Abstract
The incessant release of pharmaceuticals into the aquatic environment continues to be a subject of increasing concern. This is because of the growing demand for potable water sources and the potential health hazards which these pollutants pose to aquatic animals and humans. The inability of conventional water treatment systems to remove these compounds creates the need for new treatment systems in order to deal with these class of compounds. This review focuses on advanced oxidation processes that employ graphene-based composites as catalysts for the degradation of pharmaceuticals. These composites have been identified to possess enhanced catalytic activity due to increased surface area and reduced charge carrier recombination. The techniques employed in synthesizing these composites have been explored and five different advanced oxidation processes—direct degradation process, chemical oxidation process, photocatalysis, electrocatalyis processes and sonocatalytic/sono-photocatalytic processes—have been studied in terms of their enhanced catalytic activity. Finally, a comparative analysis of the processes that employ graphene-based composites was done in terms of process efficiency, reaction rate, mineralization efficiency and time required to achieve 90% degradation.
1. Introduction
Pharmaceuticals are compounds that are produced for the purpose of usage as medicinal drugs. They remain however as persistent, biologically active compounds which could be detected in drinking and surface waters due to their incomplete removal by conventional processes [1]. These materials have received much attention recently due to their health and environmental implication [2,3,4]. The design of pharmaceuticals is such that when taken even at low concentrations, they can effect physiological changes in humans and animals. These compounds, when excreted either as metabolites or in an unchanged state, remain in the environment for a long time because they are not biologically degraded or reduced [5,6]. In aquatic environments, direct discharge of untreated and treated wastewater from treatment plants are the major source of pharmaceuticals, while terrestrial runoff from agricultural fields, aquaculture facilities and livestock farms are some of the secondary sources as shown in Figure 1 [7,8]. Aquatic channels act as transport routes for pharmaceuticals and their bioaccumulation is mainly due to their lipophilic character, although other factors such as animal homeostasis, metabolism and inhalation exposure are also important in understanding their accumulation in organisms [9,10,11,12]. Compared to other organic contaminants, hydrophobic interactions are not the main influencing parameter on the partitioning dynamics of most pharmaceuticals. Other factors including cation exchange, surface complexation, hydrogen bonding and cation bridging also play vital roles. Thus, modelling approaches that have been created for other organic contaminants such as persistent organic pollutants are inappropriate for assessing the environmental risks of pharmaceuticals [13].
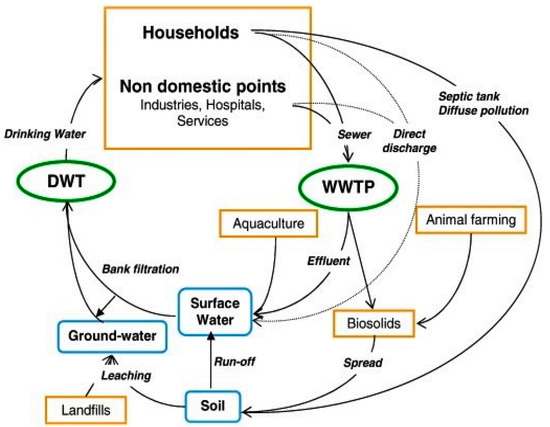
Figure 1.
Origins of pharmaceuticals and their environmental routes (WWTP—wastewater treatment plant; DWT—drinking water treatment). Reprinted form Sayadi, et al. [20].
The increasing surge in the global consumption of pharmaceuticals with the global pharmaceutical market worth expected to reach $1170 billion in 2021 from $935 billion in 2017. This implies that concerns over the pollution of the environment by the discharge of different types of pharmaceuticals will continue to grow. This account for them being tagged as part of the emerging anthropogenic hazard pollutants [14,15]. Also, their presence in the environment is due mainly to the inability of treatment plants to completely eliminate them. This, consequently, has led to their detection at concentration range of ng/L to μg/L in surface water, tap water and ground water [16,17,18]. The increase in the concentration of pharmaceuticals in the water system, implies that the reuse of treated water, which has been suggested as the adequate solution for the sustainability of water resource is also under serious treat [19].
Pharmaceuticals and their by-products could be classified into 24 therapeutic classes, with anti-inflammatory drugs (NSAIDs) (e.g., ibuprofen, gemfibrozil and diclofenac), antibiotics (e.g., sulfamethoxazole and trimethoprim), anticonvulsants (e.g., carbamazepine) and lipid regulators (propranolol, metoprolol, nadolol) being the four most studied categories. Increasing information on the potential chronic or acute effects of pharmaceuticals on the ecosystem and living organisms have begun to emerge recently. Estrogen acts as endocrine modulators or disruptors and can have adverse effect on reproductive and sexual development like feminization of male fishes even at ng/L level [18,21]. Diclofenac has been indicted for its effect on mammalian kidney and the disappearance of the Oriental White backed Vulture in India and Pakistan [22]. The increased resistance to antimicrobial agents is another risk associated with the presence of pharmaceuticals in the environment [23]. Table 1 presents some therapeutic classes of pharmaceuticals with their acute toxicity level and concentrations reported in the environment.

Table 1.
Some therapeutic classes of pharmaceuticals, toxicity level and their level of detection in the environment.
Since conventional water and wastewater treatment processes (WWTP) are incapable of completely removing pharmaceuticals, it is therefore important for advanced treatment techniques to be incorporated into WWTPs. Some of the advanced processes that have been explored for the removal of pharmaceuticals include chemical oxidation processes, membrane technologies and adsorption [36]. Membrane technologies and adsorption process are economical physical techniques, with relative ease of handling; however, these processes are highly material and energy intensive [37] and the problem of membrane retentate and spent adsorbent disposal means there is high possibility of reintroducing the removed contaminants back into the environment. Thus much focus has beenon oxidation processes, in which contaminants are degraded by highly reactive radical species species generated within the system. These processes are referred to as advanced oxidation process (AOPs) and as shown in Figure 2, there is a continual growth in the body of literature on their application in pharmaceutical degradation. Advanced oxidation process is an aqueous phase oxidation technique that is based on the action of highly reactive species such as hydroxyl radical (•OH), superoxide anion radical (, singlet oxygen ( and hydrogen peroxide [38]. Contaminants’ degradation in AOPs are stimulated either through radiolysis, sonolysis or photolysis. While radiolysis and sonolysis generate radicals in aqueous media without the use of chemical oxidants, photolysis may require the involvement of a catalyst or precursor. AOPs is comprised of a large range of technologies such as electrolysis, Fenton and photo-Fenton processes, wet air oxidation, ionization radiation, pulsed plasma, microwave, chemical oxidation processes, UV-based processes, supercritical water oxidation, and photocatalytic redox processes [39,40]. Also, hybrid AOPs involving the combination of two or more processes such electro-Fenton, and photo-Fenton processes have also been explored for the degradation of contaminants.
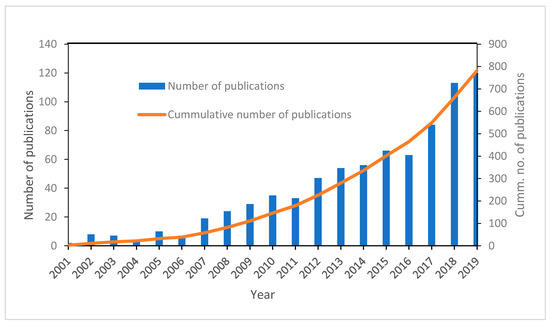
Figure 2.
Timeline distribution of the annual and cumulative number of publications on the use of advanced oxidation processes for the degradation of pharmaceuticals.
Advanced oxidation processes have generated a lot of attention because of their rapid degradation rates for a wide range of chemicals, the ability to convert pollutants into simpler and biodegradable forms and the potential for complete mineralization of most pollutants. In addition, there is no need for secondary treatment of residual solids since no residual solid is generated and no regeneration is required to sustain process efficiency. Despite these advantages of AOPs, the process suffer from some disadvantages, which include: generation of unknown and undesirable transformation products; process may be capital intensive; water quality significantly impede the efficiency of the process and secondary processes for residual oxidant quenching may be required [41].
The use of heterogeneous photocatalytic process (HPPs), among other advanced oxidation processes, has gained much attention in the past three decades and has proven to be a suitable technique for the degradation and mineralization of different contaminants via a series of multi-step reactions to form low molecular weight end products, such as carboxylic acid, CO2 and H2O [42,43]. The preference for HPPs is due mainly to their important, characteristic features which include: (i) ambient operating conditions, such as temperature and pressure (ii) potential mineralization of parent and transformation products without secondary pollution and (iii) economic feasibility and efficiency [44]. A broad class of semiconductor nanomaterials have been explored for HPP processes and could be broadly classified as metals, sulfides [45,46], oxides [47,48], nitrides [49,50], oxysulfides [51], oxyhalides [52,53] and oxynitrides [54,55]. The activity of semiconductors in light-induced redox process sensitization arise from the electronic structure of the metal atoms in chemical compounds. The electronic structure of these compounds is made up of filled valence band (VB) and an empty conduction band (CB) [43].
Supporting catalysts on suitable materials provides routes for the improvement of properties such as quantum efficiency, adsorption capacity, electronic band structure, stability, dispensability and the overall reaction rate of the process. Several class of materials currently being explored as catalyst-support include [56]: (i) carbon-based supports e.g., activated carbon, carbon nanotubes, graphite, graphene/graphene derivatives; (ii) ceramic-based supports e.g., silica, alumina, zirconia, zeolite, and silica carbide; (iii) metal-based supports e.g., chromium, platinum and cupper; and (iv) polymeric-based supports e.g., chitosan, polylactic acid, polyester and polyamides. Graphene or graphene derivatives such graphene oxide, reduced graphene oxide and doped graphene oxides are important class of support materials for catalytic degradation of contaminants in water. Incorporation of catalysts into graphene offer advantages such as high surface area and tensile strength, improved mobility and electron conductivity, lightweight, and stability [57].
There are several reviews on the degradation of pharmaceuticals in water [36,58,59,60], with focus mainly on adsorption and photocatalytic processes. In this present review, the degradation activity of graphene and graphene-based composites in AOPs such as direct catalytic degradation, electron-Fenton and chemical oxidation process, and sonocatalytic/sonophotocatalytic processes will be explored in addition to photocatalytic processes. Also, a comparative analysis of the identified processes will be carried out.
2. Graphene/Graphene Derivatives
Graphene is regarded as the strongest, thinnest and lightest compound known till date, and these properties are related to its structural features such as interlayer crosslinks arising either from covalent bonding between side atoms of different sheets or Van der Waals forces arising from interactions between carbon atoms of different layers of the graphene sheet. Also, intralayer forces, such as the sp2 carbon-carbon covalent bonds and crosslinks occurring at the graphene sheet edges contributes to the unique properties of graphene and its derivatives. Graphene-based materials are therefore outstanding materials for catalyst support in catalysis as they improve the stability and surface area of the catalyst, which is important in bringing the pollutants closer to the catalyst surface where catalytic activities occur.
Perfect graphene has a zero bandgap, and this makes pristine graphene a perfect conductor with high conductivity. The zero Eg of graphene is due to the anti-bonding ℼ* orbitals and bonding ℼ degenerate that meets at the Brillouin zone corners [61]. Doping of graphene leads to the formation of bandgap between the ℼ* and ℼ orbital, thereby converting graphene into a semiconductor and creating more active sites for reactions. Large bandgap could be achieved by high dopant concentration, thus improving the electrical property of graphene [62,63].
