. The association of blood concentrations of heavy metals and blood pressure in residents living near Janghang copper smelter in Korea. J. Agric. Med. Community Health 2017, 42, 13–23. [Google Scholar] [CrossRef][Green Version]
- Karita, K.; Shinozaki, T.; Yano, E.; Amari, N. Blood lead levels in copper smelter workers in Japan. Ind. Health 2000, 38, 57–61. [Google Scholar] [CrossRef]
- Garçon, G.; Leleu, B.; Marez, T.; Zerimech, F.; Haguenoer, J.M.; Furon, D.; Shirali, P. Biomonitoring of the adverse effects induced by the chronic exposure to lead and cadmium on kidney function: Usefulness of alpha-glutathione S-transferase. Sci. Total Environ. 2007, 377, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wilhelma, M.; Ewersb, U.; Schulzc, C. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int. J. Hyg. Environ. Health 2004, 207, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Zhang, Z.-W.; Moon, C.-S.; Shimbo, S.; Nakatsuka, H.; Matsuda-Inoguchi, N.; Higashikawa, K.; Ikeda, M. Cadmium exposure of women in general populations in Japan during 1991–1997 compared with 1977–1981. Int. Arch. Occup. Environ. Health 2000, 73, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-W.; Pan, W.-H.; Liou, S.-H.; Sun, C.-W.; Huang, P.-C.; Wang, S.-L. Levels and temporal variations of urinary lead, cadmium, cobalt, and copper exposure in the general population of Taiwan. Environ. Sci. Pollut. Res. 2019, 26, 6048–6064. [Google Scholar] [CrossRef] [PubMed]
- Bråtveit, M.; Magerøy, N.; Gundersen, H.; Vahter, M.; Moen, B.E. Biomarker of chronic cadmium exposure in a population residing in the vicinity of a zinc producing plant. Sci. Total Environ. 2011, 409, 4222–4228. [Google Scholar] [CrossRef]
- Järup, L.; Hellström, L.; Alfvén, T.; Carlsson, M.D.; Grubb, A.; Persson, B.; Pettersson, C.; Spång, G.; Schütz, A.; Elinder, C.-G. Low level exposure to cadmium and early kidney damage: The OSCAR study. Occup. Environ. Med. 2000, 57, 668–672. [Google Scholar] [CrossRef]
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Jiao, S.; Zhao, K.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 37585. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Exposure to Cadmium: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Bernald, A. Cadmium & its adverse effects on human health. India. J. Med. Res. 2008, 128, 557–564. [Google Scholar]
- Vahter, M.; Gochfeld, M.; Casati, B.; Thiruchelvam, M.; Falk-Filippson, A.; Kavlock, R.; Marafante, E.; Cory-Slechta, D. Implications of gender differences for human health risk assessment and toxicology. Environ. Res. 2007, 104, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.; Akesson, A.; Lidén, C.; Ceccatelli, S.; Berglund, M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007, 104, 85–95. [Google Scholar] [CrossRef]
- ATSDR. Public Health Statement Cadmium; ATSDR: Atlanta, GA, USA, 2012. [Google Scholar]
- Muntner, P.; Menke, A.; DeSalvo, K.B.; Rabito, F.A.; Batuman, V. Continued decline in blood lead levels among adults in the United States: The National Health and Nutrition Examination Surveys. Int. Arch. Med. 2005, 165, 2155–2161. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. Available online: https://health.cdc.go.kr/healthinfo/biz/health/gnrlzHealthInfo/gnrlzHealthInfo/gnrlzHealthInfoView.do?cntnts_sn=16 (accessed on 13 October 2015).