Electron arrangement in the graphene lattice is dominated by the s and p orbitals. However, the low dispersibility, difficult recovery and ease of restacking are major drawbacks to its application. This informed the development of many strategies to modify it by incorporating several functional groups such as halogens, chalcogens, boron, nitrogen and phosphorus [64]. Complex functional groups such as aryl and alkyl hydrocarbons have also been reportedly incorporated [65]. The methods of incorporating functional groups into graphene are shown in Figure 3. The most studied functionalized graphenes are graphene oxide (GO) and reduced graphene oxides (rGO), which are derivatives with poorly defined compositions that are strongly influenced by the synthesis method [64]. The abundance amount of oxygen functionalities on the GO surface, coupled with other properties such as structural defects, irregularity of heteroatoms makes them potential materials for specific applications. In graphene oxide, the electronic properties is strongly determined by its chemical structure [66]. Compared to graphene, GO is hydrophilic and an insulator by nature because of the presence of numerous surface hydroxyl (−OH), keto (C=O), epoxy (C-O-C) and carboxy (−COOH) groups [67]. The formation of C-O bond significantly induces the local distortion of graphene, altering the bonding character from planar sp2 to partial sp3 hybridization. By reducing GO, partial restoration of the intrinsic structural and electronic properties of graphene can be restored to produce rGO. However, the removal of oxygen-containing functionalities can lead to the rapid re-aggregation of rGO nanosheets into graphite [68]. Considering an arrangement of epoxy functional groups in fully oxidized graphene sheet and the effect of epoxy arrangement on electronic properties, a significant induction of bandgap of 0.529 eV has been reported [69].
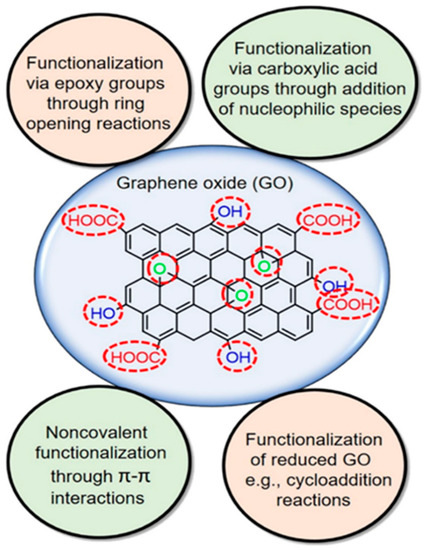
Figure 3.
Different routes to graphene/graphene derivatives functionalization. Reproduced from Bilal, et al. [70]. Copyright (2020) Elsevier.
2.1. Synthesis of Graphene Oxide and Reduced Graphene Oxide
GO is widely employed in several applications because it is easy to synthesize, process and functionalize chemically. It is generally prepared either by the Brodie, Staudenmaier, Hummers, Tours or by modified forms of any of these methods. All the methods involve the oxidation of graphite to varying levels. The wet-chemical oxidative process used to convert graphite into GO involves four steps: (i) intercalation of graphite, (ii) oxidation, (iii) oxidized sheets exfoliation, and (iv) impurity removal [71,72,73,74,75,76]. The intercalation step involves the insertion of small molecules within the graphite layers. Alkali compounds such as NaNO3 and KClO3 are usually intercalated into the graphite layers, which is then subsequently oxidized by acids such as HNO3, HCl and H2SO4. The properties of the final product are influenced by several factors such as graphite’s nature and source, nature and concentration of hydrogen peroxide, oxidant, water and sulfuric acid and physical parameters such as reaction time and temperature. The first reliable method for GO synthesis from graphite was reported in 1855 [77]. It involves the treatment of graphite with fuming HNO3 and KClO3. This method was improved on by using H2SO4 as additive and also the use of excess HNO3 [78]. It gave highly oxidized GO ‘within a shorter period of time’ compared to the Brodie method. The widely used Hummer’s method involves the treatment of graphite with NaNO3, KMnO4 and H2SO4. Recently, the Tour’s method as described by Marcano, et al. [79], introduced the use of H3PO4 as co-acids with H2SO4 and six equivalents of KMnO4 as oxidant.
Electrochemical stripping of graphite is another well explored technique for GO synthesis. This method is a safe, pollution-free, and efficient technique involving no chemical oxidant and achievable within hours or sometimes minutes. It also offers ease of manipulating GO properties such as defect density and degree of oxidation by tuning the electrical process [80,81]. The technique explores the conductivity of graphite to intercalate anions/molecules in the electrolyte under bias current or voltage. The GO is then obtained by ultrasonication of the graphite oxide formed through the oxidation of the graphite intercalation compound by oxygenated species produced by the electrolysis of water [82]. The mechanism of formation of graphene oxide via the electrochemical stripping process is shown in Figure 4. The mechanism could either be anodic or cathodic based on the applied potential. In anodic process, electrons are withdrawn from the graphite electrode leading to the creation of positive charge, allowing for the intercalation of negatively charged ions like sulfate anions. In cathodic process, negative charges created at the graphite electrode attracts positively charge ions. Incorporation of these ions increase the interlayer spacing between the graphene sheets and enhance its exfoliation [83].
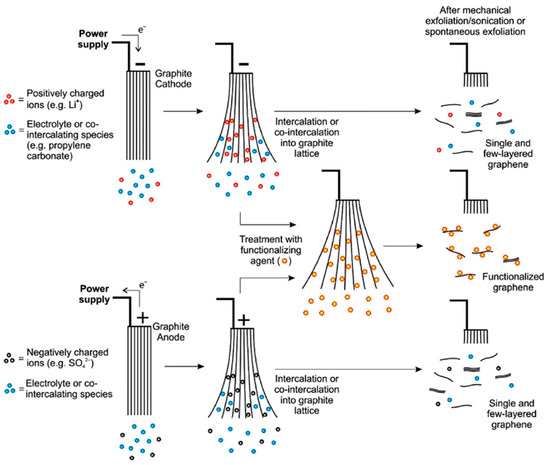
Figure 4.
A schematic overview of mechanism of cathodic and anodic exfoliation. Functionalization of graphene may be achieved either during exfoliation by introducing functionalizing agent or after exfoliation. Reprinted with permission from Yu, et al. [83]. Copyright (2015) Elsevier.
The removal of oxygen functional groups from GO is a subject of continued research, with different methods such as thermal, photochemical, biological and chemical methods some of the already established routes as depicted in Figure 5 [84]. Each method produces rGO of varying properties such as morphology, dispersibility, conductivity and mechanical strength. The choice of the reduction method is influenced by factors such as C/O ratio of end product, selectivity in removal of either −OH, −COOH or C-O-C functionalities, choice of reducing agent, surface defect reduction and improvement in chemical and physical properties [85]. The chemical method is the most explored routes to rGO and it involves treating GO suspension with reducing agents such as hydrazine [86,87,88], ascorbic acid [89,90,91,92], sodium borohydride [93,94,95,96] and sodium hypophosphite monohydrate [97,98]. In a report by Luo, et al. [99], the reduction of GO by N2H4•H2O, NaBH4, NaOH, solvothermal, high-temperature, and a two-step method combining NaBH4 and high temperature was comparatively studied using four criteria: degree of reduction, level of defect repair, dispersibility, and electrical conductivity. The study showed that by combining two reduction processes, the properties of rGO could be greatly enhanced.
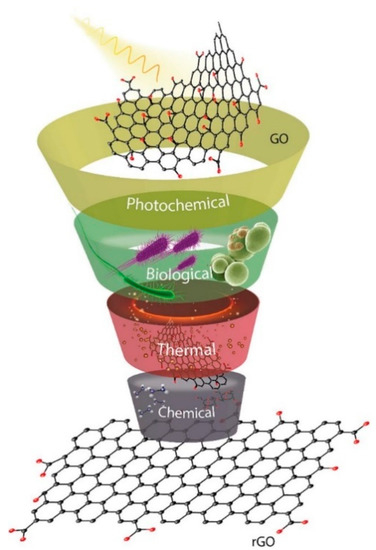
Figure 5.
Routes to reduction of graphene oxide. Reprinted with permission from Agarwal and Zetterlund [100]. Copyright (2021) Elsevier.
Recently, the use of plant extracts as green reductants for rGO synthesis has been studied. The method involves the use of extracts from different plant parts such as seeds, roots, flowers and fruits. The reduction could be initiated by polyphenols (such as catechol and pyrogallol) or flavonoids (glucoside, diosmetin and apigenin) in the plant extracts. According to the mechanism proposed by Bhattacharya, et al. [101], shown in Figure 6, the carboxylic groups in GO reacts with reduced species in plant extracts to form esters by condensation reaction, which is further reduced by ring opening reaction. The hydroxyl groups are reduced by undergoing an initial condensation reaction, which is also followed by ring cleavage, while epoxy group reacts with polyhydroxy groups in extracts to form an intermediate that undergoes subsequent ring formation and cleavage.
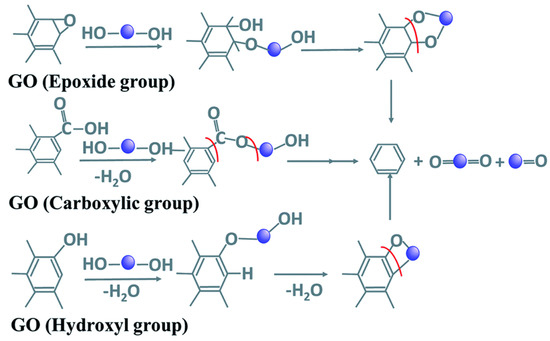
Figure 6.
Reduction of oxygen functionality in GO by plant extracts. Adapted with permission from Bhattacharya, et al. [101] under Creative Commons Attribution 4.0 International.
2.2. Composites of Graphene/Graphene Derivatives
Increased utilization of graphene and its derivatives have motivated the exploration of different functionalization routes. Compositing of graphene with other nanomaterials take advantage of its superior properties to enhance catalytic activities. Graphene possess a two-dimensional carbon network with sp2 hybridization and it exhibits unique properties including high specific surface area, high intrinsic electron mobility and high thermal conductivity [102]. High degradation of pollutants could be achieved by hybridizing graphene with catalysts due to its effective electron conductivity and effective minimization of charge carrier’s recombination via its acceptance of photogenerated electrons.
The improvement of catalytic activity by graphene and its derivatives is mainly due to their ability to extent light absorption range, absorptivity enhancement, enhanced surface area and improved charge separation and transport [103]. The compositing of a photocatalyst results in decreased h+/e− recombination and enhances the electron transfer rate, while also enhancing chemical species adsorption via ℼ-ℼ interactions [104]. Generally, in graphene-based composites, graphene can either act as a support [105], dopant or a coating [106,107]. The three classes of composite could be prepared either via “ex situ” or “in situ” compositing as shown in Figure 7.
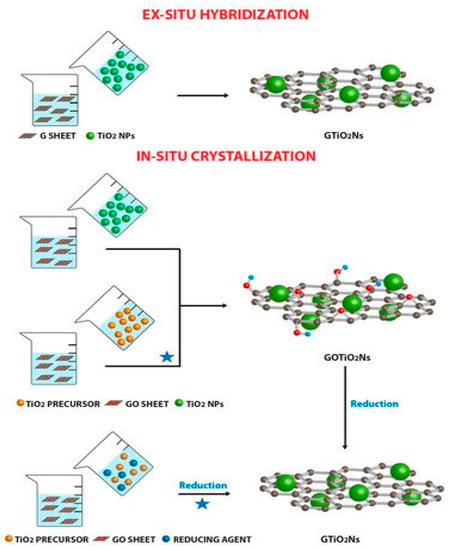
Figure 7.
In-situ and Ex-situ compositing of metal salt with graphene and graphene derivatives. Reproduced with permission from Giovannetti, et al. [108] under Creative Commons Attribution 4.0 International.
Ex situ compositing process involves the mixing of nanoparticles with graphene dispersions. To enhance the process, the graphene sheets may be functionalized through covalent C-C coupling or non-covalent ℼ-ℼ stacking reactions [109]. Ex situ compositing may sometimes result in low density and non-uniform distribution of the nanoparticles on the graphene. Several synthetic routes have been developed based on the ex situ process. AbdelDayem, et al. [110] reported the ex situ compositing of alumina with graphene oxide by mixing pre-prepared alumina with GO solution and refluxing the mixture at 80 °C for 8 h. A two-phase method to the compositing of GO and CdS was reported by Gao, et al. [111]. In this method, synthesized GO and CdS were dispersed in water and toluene respectively. The two solutions were then mixed together and stirred continuously for 24 h, followed by washing and drying. The interaction between the GO functional groups and CdS led to the distribution of the CdS on the GO surface and the aggregation of the CdS particles was minimized by the process. The synthesis of TiO2@rGO through mechanochemical synthesis involving the grinding of TiO2 nanoparticles and rGO was also reported by Deng, et al. [112].