- Bæcklund, M.; Pedersen, N.L.; Björkman, L.; Vahter, M. Variation in blood concentrations of cadmium and lead in the elderly. Environ. Res. 1999, 80, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Kim, K.-Y.; Kim, D.-W.; Choi, S.-J.; Kim, H.-S.; Choi, B.-S.; Choi, M.-K.; Park, J.-D. The relation between blood lead concentration, epidemiologic factors and body iron status. J. Environ. Toxicol. 2006, 21, 153–163. [Google Scholar]
- Yun, S.-H.; Kim, C.-Y.; Hwang, T.-Y.; Won, K.-C.; Do, J.-Y.; Lee, S.-J.; Park, Y.-M.; Jun, K.-S.; Lee, G.-H.; Lee, D.-Y.; et al. The concentration of cadmium in urine, and its role in health-risk assessment of residents in the vicinity of abandoned mines in Gyeongsangbuk-do, Korea. Korean J. Occup. Environ. Med. 2010, 22, 251–261. [Google Scholar] [CrossRef]
- Joo, Y.-K.; Kwon, Y.-M.; Kim, S.-Y.; Choi, K.-H.; Lee, C.-W.; Yu, S.-D.; Yoo, J.-Y. A study on heavy metals exposure and major sociodemographic influence factors among Korean adults—Korean National Environmental Health Survey (2009–2017). J. Environ. Health Sci. 2019, 45, 541–555. [Google Scholar]
- Im, J.-Y.; Chung, E.-K.; Park, H.-J.; Yu, S.-D.; Jang, B.-K.; Son, B.-S. A study on concentrations of heavy metal in blood and urine of local area in Korea. J. Environ. Sci. 2013, 22, 59–72. [Google Scholar]
- Vahter, M.; Berglund, M.; Åkesson, A.; Lidén, C. Metals and women’s health. Environ. Res. 2002, 88, 145–155. [Google Scholar] [CrossRef]
- World Health Organization. Inorganic Lead; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Kim, N.-S.; Lee, B.-K. National estimates of blood lead, cadmium, and mercury levels in the Korean general adult population. Int. Arch. Occup. Environ. Health 2011, 84, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Lee, S.-W.; Ahn, R.-M.; Kim, J.-H.; Son, B.-S. A study on the concentration of biomarkers for heavy metals and VOCs in the residents living in the vicinity of Gwangyang Industrial Complex in Korea. J. Odor Indoor Environ. 2019, 18, 228–235. [Google Scholar] [CrossRef]
- Rossi, E. Low level environmental lead exposure—A continuing challenge. Clin. Biochem. Rev. 2008, 29, 63–70. [Google Scholar]
- Riederer, A.M.; Belova, A.; George, B.J.; Anastas, P.T. Urinary cadmium in the 1999–2008 U.S. National Health and Nutrition Examination Survey (NHANES). Environ. Sci. Technol. 2013, 47, 1137–1147. [Google Scholar] [CrossRef]
- Moon, S.-W.; Gwak, J.-I.; Park, Y.-H. The effect of smoking and second-hand smoking on the concentration of mercury, lead and cadmium in the blood: Based on the Fifth Korea National Health and Nutrition Examination Survey. Korean J. Fam. Pract. 2016, 6, 44–48. [Google Scholar] [CrossRef]
- Al-Ghabban, S.I. Blood lead levels among cigarette smokers. Kufa Med. J. 2018, 9, 431–435. [Google Scholar]
- Mannino, D.M.; Homa, D.M.; Matte, T.; Hernandez-Avila, M. Active and passive smoking and blood lead levels in U.S. adults: Data from the Third National Health and Nutrition Examination Survey. Nicotine Tobacco Res. 2005, 7, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-Y.; Lee, J.-H.; Paek, D.-M.; Lee, J.-T. Blood levels of lead, cadmium, and mercury in the Korean population: Results from the Second Korean National Human Exposure and Bio-monitoring Examination. Environ. Res. 2009, 109, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Effects of Smoking on Concentration of Heavy Metals in Blood and Clinical Characteristics. Available online: http://www.nih.go.kr/filepath/boardDownload.es?bid=0034&list_no=75528&seq=1 (accessed on 20 July 2017).