The in-situ method is the most explored for graphene/nanoparticle composites and it involves direct reaction of GO/rGO sheets and nanoparticles’ precursors in solution. The growth of nanoparticles on the surface of GO/rGO have been explored widely through solvothermal and/or hydrothermal techniques. It has been explored frequently in the synthesis of GO/rGO-metal oxide/sulphide nanocomposites. In the in-situ method, nanoparticle nucleation and growth occur on the GO/rGO surface under high-temperature and pressure, with the hydroxyl and carboxyl functional groups acting as nucleation sites for nanoparticles growth via metal-O-C bond formation [113,114,115]. A microwave assisted reduction synthetic method via a mixture of PdCl2, RuCl3 and GO, was employed for the synthesis of bimetallic-graphene composite. Reduction of the metal salts to metallic state was achieved with NaBH4, which was enhanced by holding the mixture in a microwave oven [116]. A method involving the mixture of GO/rGO precursor and nanoparticle precursors was described by Anand, et al. [117] for the synthesis of rGO/Bi2Al4O9. In the synthesis [Bi(NO3)•5H2O] and AlNO3•9H2O were dissolved into the graphene oxide synthesis set-up. The mixture was then stirred for 30 min and further sonicated for 5 min. The process of stirring and sonicating was repeated for 4 h, and the reaction was quenched afterwards. GO reduction to rGO was achieved by adding 10% ascorbic acid solution to the reaction system and heating at 95 °C for 1 h. Cao, et al. [118] reported the combination of precursor mixing and annealing for the in-situ synthesis of ZnO/rGO. In this synthesis route, ZnO powder was firstly mixed with GO solution and the mixture stirred vigorously for 3 h. The mixture was further kept at room temperature for 3 h and the product was collected by centrifugation, then dried at 60 °C for 12 h. The product was annealed for 2 h at 350 °C at a heating rate of 2 °C/min to obtain the ZnO/rGO.
Electrochemical exfoliation method is another simple and one-step ex-situ technique used in preparing GO/rGO-composites. Ansari and Payami [119] reported the synthesis of magnetic graphene-Fe2O3 nanocomposite using this method. The electrolytic cell was made with graphite foil anode and iron plate as cathode, and applying a voltage of 10 V DC for 3 h. In a study to evaluate the effect of synthesis method on the properties of GO/rGO-composite, Aquino, et al. [120] reported the synthesis of GO/WO3NW/PANI through chemical and electrochemical routes. It was reported that the electrochemical process resulted in a composite with disorganized structure, which however, increased the doping level in the polymer chain, enhanced porosity and also permitted higher synergistic effect among the components of the composites, when compared to the nanocomposites prepared via the chemical route.
The growth of nanoparticles on GO/rGO sheets is influenced by the amount of functional group present on the sheet [121]. So, compared to rGO, GO can facilitate the growth of nanoparticles leading to higher density and smaller nanoparticle size [122]. Therefore, to overcome low particle loading and poor solubility of rGO, surfactant molecules or polymers are employed in composite synthesis. The synthesis of Pd-CuO/rGO and Au-CuO/rGO via an ionic liquid-assisted approach was reported by Alhumaimess, et al. [123]. The addition of little amount of the ionic liquid 1-butyl-3methyl imidazolium tetrafluoroborate resulted in uniformly dispersed nanoparticles, with simultaneous reduction of GO.
Graphene doped with heteroatoms such as nitrogen (N), sulphur (S), boron (B) have also been explored in synthesis of composites with semiconductors. Ex-situ synthesis of doped-GO/rGO-composite such as N-graphene, and B-graphene have also been reported. Liu, et al. [124] reported the solvothermal synthesis of NG/TiO2 by mixing N-graphene oxide and TiO2 in a Teflon-lined reactor. The synthesis of Co3Sn2@Co-NG was also reported by Mahmood, et al. [125] via the in-situ approach.
3. Catalytic Activity of Graphene-Based Composites in the Degradation of Pharmaceuticals
The enhanced catalytic properties of graphene-supported composites have been studied in different technologies for the degradation of pharmaceuticals. While the photocatalytic process is the most explored technique, other degradation processes such as direct degradation, chemical oxidation, electrochemical and sonochemical/sono-photochemical processes have also been identified; thus, affirming the versatility of this composites as catalysts for pharmaceutical degradation.
3.1. Direct Degradation Processes
Although, most catalytic application of graphene composites in degradation of pollutants have focused on processes involving the addition of chemicals such as persulfate or light energy, few processes have been explored without these complementary components. Saroyan, et al. [126] reported the degradation of bisphenol-A by graphite oxide composited with mixed oxidation state manganese oxide. The improved catalytic activity was correlated with the improved adsorption of bisphenol-A unto the composite, which brought the BPA into close proximity with the Mn3O4. The activity of the composite was optimal at pH of 3, reaching a degradation efficiency of 93%. The degradation of tetracycline by MnO2 supported on a three-dimensional graphene was also reported by Song, et al. [127] with efficiency of up to 91% and rate constant of 0.136 μM/min.
3.2. Chemical Oxidation Processes
Peroxymonosulfate (PMS)- or persulfate (PS)-based oxidation processes are effective routes to the degradation of recalcitrant and refractory pollutants because of their great oxidation capacity and high selectivity. The activation of persulfate by processes such as heating, ultrasound, UV light, transition metal ion and alkaline conditions leads to the generation of . Due to the recent interest in heterogeneous catalysis, the use of solid catalysts has gained much focus because of their excellent catalytic activity, stability and ease of separation. Solid catalysts based on magnetic spinel-type ferrite materials (MFe2O4, M =Co, Fe, Ni, Zn, and Cu) can act as heterogeneous Fenton-like catalysts for the activation of PS. Recent studies have shown that the compositing of these catalysts with GO/rGO could enhance the PS activation activity of these heterogeneous catalysts.
The primary mechanism of persulfate activation by carbonaceous materials as shown in Figure 8 is based on the one-electron transfer route (Equation (1)) [128,129]. The generation of •OH is also possible via the reaction expressed in Equation (2). In both reactions, radical species generated are responsible for the degradation of organic compounds. Non-radical routes involving transfer of electrons from organic compounds to persulfate and/or singlet oxygen generation could also occur. The ability of doped graphene to effectively activate PS was reported by Chen and Carroll [130] in which 99.9% degradation of sulfamethoxazole was achieved by N-doped graphene. The enhanced activity was attributed to the increased nitrogen groups such as pyridinic-N and quaternary-N, which acts as adsorption sites and source of free-flowing ℼ-electrons for PS activation.
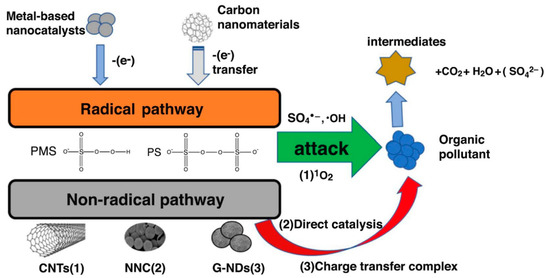
Figure 8.
Mechanism of persulfate activation by metal-based catalysts and carbonaceous materials. Reproduced with permission from Xiao, et al. [128]. Copyright (2018) Elsevier.
The degradation of methylparaben by CuFe2O4-rGO in the presence of PS showed a degradation efficiency of 95% with mineralization efficiency of 73% compared to 38 and 20% degradation and mineralization efficiencies respectively observed for CuFe2O4. The reaction rate constant for the rGO supported catalyst was almost 7-fold the value observed for bare CuFe2O4. The improved activity of the supported catalyst was attributed to the direct activation of PS via electron transfer reactions involving the -COOH and O-C=O groups on the rGO surface [131]. The activation of PS by Ni2SnO4-rGO for degradation of bisphenol A was reported by Jiang et al. [132]. Compositing of the catalyst with rGO reportedly increased the degradation efficiency of the process by 32%. This was attributed to the ability of rGO to prevent the aggregation of the Ni2SnO4 nanoparticles; thus, increasing the active sites of the catalyst.
Vieira et al. [133] reported the synthesis of a membrane based on carbon-polymer nanocomposite obtained by compositing nitrogen-doped reduced graphene oxide with poly(vinylidene fluoride) (rGO-M-PVDF). The composite was explored for PS activation in the degradation of ofloxacin (OFX), ciprofloxacin (CIP) and enrofloxacin (EFX), with degradation efficiencies of 54, 77 and 91% respectively. The degradation pathway was observed to be by both radical and non-radical oxidation routes. Evaluation of the surface group present in the spent membrane showed that a loss in the N-pyridinic group was observed, while the N-pyrrolic and N-quaternary group remained unchanged in the membrane. This confirmed that the N-pyridinic group accounts for the catalytic activity of the membrane. Also, the non-radical process occurred via the singlet oxygen generated in the reaction system. The degradation of bisphenol, phenol, acetaminophen and sulfamethoxazole by visible-light activated persulfate using Ti3+ self-doped TiO2/GO composite was reported by Yang, et al. [134]. The efficiency of the process reached 99% after just 12 min for bisphenol degradation, implying the enhanced activation of persulfate by the TiO2-x/rGO composite. As shown in Figure 9A,B, the synergy between the self-doped TiO2 and rGO was important for enhanced degradation efficiency and high reaction rate. The process showed higher degradation and mineralization efficiency towards bisphenol and acetaminophen, with almost complete degradation achieved as shown in Figure 9C,D. Studies carried out on the degradation process showed that persulfate was activated by electrons generated by the photocatalyst, leading to the generation of •OH and , which attacks the contaminants as shown in Figure 9F. Photoluminescence spectra of the composite (Figure 9E) showed a significant reduction in e−/h+ recombination, which accounts for high efficiency of the process.
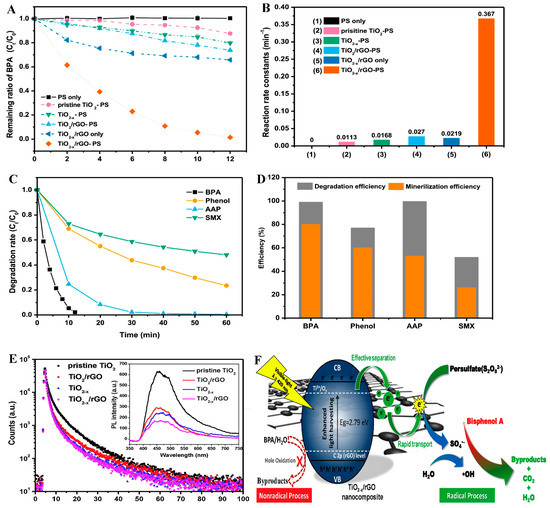
Figure 9.
Degradation of bisphenol, phenol, acetaminophen and sulfamethoxazole by TiO2−x/rGO (A) degradation efficiency of bisphenol by PS only, pristine TiO2 +PS, TiO2−x + PS, TiO2−x + PS, TiO2−x/rGO, TiO2−x/rGO + PS (B) kinetic of degradation of bisphenol by PS only, pristine TiO2 + PS, TiO2−x + PS, TiO2−x + PS, TiO2−x/rGO, TiO2−x/rGO + PS (C) Efficiency of TiO2−x/rGO + PS for bisphenol, phenol, acetaminophen and sulfamethoxazole degradation (D) degradation and mineralization of TiO2−x/rGO + PS for bisphenol, phenol, acetaminophen and sulfamethoxazole degradation (E) Time resolved PL spectra of TiO2−x/rGO (inset) steady state PL spectra. (F) Mechanism of action of TiO2−x/rGO for bisphenol degradation. Adapted with permission from Yang, et al. [134].
The mechanism of PMS activation by rGO-CoFe2O4 for ofloxacin and cefazolin degradation was reported by Fan et al. [135]. The study showed that while sulfate radicals were the major active species in ofloxacin degradation, non-radical oxidation by PMS direct two-electron transfer was prevalent in cefazolin degradation. The difference in degradation routes was attributed to the difference in the nucleophilicity and electrophilicity of different sites on the antibiotics. The activation of PMS was achieved via three different redox reactions between Co(II)/Co(III), Fe(II)/Fe(III) and lattice oxygen/O2 from rGO-CoFe2O4. Complete degradation of the pharmaceuticals was achieved under 30 min using 0.1 g/L catalyst concentration and 1 mM PMS.
Other graphene-based composites employed for the activation of persulfate in pharmaceutical degradation are presented in Table 2. The suitability of this process for pharmaceutical degradation could be observed as high degradation efficiencies and reaction rates were reported for the process. Most of the reported studies were carried out under acidic conditions, thus suggesting the preference for acidic conditions for optimal activity. It is observed that significant degradation of pharmaceuticals could be achieved within relatively short reaction times, which is an important factor for adaptation in treating large volume of wastewater in treatment plants. The presence of chalcogens such as N, O and S on the composite played significant role in persulfate activation and a corresponding increase in the activity of the composites.

Table 2.
Chemical oxidation processes based on graphene-based composites for degradation of pharmaceuticals.
3.3. Photocatalysis
Photocatalysis involves the use of semiconducting materials such as oxides, sulphides and nitrides, that have the capacity to absorb light energy and generate reactive oxygen species (ROS). The generated ROS such as •OH and are responsible for the degradation of organic pollutants via a redox process [144]. For ROS generation, light of equal or higher energy compared to the band gap of the semiconductor irradiates the semiconductor surface, leading to the excitation of electrons from the valence band into the conduction band and holes are left in the conduction band. This is referred to as electron-hole (e−/h+) pairs generation. The electronhole pairs react with oxygenated species such as water and air on the surface of the catalyst to generate ROS. The electron is responsible for the formation of via dissolved oxygen reduction, while oxidation of water by hole leads to the formation of hydrogen gas and •OH. The could subsequently produce H2O2 by reacting with H2O leading to more •OH production.
3.3.1. Metal Oxide-Based Graphene Composite Photocatalysts
Several metal oxide-graphene composites have been explored as photocatalysts for degradation of pharmaceuticals. Bhatia et al. [6] reported the degradation of 25 ppm of atenolol using a GO supported TiO2, which achieved 72% degradation under a simulated solar light irradiation for 1 h. Using Degussa P25 as catalyst, a degradation efficiency of 56% was achieved by the process. Evaluation of the effect of catalyst concentration on the system shows that while the degradation of the contaminant increased with increase in catalyst’s concentration when Degussa P25 was used, a reduction in photocatalytic activity was observed for the GO supported photocatalyst. This was attributed to the reduction in light penetration into the system as a result of the shielding effect of GO on the catalyst particles. In another study to optimize the degradation process for atenolol using a graphene-TiO2 composite, Bhatia, et al. [145], studied a four-factor three-level Box-Benkhen design to determine the optimal condition for the process. The photocatalyst concentration, pH, atenolol concentration and light intensity at levels of 10–20%, 4–9, 10–30 mg/L and 60–260 W/m2 respectively were studied. The study showed that the optimum rate constant for the process was 0.667 min−1, which was achieved by catalyst concentration of 10%, pH of 6.5, light intensity of 160 W/m2 and atenolol concentration of 30 mg/L. The effect of the presence of H2O2 on the photocatalytic degradation of acetaminophen (ACT) by a TiO2@rGO composite, showed that the degradation and mineralization efficiency of the catalyst could be enhanced by a multiple of 4 and 3 respectively by the introduction of H2O2 into the system [146]. Further study on the effect of rGO on the photocatalytic process showed the catalytic activity was maximum at 3% rGO concentration, with further increase resulting in inhibition of catalytic activity.
Heterojunctions based on graphene-metal oxide composite semiconductors have also been reported as photocatalysts for pharmaceutical degradation. The degradation of three pharmaceuticals: bisphenol-A (BPA), ibuprofen (IBP) and flurbiprofen (FBP) by graphene oxide based TiO2-ZnO heterojunction was reported by Bilgin Simsek, et al. [147]. The study showed that a degradation efficiency of 99.7, 98.5 and 98.1% of BPA, IBP and FBP was achieved by subjecting 10 mg/L of the pollutant solution to light irradiation at pH of 6.0 for 2 h under UV irradiation. The enhanced activity of the composite was attributed to the electron withdrawing properties of graphene. When other combinations between the three components of the catalyst were considered, the catalytic activity of the catalysts was reported to be in the order RGO/TiO2/ZnO > RGO/TiO2 > TiO2/ZnO > TiO2 > ZnO. Photocatalytic efficiency of Ag2CrO4/Ag/BiFeO3@rGO heterojunction for ciprofloxacin (CIF) degradation under broad light spectrum was reported by Kumar, et al. [148]. Apart from the advantages of Z-scheme formation, the reductive and oxidative capabilities of the composite were triggered by the plasmonic Ag0 electron donation-mediation and the adsorption-electron mediation of reduced graphene oxide.
3.3.2. Noble Metal Based Photocatalysts
The use of noble metals such as platinum (Pt) and silver (Ag) as co-catalysts for graphene supported photocatalysts have been explored as photocatalyst for the degradation of pharmaceuticals because of their ability to enhance light absorption and improve rate of charge generation via their surface plasmon resonance (SPR) [149]. Xu, et al. [150] reported the enhanced degradation efficiency of Ag-BiOBr-rGO composite for the degradation of ketoprofen under simulated solar light irradiation. The catalytic study showed that BiOBr alone showed a degradation efficiency of 70% towards ketoprofen after 120 min, which was increased to 78% by the introduction of 5% rGO to the catalyst. Doping of Ag into the BiOBr-rGO photocatalyst at 5% mole fraction resulted in 100% degradation of the pollutant, with 65.8% mineralization efficiency. It was further observed that excess incorporation of Ag atoms into the composite resulted in decreased degradation efficiency due to electronic sinks being formed by the excess atoms. The composite also showed sufficient stability, with up to 100% efficiency still recorded after 3 cycles of usage. Similarly, Mohan, et al. [151] reported the degradation of oxytetracycline by V2O5/rGO/Pt composites. The study focused on the effect of additives such as H2O2, Na2CO3, ethanol, persulfate and NaCl on the degradation efficiency of the photocatalyst. It was reported that additives that results in radical scavenging such as NaCl and Na2CO3 inhibited the degradation of the process, while additives with potential of increasing radical generation such as persulfate and H2O2 enhanced the degradation efficiency. For H2O2, the improvement in efficiency was limited to low concentrations because H2O2 quenches the •OH generated in the system at high concentration. Evaluation of the photocatalyst for real effluent treatment showed a slightly reduction in efficiency from 99% for simulated water sample to 87%.
3.3.3. Metal Sulphide-Based Graphene Composite Photocatalyts
Recently, metal sulphides have been explored as semiconductor materials due to their abundance, non-toxicity and low melting points, which allows for low temperature processing [152]. The use of graphene-supported ternary sulphide composites have also been explored as photocatalysts for pharmaceutical degradation. The degradation of 4 nitrophenol and 2-nitrophenol was studied using rGO supported AgIn5S8 (rGO/AIS) as a photocatalyst [153]. The optimal rGO weight percentage in the composite was determined by photoluminescence (PL) quenching experiment, which showed that highest PL quenching was observed in the composite with 1% weight of rGO, signifying reduced electron-hole recombination. This confirmed that the lowest electron-hole recombination was observed in the 1% rGO/AIS. The photocatalytic study showed that 91% degradation efficiency was achieved by the catalyst compared to 30.5% achieved by pristine AIS for 4-nitrophenol. The efficiency of degradation was 72.5 and 93.2% respectively for the degradation of 2-nitrophenol using AIS and 1% rGO/AIS. The photocatalytic activity of photocatalysts with higher rGO weight percentage was reported to be lower compared to 1% rGO/AIS, which was attributed to the covering of the catalyst surface by excess rGO, resulting in a suppression of visible light absorption, reduction of active site formation and increased electron-hole recombination. The degradation of naproxen by rGO supported ZnIn2S4 (rGO/ZIS), showed that improved activity was achieved by rGO incorporation resulting in a degradation rate of 0.082 min−1, which was 1.3 times higher than the value reported for the unsupported catalyst and the degradation efficiency of the process reached 99% after 60 min [154]. Reactive species quenching experiments conducted in order to determine the active species responsible for the degradation showed that scavenging of •OH from the process did not have any effect on the efficiency of the process, while scavenging of h+ and resulted in a significant reduction in degradation efficiency.
The compositing of two or more nanomaterials with differing dimensions through covalent and noncovalent interfaces could offer unique materials with new properties for different applications [155]. By compositing 3D bismuth oxyiodide, 2D graphene oxide (GO) and 1D bismuth sulphide, Arumugam, et al. [156] reported the synthesis of Bi2S3@GO/BiOI with 2% and 1% weight composition of Bi2S3 and GO respectively for the photodegradation of tetracycline under visible light radiation. The synergistic interaction between the multidimensional components of the catalyst resulted in enhanced charge transport and separation. The optimum composition of GO was determined by evaluating the photocatalytic activity of varying amount of GO with BiOI for tetracycline degradation and the highest degradation of 82% was observed for 1% wt GO, compared to 77 and 73% degradation for 2 and 3% GO composition respectively. The 1%GO-BiOI was then coupled with varying composition of Bi2S3, with the 2% wt composition yielding the highest degradation efficiency of 98% for tetracycline degradation, compared to 88 and 89% degradation for 1 and 3% Bi2S3 composition respectively. The reaction rate constant for the photocatalyst based on differing Bi2S3 composition was 0.171. 0.350 and 0.224 min−1 for 1%-Bi2S3@1%-GO/BiOI, 2%-Bi2S3@1%-GO/BiOI and 3%-Bi2S3@1%-GO/BiOI respectively.
3.3.4. Metal-Free Composites
Kumar, et al. [157] reported a metal free self-assembled carbon quantum dots (CQD) and rGO layers modified S@g-C3N4/B@g-C3N4 composite as visible and solar light photocatalysts for the degradation of chloramphenicol (CMP). The study showed that 99.1 and 92.4% of CMP was degraded under visible and natural solar light respectively. Combination of rGO and CQD afforded the catalyst with better adsorption capacity, enhanced visible light absorption, improved charge flow via intimate interfacial contact, reduced charge recombination and increased ROS generation. The doping of g-C3N4 with different atoms resulted in different band structures, which grew into an effective Z-scheme when combined together. The formation of heterojunctions increases the chances of and •OH compared to individual g-C3N4. Similarly, composites obtained from acidified g-C3N4 (ACN), biochar, polyaniline (PANI) and rGO were explored to synthesis three metal-free photocatalysts: g-C3N4/ACN/rGO@Biochar (CARB), G-C3N4/PANI/rGO@Biochar (CPRB) and CAN/PANI/rGO@Biochar (APRB) for degradation of ibuprofen [158]. Under Xenon lamp radiation, the APRB showed the highest efficiency of 98.4% degradation for 20 mg/L of ibuprofen after 50 min, while the efficiencies of GARB and GPRB was about 70 and 62% respectively. The reaction rate for the degradation process by APRB was 0.08025 min−1, which was 2.4-fold of the value obtained for GARB. Although, the three composites showed the feasibility for Z-scheme mechanism, the favourable band edge positions, reduced charge carrier recombination, higher photo-response, and ordered structure resulting in improved charge flow along interfaces accounts for the higher activity of APRB.
Table 3 presents an overview of some photocatalytic processes that employ graphene-based composite. The photocatalytic processes are the most explored application of graphene-based composites in pharmaceutical degradation. It is observed that UV light source are still the most explored energy source for photocatalytic process, with only few studies employing visible/solar light sources. This shows the need for improved band gap tuning techniques which could enhance the utilization of visible/solar light sources, leading to an improvement in the economic visibility of this process. The wide range of pharmaceuticals that have been studied, the short reaction time and the high degradation efficiency of this process as presented in the table, is a proof of the great potential of this process for removal of pharmaceuticals from the environment.

Table 3.