- Polańska, K.; Hanke, W.; Sobala, W.; Trzcinka-Ochocka, M.; Ligocka, D.; Strugała-Stawik, H.; Magnu, P. Predictors of environmental lead exposure among pregnant women—A prospective cohort study in Poland. Ann. Agric. Environ. Med. 2014, 21, 49–54. [Google Scholar]
- Li, L.; Zhang, Y.; Ippolito, J.A.; Xing, W.; Qiu, K.; Yang, H. Lead smelting effects heavy metal concentrations in soils, wheat, and potentially humans. Environ. Pollut. 2020, 257, 113641. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.J.; Betty, E.E.; Page, A.L.; Elliott, R.A. Occurrence of naturally high cadmium levels in soils and its accumulation by vegetation. J. Environ. Qual. 1981, 10, 551–556. [Google Scholar] [CrossRef]
- Democratic Party of Korea. Available online: https://www.hanjeoungae.com/category/%EC%9D%98%EC%A0%95%ED%99%9C%EB%8F%99/%EB%B3%B4%EB%8F%84%EC%9E%90%EB%A3%8C?page=92 (accessed on 23 October 2014).
- Korea Ministry of Environment. Soil Environment Conservation Act and the Enforcement Decree of the Soil Environment Conservation Act; Korea Ministry of Environment: Sejong Special Self-Governing City, Korea, 2009.
- Saha, N.; Zaman, M.R. Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 2013, 185, 3867–3878. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Zhu, G.; Nordberg, G.F.; Jin, T.; Ding, X. The association between cumulative cadmium intake and osteoporosis and risk of fracture in a Chinese population. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 435–443. [Google Scholar] [CrossRef]
- Yang, J.; Ma, S.; Zhou, J.C.; Song, Y.W.; Li, F. Heavy metal contamination in soils and vegetables and health risk assessment of inhabitants in Daye, China. J. Int. Med. Res. 2018, 46, 3374–3387. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S. Response and follow-up bias in cohort studies. Am. J. Epidemiol. 1977, 106, 184–187. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Exposure Group | Control Group | Total | p-Value |
|---|---|---|---|---|
| Total | 549 (67.4%) | 265 (32.6%) | 814 (100%) | |
| Sex | ||||
| Men | 236 (43.0%) | 99 (37.4%) | 335 (41.2%) | 0.124 |
| Women | 313 (57.0%) | 166 (62.6%) | 479 (58.8%) | |
| Age (years) | ||||
| 20 to 49 | 140 (25.5%) | 22 (8.3%) | 162 (19.9%) | <0.001 |
| 50 to 59 | 104 (18.9%) | 37 (14.0%) | 141 (17.3%) | |
| 60 to 69 | 139 (25.3%) | 75 (28.3%) | 214 (26.3%) | |
| ≥70 | 166 (30.2%) | 131 (49.4%) | 297 (36.5%) | |
| Mean ± SD | 60.3 ± 15.0 | 67.7 ± 12.1 | 62.7 ± 14.5 | <0.001 |
| Duration of Residence | ||||
| < 10 years | 167 (30.4%) | 56 (21.1%) | 223 (27.3%) | 0.005 |
| ≥ 10 years | 380 (69.2%) | 209 (78.9%) | 589 (72.3%) | |
| Missing data | 3 (0.4%) | 0 (0%) | 3 (0.4%) | |
| Mean ± SD | 24.0 ± 18.3 | 33.7 ± 22.1 | 27.1 ± 20.1 | <0.001 |
| Experience of Work in a Smelter or Mine | ||||
| No | 270 (49.2%) | 254 (95.8%) | 524 (64.4%) | <0.001 |
| Yes | 277 (50.5%) | 11 (4.2%) | 288 (35.4%) | |
| Missing data | 2 (0.3%) | 0 (0%) | 2 (0.2%) | |
| Smoking | ||||
| Non-smoker | 372 (67.8%) | 188 (70.9%) | 560 (68.8%) | 0.114 |
| Smoker (~20 packs) | 173 (31.5%) | 77 (29.1%) | 250 (30.7%) | |
| Missing data | 4 (0.7%) | 0 (0%) | 4 (0.5%) | |
| Alcohol Drinking | ||||
| No | 307 (55.9%) | 132 (49.8%) | 439 (53.9%) | 0.414 |
| Yes | 239 (43.5%) | 132 (49.8%) | 371 (45.6%) | |
| Missing data | 3 (0.6%) | 1 (0.4%) | 4 (0.5%) | |
| Beans | ||||
| Store-bought | 389 (70.9%) | 122 (46.0%) | 511 (62.8%) | 0.001 |
| Locally grown | 125 (22.8%) | 139 (52.5%) | 264 (32.4%) | |
| Not consumed | 27 (4.9%) | 3 (1.1%) | 30 (3.7%) | |
| Missing data | 8 (1.5%) | 1 (0.4%) | 9 (1.1%) | |
| Vegetables | ||||
| Store-bought | 283 (51.5%) | 79 (29.8%) | 362 (44.5%) | <0.001 |
| Locally grown | 255 (46.4%) | 183 (69.1%) | 438 (53.8%) | |
| Not consumed | 7 (1.3%) | 2 (0.8%) | 9 (1.1%) | |
| Missing data | 4 (0.7%) | 1 (0.4%) | 5 (0.6%) | |
| Potatoes | ||||
| Store-bought | 318 (57.9%) | 90 (34.0%) | 408 (50.1%) | <0.001 |
| Locally grown | 213 (38.8%) | 174 (65.7%) | 387 (47.5%) | |
| Not consumed | 13 (2.4%) | 1 (0.4%) | 14 (1.7%) | |
| Missing data | 5 (0.9%) | 0 (0%) | 5 (0.6%) |
| GM ± GSD | Percentiles | ||||||
|---|---|---|---|---|---|---|---|
| 10th | 25th | 50th | 75th | 90th | |||
| All participants | Exposure Group | ||||||
| B_Pb [μg/dL] | 4.19 ± 1.87 * | 1.96 | 2.88 | 4.20 | 6.22 | 8.68 | |
| U_Cd [μg/L] | 1.32 ± 2.63 * | 0.39 | 0.73 | 1.43 | 2.47 | 4.12 | |
| U_Cd [μg/g-cr] | 1.47 ± 2.36 * | 0.50 | 0.89 | 1.47 | 2.73 | 4.03 | |
| Control Group | |||||||
| B_Pb [μg/dL] | 2.70 ± 1.39 | 1.78 | 2.17 | 2.69 | 3.30 | 4.15 | |
| U_Cd [μg/L] | 0.80 ± 2.57 | 0.23 | 0.45 | 0.81 | 1.55 | 2.60 | |
| U_Cd [μg/g-cr] | 1.14 ± 1.86 | 0.52 | 0.75 | 1.17 | 1.73 | 2.44 | |
| Participants without occupational exposure (history) | Exposure Group | ||||||
| B_Pb [μg/dL] | 3.47 ± 1.70 * | 1.77 | 2.53 | 3.41 | 4.96 | 6.76 | |
| U_Cd [μg/L] | 1.36 ± 2.58 * | 0.41 | 0.76 | 1.48 | 2.43 | 4.13 | |
| U_Cd [μg/g-cr] | 1.57 ± 2.33 * | 0.55 | 0.94 | 1.69 | 2.85 | 4.06 | |
| Control Group | |||||||
| B_Pb [μg/dL] | 2.67 ± 1.39 | 1.78 | 2.14 | 2.64 | 3.27 | 4.09 | |
| U_Cd [μg/L] | 0.80 ± 2.60 | 0.22 | 0.45 | 0.82 | 1.61 | 2.61 | |
| U_Cd [μg/g-cr] | 1.16 ± 1.86 | 0.52 | 0.75 | 1.19 | 1.80 | 2.47 | |