Photocatalytic degradation of pharmaceuticals using graphene-based composites.
3.4. Electrocatalytic Process
The Fenton process is one of the prominent AOPs for the degradation of contaminants. The process involves the generation of reactive •OH radicals by the reaction of hydrogen peroxide with ferrous iron catalyst (Equations (3) and (4)). The formation of large ferric hydroxide sludge, narrow pH requirement, large Fenton reagent consumption and high risk involved in production, transportation and storage of H2O2 are serious drawbacks for the process despite its high potential [180].
With the electro-Fenton process (EF), H2O2 for the Fenton process is electrogenerated in the reaction system via the two-electron oxygen reduction reaction at the cathode (Equation (5)). Thus, circumventing the challenges associated with H2O2 addition in the Fenton process [181]. The continuous reduction of Fe3+ to Fe2+ at the surface of the cathode via the direct one electron reduction or by reaction with H2O2 (Equation (4)) is the main driving force behind the EF process [182]. The EF process has gained much attention due to its high efficiency, low cost and facile operating conditions [183,184]. Also, advances in electro-Fenton electrode development has led to composite electrodes into which solid Fenton catalysts are immobilized such as ferrite-carbon aerogel [185], Fe3O4@carbon fiber paper@polyaniline [186], Fe3O4@Fe2O3/activated carbon aerogel [187]. These functionalized cathodes have the potential of achieving the Fenton reaction over a wide pH range.
The degradation of acetaminophen in an electro-Fenton process employing heteroatom-doped graphene aerogel cathode and carbon-magnetite catalyst, showed complete degradation was achieved in 240 min, while 45.5% mineralization efficiency was achieved in 360 min [188]. The degradation efficiency was influenced by the amount and nature of heteroatom, while the mineralization efficiency was strongly correlated to the amount of iron leached from the catalyst. The degradation efficiency of homogeneous and heterogeneous electro-Fenton process using FeSO4•7H2O and graphene oxide-Fe3O4 composite respectively as catalyst for the degradation of chloramphenicol and metronidazole was reported by Görmez, et al. [189]. The study showed that 57 and 71% mineralization was obtained for the degradation of 80 g/L−1 metronidazole and chloramphenicol after 300 min for the homogenous process. Under the same reaction condition, the graphene oxide-Fe3O4 heterogeneous catalyst achieved 73 and 86% mineralization efficiency for metronidazole and chloramphenicol respectively, with degradation efficiency reaching above 99%. The process was observed to be greatly influenced by the pH of the system, with optimum pH being 3. This was attributed to the positive charge induced on the surface of the catalyst at low pH, thus favouring an electrostatic interaction with the pollutant molecules which were also present in their ionic form.
A novel γ-FeOOH graphene polyacrylamide carbonized aerogel was reported as air-cathode for the degradation of sulfamethoxazole (SMX) [190]. The study showed a gradual increase in the conductivity, and specific surface area of the cathode material until an optimum concentration of 5 mg/mL−1 GO was reached. Above this optimum GO concentration, a notable decrease in surface area was observed despite the increase in conductivity. This was attributed to the reduced incorporation of GO into the hydrogel when excess GO was employed. When the catalytic activity of the catalyst was evaluated using 0.15 g of the catalyst on 500 mL of 0.1 mM SMX at 200 mA, a complete degradation was achieved after about 120 min with a degradation rate constant of 0.51 × 10−1 min−1. In another study, Mi, et al. [191], reported the mechanism of degradation of ciprofloxacin (CIP) by RGO-Ce/WO3 nanosheets modified carbon felt cathode. It was observed that the degradation process progressed by the oxidative degradation of the piperazine ring and oxidative cleavage of the cyclopropane ring of CIP. As shown in Figure 10, the oxidative degradation process was achieved via hydroxylation, decarboxylation and N-dealkylation processes.
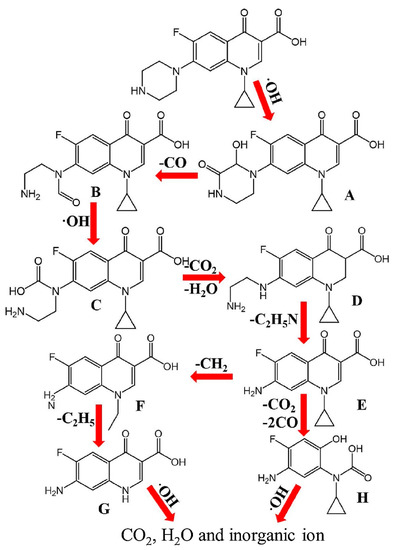
Figure 10.
Mechanism of degradation of ciprofloxacin in an RGO-Ce/WO3 nanosheets modified carbon felt cathode based Electro-Fenton process. Reprinted with permission from Mi, et al. [191]. Copyright (2019) Elsevier.
Aside from electro-Fenton processes, graphene-based composites have also been explored as anodic membranes for the electrochemical oxidation of pharmaceuticals. The excellent electrical conductivity of graphene and electrooxidation capacity of SnO2 were explored in a G/SnO2/CFs composite for the electrochemical degradation of SMX [192]. The membrane showed improved degradation efficiency of 85% and high stability, withstanding 10 cycles without any significance loss of activity. Mechanism study showed that two transformation pathways: hydroxyl radical attack and ring cleavage were the identified routes for the degradation process. Presented in Table 3, are the process overview of other electro-Fenton process employing graphene-based composites are presented in Table 4 and the potential application of graphene-based composites as catalysts, electrodes or membranes in this process, shows the significant role these composites can play in the further development of these AOP technique.

Table 4.
Fenton processes based on graphene composites for degradation of pharmaceuticals.
3.5. Sonocatalytic/Sono-Photocatalytic Process
Different studies have shown that by combining ultrasonic irradiation with other AOPs such as ultrasonic/photocatalysis, ultrasonic/Fenton process and ultrasonic/catalysis, the efficiency of contaminant sdegradation could be enhanced. Coupling of ultrasonication with other AOPs often leads to: (i) an increase in mass transfer of pollutants between catalysts surface and liquid phase, (ii) enhancement of reactive radical generation, and (iii) de-aggregation of the nanocomposite particles. The sonocatalytic degradation of sulfasalazine and diclofenac by Ag2O/CdO/CeO2/rGO was reported by Mirzazadeh and Lashanizadegan [197] with efficiencies of 100% and ~85% reported for sulfasalazine and diclofenac respectively. The efficiency of the process was significantly influenced by the weight percent of Ag in the composite and the optimum composition of Ag was reported to be 5%. Kinetic studies showed a reaction rate constant of 0.11 and 0.018 min−1 for sulfasalazine and diclofenac respectively. The effect of sonolytic frequency on the degradation of carbamazepine and acetaminophen by GO/β-Bi2O3/TiO2/Bi2Ti2O/heterojunction was reported by Lee, et al. [198]. Although, increasing the frequency leads to the enhancement of acoustic cavitation and subsequently the catalytic activity, increasing the frequency beyond an optimal level resulted in reduced activity due to the reduction in the occurrence of collapsing events, which may result to reduced number of small cavity bubbles. Comparing degradation at frequencies of 28, 580 and 970 kHz, the process at 580 kHZ was found to be most effective for carbamazepine and acetaminophen degradation.
Moradi, et al. [199] reported the sonophotocatalytic degradation of sulfamethoxazole using MgO/ZnO/Graphene nanocomposite. Incorporation of graphene and ZnO with MgO enhanced its catalytic activity under both UV and visible light radiation. Complete degradation of SMX was achieved after 120 min. The ultrasound played an important role in ensuring clean-up of the catalyst surface and jointly enhanced the production of reactive radicals with the light source. The photocatalytic interaction between the composite and light source was also significant in enhancing the efficiency of the process.
4. Factors Influencing the Degradation of Pharmaceuticals
Generally, the efficiency of any advanced oxidation process is significantly influenced by factors that may influence the reactive radical generation. These factors are often referred to as controlling parameters and the most studied parameters include concentration of the pharmaceutical, catalyst concentration, dissolved oxygen levels, pH, concentration of oxidants, water matrix, light source and intensity.
4.1. Concentration of Pharmaceuticals
High initial concentration of pollutants usually lead to a higher rate of adsorption on the catalyst surface. However, a commensurate increase in reactive radical generation is usually needed to sustain the degradation process. Therefore, increasing the initial concentration of the pollutant usually result in increased degradation burden on a unit catalyst due to limitation in the amount of reactive species generated in the process. Also, the coverage of the catalyst surface by the adsorbed molecules have also been suggested to be capable of blocking the catalyst surface from receiving sufficient light energy for generation of reactive radical species in photon-based processes [200]. Therefore, Increasing the concentration of pollutant has been found to generally result in process efficiency reduction.
4.2. Effect of pH
It is well established that the adsorption and surface charge of a catalyst is greatly influenced by the system pH. Also, the ionic form of the pollutant in solution is significantly influenced. At pH value equal to the point of zero charge (pHpzc) of the catalyst, surface charge is usually zero, while pH values lower or higher than the pHpzc leaves a net positive or negative charge on the catalyst [201]. Kaur, et al. [202] confirmed this trend in the study of the effect of pH on the degradation of triclosan using rGO-TiO2 composite. At a pH value below the pHpzc of the catalyst complete degradation was achieved, while the degradation was significantly suppressed at higher pH. In the degradation of amoxillin by graphene-titanium oxide nanotubes composites, Song, et al. [203] reported a gradual increase in degradation efficiency with increase in pH from 3 to 9; however, increasing the pH to11 resulted in a decrease in efficiency due to the similar charge on the catalyst and amoxicillin molecule. Therefore, depending on the ionic state of the pollutant and the net charge on the catalyst surface, the adsorption of the pollutant which is significant for the degradation process needs to be altered towards the enhancement of the degradation process.
4.3. Effect of Catalyst Concentration
Effect of catalyst concentration on the degradation efficiency usually varies for photon-based processes and non-photo-based processes. While in general, the increase in the catalyst concentration increases the quantity of reactive radical species generated in a system, in photon-based processes, increasing the catalyst concentration beyond certain concentration level is found to reduce the degradation efficiency. This is due to reduced light peneteration into the reaction system, leading to reduced radical generation. In the study by Sayadi, et al. [175], increasing the catalyst concentration from 0.25 g/L to 0.75 g/L led to an increase in degradation efficiency. However, beyond this concentration level, a reduction in catalytic activity was observed due to increased turbidity of the solution, which reduces light penetration and dispersion.
4.4. Dissolved Oxygen
Dissolved oxygen (DO) in a catalytic system acts as an electron acceptor, thus reducing the rate of electron-hole recombination, which enhances the degradation efficiency of a process. Also, dissolved oxygen could help in radical intermediate stabilization; aromatic ring cleavage induction and mineralization [204,205]. Azim, et al. [206] reported an increase in the catalytic activity of GO as the level of DO in the system was increased. However, Lee, et al. [207] observed that in processes that employed DO generator in order to improve level of DO, bubbles formed by the generator got attached to the catalyst surface, thereby reducing the efficiency of the process. At high DO concentration level, this effect was mitigated and enhanced catalytic activity was observed. Subramanian and Kannan [208], reported that the level of DO in a system is important for processes involving high light intensities due to the large quantities of holes and electrons generated, as the electron scavenging role of dissolved oxygen becomes significant.
4.5. Light Intensity
Light intensity is an important factor to be optimized for a photocatalytic process as it contirbutes significantly to the overall cost of a process. Thus, its effective use is a major factor to be considered in the design and operation of a process. In terms of the effect of light intensity on reaction rate, three types of relationships have been identified. A linear relationship is generally observed between degradation rate and light intensity, when low intensity light is used. At high light intensity, the degradation rate is independent of the light intensity. For intermediate light intensities, the power-law between light intensity and reaction rate holds with an exponent varying between 0.5–0.8 [209]. Malekshoar, et al. [209], studied the effect of light intensity on the activity of Graphene-TiO2 composite on degradation of phenol, by varying the light intensity between 20–10 mW/cm2. The authours observed a linear relationship between the intensity and reaction rate at light intensities below 50 mW/cm2, while at higher intensities the rate increased with a power of 0.7.