| Number | B_Pb [μg/dL] | U_Cd [μg/L] | U_Cd [μg/g-cr] | |
|---|---|---|---|---|
| Sex | ||||
| Men | 236 | 5.31 | 1.08 | 1.05 |
| Women | 313 | 3.50 | 1.54 | 1.91 |
| p-value | <0.001 | <0.001 | <0.001 | |
| Age (years) | ||||
| 20 to 49 | 140 | 3.53 | 0.67 | 0.68 |
| 50 to 59 | 104 | 4.77 | 1.38 | 1.55 |
| 60 to 69 | 139 | 4.57 | 1.54 | 1.77 |
| ≥70 | 166 | 4.15 | 2.02 | 2.30 |
| p-value | 0.015 | <0.001 | <0.001 | |
| Duration of Residence | ||||
| <10 years | 167 | 4.03 | 1.01 | 1.03 |
| ≥10 years | 380 | 4.26 | 1.49 | 1.76 |
| p-value | 0.849 | <0.001 | <0.001 | |
| Experience of Work (in smelter or mine) | ||||
| No | 270 | 3.47 | 1.36 | 1.03 |
| Yes | 277 | 5.03 | 1.29 | 1.72 |
| p-value | <0.001 | 0.556 | 0.093 | |
| Smoking (~20 packs) | ||||
| Non-smoker | 369 | 3.63 | 1.46 | 1.68 |
| Smoker | 177 | 5.66 | 1.07 | 1.13 |
| p-value | <0.001 | 0.001 | <0.001 | |
| Alcohol Drinking | ||||
| No | 307 | 4.50 | 1.20 | 1.30 |
| Yes | 239 | 3.84 | 1.51 | 1.72 |
| p-value | 0.001 | 0.016 | <0.001 | |
| Beans | ||||
| Store-bought | 389 | 4.27 | 1.30 | 1.41 |
| Locally grown | 125 | 4.04 | 1.54 | 1.76 |
| p-value | 0.072 | 0.035 | 0.017 | |
| Vegetables | ||||
| Store-bought | 283 | 4.04 | 1.18 | 1.32 |
| Locally grown | 255 | 4.38 | 1.54 | 1.70 |
| p-value | 0.420 | 0.001 | 0.001 | |
| Potatoes | ||||
| Store-bought | 318 | 4.18 | 1.17 | 1.30 |
| Locally grown | 213 | 4.24 | 1.65 | 1.82 |
| p-value | 0.574 | <0.001 | <0.001 |
| Number | B_Pb [μg/dL] | U_Cd [μg/L] | U_Cd [μg/g-cr] | |
|---|---|---|---|---|
| Sex | ||||
| Male | 99 | 3.06 | 0.73 | 0.82 |
| Female | 166 | 2.50 | 0.84 | 1.40 |
| p-value | 0.005 | 0.517 | <0.001 | |
| Age (years) | ||||
| 20 to 49 | 22 | 2.50 | 0.63 | 0.80 |
| 50 to 59 | 37 | 2.79 | 0.77 | 1.15 |
| 60 to 69 | 75 | 2.81 | 0.78 | 1.08 |
| ≥70 | 131 | 2.64 | 0.85 | 1.29 |
| p-value | 0.099 | 0.595 | 0.019 | |
| Duration of Residence | ||||
| <10 years | 55 | 2.82 | 0.81 | 1.11 |
| ≥10 years | 209 | 2.66 | 0.79 | 1.17 |
| p-value | 0.921 | 0.705 | 0.587 | |
| Experience of Work (in smelter or mine) | ||||
| No | 254 | 2.67 | 0.80 | 1.15 |
| Yes | 11 | 3.35 | 0.80 | 0.88 |
| p-value | 0.162 | 0.806 | 0.145 | |
| Smoking (~20 packs) | ||||
| Non-smoker | 188 | 2.58 | 0.75 | 1.21 |
| Smoker | 77 | 2.99 | 0.91 | 1.02 |
| p-value | 0.146 | 0.113 | 0.052 | |
| Alcohol Drinking | ||||
| No | 132 | 2.94 | 0.81 | 1.08 |
| Yes | 132 | 2.48 | 0.79 | 1.23 |
| p-value | 0.012 | 0.545 | 0.063 | |
| Beans | ||||
| Store-bought | 122 | 2.65 | 0.86 | 1.18 |
| Locally grown | 139 | 2.75 | 0.75 | 1.14 |
| p-value | 0.128 | 0.277 | 0.684 | |
| Vegetables | ||||
| Store-bought | 79 | 2.67 | 0.84 | 1.15 |
| Locally grown | 183 | 2.71 | 0.79 | 1.17 |
| p-value | 0.572 | 0.745 | 0.838 | |
| Potatoes | ||||
| Store-bought | 90 | 2.69 | 0.90 | 1.25 |
| Locally grown | 174 | 2.69 | 0.75 | 1.10 |
| p-value | 0.328 | 0.391 | 0.156 |
| Heavy Metals | Exposure Factors | Unstandardized Beta | Standardized Beta | t | p-Value |
|---|---|---|---|---|---|
| B_Pb [μg/dL] | Sex (vs. male) | −0.163 | −0.137 | −3.204 | 0.001 |
| Age | 0.005 | 0.121 | 3.611 | <0.001 | |
| Occupational history at smelter or mine (vs. no) | 0.247 | 0.201 | 5.230 | <0.001 | |
| Smoking (vs. non-smoker) | 0.173 | 0.136 | 3.361 | 0.001 | |
| Alcohol drinking (vs. no) | −0.086 | −0.072 | −2.195 | 0.028 | |
| Distance from smelter (vs. ≥30 km) | |||||
| (vs. ≤0.5 km) | 0.408 | 0.275 | 7.104 | <0.001 | |
| (vs. 0.6–1.0 km) | 0.307 | 0.251 | 6.065 | <0.001 | |
| (vs. 1.1–1.5 km) | 0.335 | 0.141 | 4.139 | <0.001 | |
| (vs. 1.6–3.0 km) | 0.311 | 0.101 | 3.111 | 0.002 | |
| U_Cd [μg/g-cr] | Sex (vs. male) | 0.571 | 0.359 | 10.712 | <0.001 |
| Age | 0.024 | 0.425 | 12.906 | <0.001 | |
| Occupational history at smelter or mine (vs. no) | 0.159 | 0.098 | 2.601 | 0.009 | |
| Distance from smelter (vs. ≥30 km) | |||||
| (vs. ≤0.5 km) | 0.515 | 0.263 | 6.714 | <0.001 | |
| (vs. 0.6–1.0 km) | 0.345 | 0.212 | 5.047 | <0.001 | |
| (vs. 1.1–1.5 km) | 0.500 | 0.159 | 4.729 | <0.001 | |
| (vs. 1.6–3.0 km) | 0.317 | 0.074 | 2.279 | 0.023 | |
| Locally grown vegetable (vs. buying) | 0.103 | 0.065 | 2.057 | 0.040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Kim, G.; Chang, J.; Lee, K.; Lee, C.; Lee, B. Chronic Exposure to Lead and Cadmium in Residents Living near a Zinc Smelter. Int. J. Environ. Res. Public Health 2021, 18, 1731. https://doi.org/10.3390/ijerph18041731
Jo H, Kim G, Chang J, Lee K, Lee C, Lee B. Chronic Exposure to Lead and Cadmium in Residents Living near a Zinc Smelter. International Journal of Environmental Research and Public Health. 2021; 18(4):1731. https://doi.org/10.3390/ijerph18041731
Chicago/Turabian StyleJo, HyeJeong, GeunBae Kim, JunYoung Chang, Kwan Lee, ChulWoo Lee, and BoEun Lee. 2021. "Chronic Exposure to Lead and Cadmium in Residents Living near a Zinc Smelter" International Journal of Environmental Research and Public Health 18, no. 4: 1731. https://doi.org/10.3390/ijerph18041731
APA StyleJo, H., Kim, G., Chang, J., Lee, K., Lee, C., & Lee, B. (2021). Chronic Exposure to Lead and Cadmium in Residents Living near a Zinc Smelter. International Journal of Environmental Research and Public Health, 18(4), 1731. https://doi.org/10.3390/ijerph18041731