4.6. Water Matrix
To determine the suitability of any AOP for wastewater treatment, it is important to evaluate the effect of co-existing susbstances such as organic acids (e.g., acetic and formic acids), natural organic matters (NOM) (e.g., fulvic and humic acids) and inorganic ions (e.g., Cl-, , and on the removal efficiency of pollutants. The water matrix also significantly influence the optimal condition for any degradation process. The presence of co-existing substances could either have a negative or positive influence on the degradation efficiency of a process. The negative influence of co-existing substances may arise from their undesirable reactions with generated radical species and the light scattering ability of some suspendeded solids. Meanwhile, the positive effect of co-existing substances arises due to the oxidation and reduction properties of some of these compounds, which could serve either as oxidants or reductants in the catalytic process. Furthermore, compounds with carboxylic and hydroxy groups could form complexes with high quantum yield, leading to enhanced photo-reduction reaction [210,211,212]. Tokumura, et al. [212] explored the effect of water matrix on the degradation of carbamazepine and diclofenac using three AOPs: photo-Fenton, photocatalysis and combined oxine and hydrogen peroxide oxidation process. The authors reported an increase in the efficiency of the Fenton process due to additional iron ions from the water matrix. However, the photo-reduction reaction was inhibited by the light scattering effect and complex-formation of co-existing substances. For the photocatalytic and combined ozone and hydrogen peroxide oxdiation process, the scavenging effect of the co-existing substances was a main inhibiting factor to the efficiency of the processes.
4.7. Comparative Analysis of Graphene-Based Catalytic Process for Degradation of Pharmaceuticals
The comparative analysis of the identified degradation techniques for degradation of pharmaceuticals by graphene-based catalytic processes, was carried out in terms of efficiency, reaction rate, mineralization rate and the time required to reach 90% degradation. The result of the analysis is shown in Table 5. All the five degradation techniques showed very close degradation efficiency above 90%, which confirms the potential of these techniques in pharmaceutical degradation. In terms of the reaction rate for each process, the chemical oxidation process showed the highest average reaction rate, with photocatalysis and electrocatalysis showing very similar degradation rates. The direct degradation and sonocatalytic/sonophotocatalytic processes showed low degradation rates. Comparing the average mineralization of each process, it is observed that electrocatalysis and sonocatalytic processes had the highest potential for conversion of pharmaceuticals to CO2 and water.

Table 5.
Comparative analysis of graphene-based composite techniques for pharmaceutical degradation.
The time required to attain 90% degradation for each process was evaluated using the equation:
where k is the reaction rate constant for each of the study. The average of the t0.9 for each process, showed that photocatalytic process had the lowest required time to achieve 90% degradation compared to other processes. This was followed by electrocatalysis and chemical oxidation processes.
5. Future Perspectives
The excellent catalytic activity of graphene-based composites for pharmaceutical degradation has been discussed in this review. In many of the explored studies, graphene/graphene derivatives have been used as a minor component of the catalytic materials. However, few studies have shown that composites constituting graphene/graphene oxide as major components have also shown good catalytic activity. It becomes important for the optimal graphene/graphene derivative to be explored and important factors such as cost, sustainability, efficiency in determining the preferred constitution. Also, discussed in this review were mainly bench scale studies with limited studies available for pilot scale studies. With the large volume of bench scale results obtained so far, studies on graphene-based composites for pharmaceutical degradation seems ripe for pilot scale studies, which might set it on the part to full commercialization.
6. Conclusions
A review of processes employing graphene-based composites as catalysts for degradation of pharmaceuticals have been presented in this study and five different degradation processes—direct catalytic degradation, chemical oxidation process, photocatalysis, electrocatalytic processes and sonocatalytic/sono-photocatalytic processes—have been identified in the explored literatures. Each of the process showed high degradation efficiencies, with the generation of reactive radical species being responsible for the activity of each process. Incorporation of graphene/graphene derivatives, was observed to enhance the degradation efficiency by improving on the generation of radical species, through improved surface area, light absorption and reduce recombination of generated charge carriers. A key factor in enhancing the activity of the composite is the determination of the optimal weight percentage of graphene required for improving the catalytic activity. Comparative analysis of these techniques showed that while they exhibit similar degradation efficiency, the photocatalytic process requires the least amount of time to attain 90% degradation, while the electrocatalytic and sonocatalytic processes are the most desirable in terms of mineralization potential.
Author Contributions
Conceptualization, O.C.O. and D.C.O.; Supervision, D.C.O.; writing—original draft, O.C.O.; writing—review and editing, D.C.O.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research Foundation (NRF), South Africa, Grant Number [UID109333 and UID 116338]. The APC was funded by the North-West University, South Africa.
Data Availability Statement
Not available.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Lin, L.; Wang, H.; Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 2017, 310, 389–398. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.J.; Zhang, Y.; Ge, F.; Steel, R.M.; Sun, L.Y. Advances in technologies for pharmaceuticals and personal care products removal. J. Mater. Chem. A 2017, 5, 12001–12014. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef]
- Wongso, V.; Chung, H.K.; Sambudi, N.S.; Sufian, S.; Abdullah, B.; Wirzal, M.D.H.; Ang, W.L. Silica–carbon quantum dots decorated titanium dioxide as sunlight-driven photocatalyst to diminish acetaminophen from aquatic environment. J. Photochem. Photobiol. A Chem. 2020, 394, 112436. [Google Scholar] [CrossRef]
- Zur, J.; Michalska, J.; Pinski, A.; Mrozik, A.; Nowak, A. Effects of Low Concentration of Selected Analgesics and Successive Bioaugmentation of the Activated Sludge on its Activity and Metabolic Diversity. Water 2020, 12, 4113. [Google Scholar] [CrossRef]
- Bhatia, V.; Malekshoar, G.; Dhir, A.; Ray, A.K. Enhanced photocatalytic degradation of atenolol using graphene TiO2 composite. J. Photochem. Photobiol. A Chem. 2017, 332, 182–187. [Google Scholar] [CrossRef]
- Hong, B.; Lin, Q.; Yu, S.; Chen, Y.; Chen, Y.; Chiang, P. Urbanization gradient of selected pharmaceuticals in surface water at a watershed scale. Sci. Total Environ. 2018, 634, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. I. Rationale for and avenues toward a green pharmacy. Environ. Health Perspect. 2003, 111, 757–774. [Google Scholar] [CrossRef]
- Khan, H.K.; Rehman, M.Y.A.; Malik, R.N. Fate and toxicity of pharmaceuticals in water environment: An insight on their occurrence in South Asia. J. Environ. Manag. 2020, 271, 111030. [Google Scholar] [CrossRef]
- Franzellitti, S.; Fabbri, E. Response to Commentary on “Are some invertebrates exquisitely sensitive to the human pharmaceutical fluoxetine?”. Aquat. Toxicol. 2014, 146, 264–265. [Google Scholar] [CrossRef]
- Du, B.; Haddad, S.P.; Luek, A.; Scott, W.C.; Saari, G.N.; Kristofco, L.A.; Connors, K.A.; Rash, C.; Rasmussen, J.B.; Chambliss, C.K.; et al. Bioaccumulation and trophic dilution of human pharmaceuticals across trophic positions of an effluent-dependent wadeable stream. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20140058. [Google Scholar] [CrossRef]
- Gomez, C.F.; Constantine, L.; Moen, M.; Vaz, A.; Wang, W.; Huggett, D.B. Ibuprofen Metabolism in the Liver and Gill of Rainbow Trout, Oncorhynchus mykiss. Bull. Environ. Contam. Toxicol. 2011, 86, 247–251. [Google Scholar] [CrossRef]
- Brooks, B.W.; Huggett, D.B.; Boxall, A.B. Pharmaceuticals and personal care products: Research needs for the next decade. Environ. Toxicol. Chem. 2009, 28, 2469. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661. [Google Scholar] [CrossRef]
- Redding, A.M.; Cannon, F.S.; Snyder, S.A.; Vanderford, B.J. A QSAR-like analysis of the adsorption of endocrine disrupting compounds, pharmaceuticals, and personal care products on modified activated carbons. Water Res. 2009, 43, 3849–3861. [Google Scholar] [CrossRef]
- Valcarcel, Y.; Alonso, S.G.; Rodriguez-Gil, J.L.; Maroto, R.R.; Gil, A.; Catala, M. Analysis of the presence of cardiovascular and analgesic/anti-inflammatory/antipyretic pharmaceuticals in river- and drinking-water of the Madrid Region in Spain. Chemosphere 2011, 82, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kushor, K.; Msna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in Aquatic Systems: Chemistry, Occurrence, Effects and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Radjenovic, J.; Petrovic, M.; Barcelo, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Sayadi, H.; Trivedy, R.K.; Pathak, R.K. Pollution of Pharmaceuticals in Environment. J. Ind. Pollut. Control 2010, 26, 89–94. [Google Scholar]
- Robinson, I.; Junqua, G.; Van Coillie, R.; Thomas, O. Trends in the detection of pharmaceutical products, and their impact and mitigation in water and wastewater in North America. Anal. Bioanal. Chem. Res. 2007, 387, 1143–1151. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Kümmerer, K. Pharmaceuticals in the Environment—Scope of the Book and Introduction. In Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 3–11. [Google Scholar]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Memmert, U.; Peither, A.; Burri, R.; Weber, K.; Schmidt, T.; Sumpter, J.P.; Hartmann, A. Diclofenac: New data on chronic toxicity and bioconcentration in fish. Environ. Toxicol. Chem. 2013, 32, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.J.; de Pedro, C.; Paxeus, N.J. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.-Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef]
- De Lange, H.J.; Noordoven, W.; Murk, A.J.; Lürling, M.; Peeters, E.T.H.M. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 2006, 78, 209–216. [Google Scholar] [CrossRef]
- Nikolai, L.N.; McClure, E.L.; MacLeod, S.L.; Wong, C.S. Stereoisomer quantification of the β-blocker drugs atenolol, metoprolol, and propranolol in wastewaters by chiral high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2006, 1131, 103–109. [Google Scholar] [CrossRef]
- Fraysse, B.; Garric, J. Prediction and experimental validation of acute toxicity of beta-blockers in Ceriodaphnia dubia. Environ. Toxicol. Chem. 2005, 24, 2470–2476. [Google Scholar] [CrossRef]
- Li, M.-H. Acute toxicity of industrial endocrine-disrupting chemicals, natural and synthetic sex hormones to the freshwater planarian, Dugesia japonica. Toxicol. Environ. Chem. 2013, 95, 984–991. [Google Scholar] [CrossRef]
- Forghani, M.; Sadeghi, G.; Peyda, M. The Presence of 17 Beta-Estradiol in the Environment: Health Effects and Increasing Environmental Concerns. Int. J. Epidemiol. Res. 2018, 5, 151–158. [Google Scholar] [CrossRef]
- Nassour, C.; Barton, S.J.; Nabhani-Gebara, S.; Saab, Y.; Barker, J. Occurrence of anticancer drugs in the aquatic environment: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 1339–1347. [Google Scholar] [CrossRef]
- Jauković, Z.D.; Grujić, S.D.; Vasiljević, T.M.; Petrović, S.D.; Lausević, M.D. Cardiovascular drugs in environmental waters and wastewaters: Method optimization and real sample analysis. J. AOAC Int. 2014, 97, 1167–1174. [Google Scholar] [CrossRef]
- Scheurer, M.; Sacher, F.; Brauch, H.-J. Occurrence of the antidiabetic drug metformin in sewage and surface waters in Germany. J. Environ. Monit. 2009, 11, 1608–1613. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Larsen, T.A.; Lienert, J.; Joss, A.; Siegrist, H. How to avoid pharmaceuticals in the aquatic environment. J. Biotechnol. 2004, 113, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Khurana, P.; Thatai, S.; Kumar, D. Destruction of recalcitrant nanomaterials contaminants in industrial wastewater. In Emerging and Nanomaterial Contaminants in Wastewater; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–158. [Google Scholar]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.R.; Kasiri, M.B. Artificial neural networks modeling of contaminated water treatment processes by homogeneous and heterogeneous nanocatalysis. J. Mol. Catal. A Chem. 2010, 331, 86–100. [Google Scholar] [CrossRef]
- Linden, K.G.; Mohseni, M. 2.8—Advanced Oxidation Processes: Applications in Drinking Water Treatment. In Comprehensive Water Quality and Purification; Ahuja, S., Ed.; Elsevier: Waltham, MA, USA, 2014; pp. 148–172. [Google Scholar]
- de Luna, M.D.G.; Lin, J.C.-T.; Gotostos, M.J.N.; Lu, M.-C. Photocatalytic oxidation of acetaminophen using carbon self-doped titanium dioxide. Sustain. Environ. Res. 2016, 26, 161–167. [Google Scholar] [CrossRef]
- Khataee, A.R.; Fathinia, M. Recent Advances in Photocatalytic Processes by Nanomaterials. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 267–288. [Google Scholar]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Tian, W.; Lv, X.; Zhang, H.; Hu, Z.; Zhang, Y.; Ji, J.; Jiang, W. Co-doped Ni3S2@ CNT arrays anchored on graphite foam with a hierarchical conductive network for high-performance supercapacitors and hydrogen evolution electrodes. J. Mater. Chem. A 2018, 6, 10490–10496. [Google Scholar] [CrossRef]
- Isac, L.; Cazan, C.; Enesca, A.; Andronic, L. Copper Sulfide Based Heterojunctions as Photocatalysts for Dyes Photodegradation. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Alenizi, M.A.; Kumar, R.; Aslam, M.; Alseroury, F.A.; Barakat, M.A. Construction of a ternary g-C3N4/TiO2@polyaniline nanocomposite for the enhanced photocatalytic activity under solar light. Sci. Rep. 2019, 9, 12091. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Mamba, B.B.; Sciences, M. TiO2-based photocatalysis: Toward visible light-responsive photocatalysts through doping and fabrication of carbon-based nanocomposites. Crit. Rev. Solid State Mater. Sci. 2017, 42, 295–346. [Google Scholar] [CrossRef]
- Rattan Paul, D.; Nehra, S.P. Graphitic carbon nitride: A sustainable photocatalyst for organic pollutant degradation and antibacterial applications. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Darkwah, W.K.; Oswald, K.A. Photocatalytic Applications of Heterostructure Graphitic Carbon Nitride: Pollutant Degradation, Hydrogen Gas Production (water splitting), and CO2 Reduction. Nanoscale Res. Lett. 2019, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Oxysulfide Sm2Ti2S2O5 as a Stable Photocatalyst for Water Oxidation and Reduction under Visible Light Irradiation (λ ≤ 650 nm). J. Am. Chem. Soc. 2002, 124, 13547–13553. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Krishnan, V. Influence of different bismuth oxyhalides on the photocatalytic activity of graphitic carbon nitride: A comparative study under natural sunlight. Mater. Adv. 2020, 1, 1262–1272. [Google Scholar] [CrossRef]
- Jung, B.; Deng, W.; Li, Y.; Batchelor, B.; Abdel-Wahab, A. Simulated solar light-driven photocatalytic degradation of trichloroethylene in water using BiOBr promoted by sulfite addition. Environ. Sci. Eur. 2020, 32. [Google Scholar] [CrossRef]
- Cosham, S.D.; Celorrio, V.; Kulak, A.N.; Hyett, G. Observation of visible light activated photocatalytic degradation of stearic acid on thin films of tantalum oxynitride synthesized by aerosol assisted chemical vapour deposition. Dalton Trans. 2019, 48, 10619–10627. [Google Scholar] [CrossRef]
- Boppana, V.B.R.; Doren, D.J.; Lobo, R.F. A Spinel Oxynitride with Visible-Light Photocatalytic Activity. ChemSusChem 2010, 3, 814–817. [Google Scholar] [CrossRef]
- Mohd Adnan, M.A.; Muhd Julkapli, N.; Amir, M.N.I.; Maamor, A. Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: A review. Int. J. Environ. Sci. Technol. 2019, 16, 547–566. [Google Scholar] [CrossRef]
- Anku, W.W.; Kiarii, E.M.; Sharma, R.; Joshi, G.M.; Shukla, S.K.; Govender, P.P. Photocatalytic degradation of pharmaceuticals using graphene based materials. In A New Generation Material Graphene: Applications in Water Technology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 187–208. [Google Scholar]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Carmalin Sophia, A.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater—A review. Desalin. Water Treat. 2016, 57, 27573–27586. [Google Scholar] [CrossRef]
- Mester, A.S.; Carvalho, A.P. Photocatalytic degradation of pharmaceuticals carbamazepine, Diclofenac and Sulfamethoxazole by semiconductor and carbon materials: A Review. Molecules. 2019, 24, 3702. [Google Scholar] [CrossRef] [PubMed]
- Putri, L.K.; Ong, W.-J.; Chang, W.S.; Chai, S.-P. Heteroatom doped graphene in photocatalysis: A review. Appl. Surf. Sci. 2015, 358, 2–14. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. Int. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Rani, P.; Jindal, V.K. Designing band gap of graphene by B and N dopant atoms. RSC Adv. 2013, 3, 802–812. [Google Scholar] [CrossRef]
- Sturala, J.; Luxa, J.; Pumera, M.; Sofer, Z. Chemistry of Graphene Derivatives: Synthesis, Applications, and Perspectives. Chem. Eur. J. 2018, 24, 5992–6006. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bardhan, N.M.; Tongay, S.; Wu, J.; Belcher, A.M.; Grossman, J.C. Scalable enhancement of graphene oxide properties by thermally driven phase transformation. Nat. Chem. 2014, 6, 151–158. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390. [Google Scholar] [CrossRef]
- Han, C.; Zhang, N.; Xu, Y.-J. Structural diversity of graphene materials and their multifarious roles in heterogeneous photocatalysis. Nano Today 2016, 11, 351–372. [Google Scholar] [CrossRef]
- Ye, L.-H.; Liu, B.-G.; Ding-Sheng, W. Ab initio Molecular Dynamics Study on Small Carbon Nanotubes. Chin. Phys. Lett. 2001, 18, 1496. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Mehmood, S.; Tang, H.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Mitigation of environmentally-related hazardous pollutants from water matrices using nanostructured materials—A review. Chemosphere 2020, 253, 126770. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 2015, 81, 826–834. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Z.; Lin, S.; Wang, K.; Ma, C.; Lu, H. Reactivity-Controlled Preparation of Ultralarge Graphene Oxide by Chemical Expansion of Graphite. Chem. Mater. 2017, 29, 564–572. [Google Scholar] [CrossRef]
- Liu, Z.; Duan, X.; Zhou, X.; Qian, G.; Zhou, J.; Yuan, W. Controlling and formation mechanism of oxygen-containing groups on graphite oxide. Ind. Eng. Chem. Res. 2014, 53, 253–258. [Google Scholar] [CrossRef]
- Morimoto, N.; Kubo, T.; Nishina, Y. Tailoring the oxygen content of graphite and reduced graphene oxide for specific applications. Sci. Rep. 2016, 6, 21715. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.; Sun, H.; Li, Z.; Zhao, X.; Gao, C. An iron-based green approach to 1-h production of single-layer graphene oxide. Nat. Commun. 2015, 6, 5716. [Google Scholar] [CrossRef] [PubMed]
- Pendolino, F.; Armata, N.; Masullo, T.; Cuttitta, A. Temperature influence on the synthesis of pristine graphene oxide and graphite oxide. Mater. Chem. Phys. 2015, 164, 71–77. [Google Scholar] [CrossRef]
- Brodie, B. Note sur un nouveau procédé pour la purification et la désagrégation du graphite. J. Ann. Chim. Phys. 1855, 45, 351–353. [Google Scholar]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.-M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Muthoosamy, K.; Manickam, S. State of the art and recent advances in the ultrasound-assisted synthesis, exfoliation and functionalization of graphene derivatives. Ultrason. Sonochem. 2017, 39, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, D.; Deng, J.; Kang, Q.; Liu, Z.; Fang, J.; Gou, Y. Review—Progress of Research on the Preparation of Graphene Oxide via Electrochemical Approaches. J. Electrochem. Soc. 2020, 167, 155519. [Google Scholar] [CrossRef]
- Yu, P.; Lowe, S.E.; Simon, G.P.; Zhong, Y.L. Electrochemical exfoliation of graphite and production of functional graphene. Curr. Opin. Colloid Interface Sci. 2015, 20, 329–338. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Gholampour, A.; Valizadeh Kiamahalleh, M.; Tran, D.N.H.; Ozbakkaloglu, T.; Losic, D. From Graphene Oxide to Reduced Graphene Oxide: Impact on the Physiochemical and Mechanical Properties of Graphene–Cement Composites. ACS Appl. Mater. Interfaces 2017, 9, 43275–43286. [Google Scholar] [CrossRef] [PubMed]
- Venkanna, M.; Chakraborty, A.K. Synthesis and characterizations of graphene oxide and reduced graphene oxide nanosheets. AIP Conf. Proc. 2014, 1591, 574–576. [Google Scholar] [CrossRef]
- Minitha, C.R.; Rajendrakumar, R.T. Synthesis and Characterization of Reduced Graphene Oxide. Adv. Mat. Res. 2013, 678, 56–60. [Google Scholar] [CrossRef]
- Habte, A.T.; Ayele, D.W. Synthesis and Characterization of Reduced Graphene Oxide (rGO) Started from Graphene Oxide (GO) Using the Tour Method with Different Parameters. Adv. Mater. Sci. Eng. 2019, 2019, 5058163. [Google Scholar] [CrossRef]
- Andrijanto, E.; Shoelarta, S.; Subiyanto, G.; Rifki, S. Facile synthesis of graphene from graphite using ascorbic acid as reducing agent. AIP Conf. Proc. 2016, 1725. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, C.; Li, F.; Chen, J.; Zhou, Q. Photo-reductive defluorination of perfluorooctanoic acid in water. Water Res. 2010, 44, 2939–2947. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Zheng, Q.-B.; Qiu, H.-X.; Li, J.; Yang, J.-H. A simple method for the reduction of graphene oxide by sodium borohydride with CaCl2 as a catalyst. New Carbon Mater. 2015, 30, 41–47. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Reduction of graphene oxide with substituted borohydrides. J. Mater. Chem. A 2013, 1, 1892–1898. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Berzina, A.; Tupureina, V.; Orlovs, R.; Saharovs, D.; Bitenieks, J.; Knite, M. Increase of Electrical Conductivity due to Chemical Reduction of Pre-Exfoliated Graphene Oxide by Sodium Borohydride. Adv. Mat. Res. 2015, 1117, 187–190. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Yu, B.; Feng, X.; Song, L.; Hu, Y. A facile and cost-effective approach to the reduction of exfoliated graphite oxide using sodium hypophosphite under acidic conditions. J. Mater. Chem. C 2013, 1, 690–694. [Google Scholar] [CrossRef]
- Liu, C.; Han, G.; Chang, Y.; Xiao, Y.; Li, Y.; Li, M.; Zhou, H. Capacitive Performances of Reduced Graphene Oxide Hydrogel Prepared by Using Sodium Hypophosphite as Reducer. Chin. J. Chem. 2016, 34, 89–97. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, G.; Liu, J.; Sun, X. Evaluation Criteria for Reduced Graphene Oxide. J. Phys. Chem. 2011, 115, 11327–11335. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide—A comprehensive review. Chem. Eng. J. 2021, 405. [Google Scholar] [CrossRef]
- Bhattacharya, G.; Sas, S.; Wadhwa, S.; Mathur, A.; McLaughlin, J.; Roy, S.S. Aloe vera assisted facile green synthesis of reduced graphene oxide for electrochemical and dye removal applications. RSC Adv. 2017, 7, 26680–26688. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Wang, H.L.; Casalongue, H.S.; Chen, Z.; Dai, H.J. TiO2 Nanocrystals Grown on Graphene as Advanced Photocatalytic Hybrid Materials. Nano Res. 2010, 3, 701–705. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Tang, Z.-R.; Xu, Y.-J. Noncovalently Functionalized Graphene-Directed Synthesis of Ultralarge Graphene-Based TiO2 Nanosheet Composites: Tunable Morphology and Photocatalytic Applications. J. Phys. Chem. 2014, 118, 27325–27335. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Kamegawa, T.; Yamahana, D.; Yamashita, H. Graphene Coating of TiO2 Nanoparticles Loaded on Mesoporous Silica for Enhancement of Photocatalytic Activity. J. Phys. Chem. 2010, 114, 15049–15053. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Zou, J.; Cui, Z.; Zhang, Q.; Schiettekatte, F.; Barba, D.; Liu, B.; Wang, Y. Graphene-Wrapped ZnMn2O4 Nanoparticles with Enhanced Performance as Lithium-Ion Battery Anode Materials. Nano 2020, 15. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- He, F.; Fan, J.; Ma, D.; Zhang, L.; Leung, C.; Chan, H.L. The attachment of Fe3O4 nanoparticles to graphene oxide by covalent bonding. Carbon 2010, 48, 3139–3144. [Google Scholar] [CrossRef]
- AbdelDayem, H.M.; Salib, B.G.; El-Hosiny, F.I. Facile synthesis of hydrothermal stable hierarchically macro-mesoporous hollow microspheres gamma-Al2O3-graphene oxide composite: As a new efficient acid-base catalyst for transesterification reaction for biodiesel production. Fuel 2020, 277. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Sun, D.D.; Ng, W. Graphene oxide-CdS composite with high photocatalytic degradation and disinfection activities under visible light irradiation. J. Hazard. Mater. 2013, 250–251, 412–420. [Google Scholar] [CrossRef]
- Deng, D.R.; Cui, X.Y.; Wu, Q.H.; Zheng, M.S.; Dong, Q.F. In-situ synthesis TiO2 nanosheets@rGO for ultrafast sodium ion storage at both room and low temperatures. J. Alloys Compd. 2020, 835. [Google Scholar] [CrossRef]
- Wang, H.; Dai, H. Strongly coupled inorganic–nano-carbon hybrid materials for energy storage. Chem. Soc. Rev. 2013, 42, 3088–3113. [Google Scholar] [CrossRef]
- Li, Q.; Mahmood, N.; Zhu, J.; Hou, Y.; Sun, S. Graphene and its composites with nanoparticles for electrochemical energy applications. Nano Today 2014, 9, 668–683. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly Coupled Inorganic/Nanocarbon Hybrid Materials for Advanced Electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef]
- Acidereli, H.; Cellat, K.; Calimli, M.H.; Sen, F. Palladium/ruthenium supported on graphene oxide (PdRu@GO) as an efficient, stable and rapid catalyst for hydrogen production from DMAB under room conditions. Renew. Energy 2020, 161, 200–206. [Google Scholar] [CrossRef]
- Anand, A.; Meena, D.; Bhatnagar, M.C. Synthesis and characterization of flexible PVDF/Bi2Al4O9/RGO based piezoelectric materials for nanogenerator application. J. Alloys Compd. 2020, 843. [Google Scholar] [CrossRef]
- Cao, P.J.; Cai, Y.Z.; Pawar, D.; Navale, S.T.; Rao, C.N.; Han, S.; Xu, W.Y.; Fang, M.; Liu, X.K.; Zeng, Y.X.; et al. Down to ppb level NO2 detection by ZnO/rGO heterojunction based chemiresistive sensors. Chem. Eng. J. 2020, 401. [Google Scholar] [CrossRef]
- Ansari, N.; Payami, Z. Synthesis of Magnetic Graphene-Fe3O4 Nanocomposite by Electrochemical Exfoliation Method. J. Nanostruct. 2020, 10, 39–43. [Google Scholar] [CrossRef]
- Aquino, C.B.; Nagaoka, D.A.; Machado, M.M.; Candido, E.G.; da Silva, A.G.M.; Camargo, P.H.C.; Domingues, S.H. Chemical versus electrochemical: What is the best synthesis method to ternary GO/WO3NW/PAni nanocomposites to improve performance as supercapacitor? Electrochim. Acta 2020, 356. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.T.; Diankov, G.; Dai, H. Nanocrystal Growth on Graphene with Various Degrees of Oxidation. J. Am. Chem. Soc. 2010, 132, 3270–3271. [Google Scholar] [CrossRef]
- Huang, X.; Boey, F.; Zhang, H.U.A. A Brief Review on Graphene-Nanoparticle Composites. Cosmos 2011, 06, 159–166. [Google Scholar] [CrossRef]
- Alhumaimess, M.S.; Alsohaimi, I.H.; Alshammari, H.M.; Aldosari, O.F.; Hassan, H.M.A. Synthesis of gold and palladium nanoparticles supported on CuO/rGO using imidazolium ionic liquid for CO oxidation. Res. Chem. Intermed. 2020, 46, 5499–5516. [Google Scholar] [CrossRef]
- Liu, J.; Chen, K.-Y.; Wang, J.; Du, M.; Gao, Z.-Y.; Song, C.-X. Preparation and Photocatalytic Properties of N-Doped Graphene/TiO2 Composites. J. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Liu, F.; Zhu, J.; Hou, Y. Hybrid of Co3Sn2@Co Nanoparticles and Nitrogen-Doped Graphene as a Lithium Ion Battery Anode. ACS Nano 2013, 7, 10307–10318. [Google Scholar] [CrossRef] [PubMed]
- Saroyan, H.S.; Bele, S.; Giannakoudakis, D.A.; Samanidou, V.F.; Bandosz, T.J.; Deliyanni, E.A. Degradation of endocrine disruptor, bisphenol-A, on an mixed oxidation state manganese oxide/modified graphite oxide composite: A role of carbonaceous phase. J. Colloid Interface Sci. 2019, 539, 516–524. [Google Scholar] [CrossRef]
- Song, C.; Guo, B.-B.; Sun, X.-F.; Wang, S.-G.; Li, Y.-T. Enrichment and degradation of tetracycline using three-dimensional graphene/MnO2 composites. Chem. Eng. J. 2019, 358, 1139–1146. [Google Scholar] [CrossRef]
- Xiao, R.; Luo, Z.; Wei, Z.; Luo, S.; Spinney, R.; Yang, W.; Dionysiou, D.D. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Curr. Opin. Chem. Eng. 2018, 19, 51–58. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Chen, H.; Carroll, K.C. Metal-free catalysis of persulfate activation and organic-pollutant degradation by nitrogen-doped graphene and aminated graphene. Environ. Pollut. 2016, 215, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Hu, M.; Xing, R.; Zhou, W. Synergistic degradation of methylparaben on CuFe2O4-rGO composite by persulfate activation. J. Alloys Compd. 2020, 823. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, X.; Yuan, J.; Zuo, Y.; Tao, Y.; Yao, D.; He, G.; Chen, H. Heterogeneous activation of persulfate for the degradation of bisphenol A with Ni2SnO4–RGO. New J. Chem. 2020, 44, 6355–6361. [Google Scholar] [CrossRef]
- Vieira, O.; Ribeiro, R.S.; Pedrosa, M.; Lado Ribeiro, A.R.; Silva, A.M.T. Nitrogen-doped reduced graphene oxide—PVDF nanocomposite membrane for persulfate activation and degradation of water organic micropollutants. Chem. Eng. J. 2020, 402. [Google Scholar] [CrossRef]
- Yang, L.; Xu, L.; Bai, X.; Jin, P. Enhanced visible-light activation of persulfate by Ti3+ self-doped TiO2/graphene nanocomposite for the rapid and efficient degradation of micropollutants in water. J. Hazard. Mater. 2019, 365, 107–117. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Z.Y.; Feng, Y.; Zhou, Y.; Wen, L.; Shih, K.M. Degradation mechanisms of ofloxacin and cefazolin using peroxymonosulfate activated by reduced graphene oxide-CoFe2O4 composites. Chem. Eng. J. 2020, 383, ARTN 123056. [Google Scholar] [CrossRef]
- Solis, R.R.; Mena, I.F.; Nadagouda, M.N.; Dionysiou, D.D. Adsorptive interaction of peroxymonosulfate with graphene and catalytic assessment via non-radical pathway for the removal of aqueous pharmaceuticals. J. Hazard. Mater. 2020, 384, 121340. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, D.; Liu, C.; Cai, H.; Qu, Y.; Yang, S.; Gao, Y.; Cai, T. Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration. Chem. Eng. J. 2018, 334, 273–284. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; San Martín, I.; Carrillo, P. Effect of sodium persulfate as electron acceptor on antipyrine degradation by solar TiO2 or TiO2/rGO photocatalysis. Chem. Eng. J. 2019, 364, 257–268. [Google Scholar] [CrossRef]
- Park, C.M.; Heo, J.; Wang, D.; Su, C.; Yoon, Y. Heterogeneous activation of persulfate by reduced graphene oxide-elemental silver/magnetite nanohybrids for the oxidative degradation of pharmaceuticals and endocrine disrupting compounds in water. Appl. Catal. B 2018, 225, 91–99. [Google Scholar] [CrossRef]
- Zuo, W.; Wang, X.; Zhang, D.; Sun, Y.; Xu, J.; Guo, P. Performance and Mechanism of GO-MCM-Fe Composite Catalyst Activating Persulfate to Remove Levofloxacin Hydrochloride in Water. Water Air Soil Pollut. 2019, 230, 255. [Google Scholar] [CrossRef]
- Peng, G.; Zhang, M.; Deng, S.; Shan, D.; He, Q.; Yu, G. Adsorption and catalytic oxidation of pharmaceuticals by nitrogen-doped reduced graphene oxide/Fe3O4 nanocomposite. Chem. Eng. J. 2018, 341, 361–370. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, H.; Duan, X.; Sun, H.; Tan, X.; Wang, S. Nickel in hierarchically structured nitrogen-doped graphene for robust and promoted degradation of antibiotics. J. Clean. Prod. 2019, 218, 202–211. [Google Scholar] [CrossRef]
- Genç, N.; Durna, E.; Gengec, E. Heterogeneous Activation of Persulfate by Graphene Oxide-TiO2 Nanosheet for Oxidation of Diclofenac: Optimization by Central Composite Design. Water Air Soil Pollut. 2018, 229, 330. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Pena-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. NPJ Clean Water 2020, 3, ARTN 1. [Google Scholar] [CrossRef]
- Bhatia, V.; Manoli, K.; Dhir, A.; Ray, A.K. Response surface optimization of the photocatalytic degradation of atenolol using immobilized graphene-TiO2 composite. Can. J. Chem. Eng. 2020, 98, 1767–1775. [Google Scholar] [CrossRef]
- Cheshme Khavar, A.H.; Moussavi, G.; Mahjoub, A.R. The preparation of TiO2@rGO nanocomposite efficiently activated with UVA/LED and H2O2 for high rate oxidation of acetaminophen: Catalyst characterization and acetaminophen degradation and mineralization. Appl. Surf. Sci. 2018, 440, 963–973. [Google Scholar] [CrossRef]
- Bilgin Simsek, E.; Kilic, B.; Asgin, M.; Akan, A. Graphene oxide based heterojunction TiO2–ZnO catalysts with outstanding photocatalytic performance for bisphenol-A, ibuprofen and flurbiprofen. J. Ind. Eng. Chem. 2018, 59, 115–126. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ahamad, T.; Veses, R.C.; Stadler, F.J. Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: The triggering effect of Ag and RGO. Chem. Eng. J. 2019, 370, 148–165. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.K.; Sharma, G.; Al-Muhtaseb, A.A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Wide spectral degradation of Norfloxacin by Ag@BiPO4/BiOBr/BiFeO3 nano-assembly: Elucidating the photocatalytic mechanism under different light sources. J. Hazard. Mater. 2019, 364, 429–440. [Google Scholar] [CrossRef]
- Xu, G.; Li, M.; Wang, Y.; Zheng, N.; Yang, L.; Yu, H.; Yu, Y. A novel Ag-BiOBr-rGO photocatalyst for enhanced ketoprofen degradation: Kinetics and mechanisms. Sci. Total Environ. 2019, 678, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Mohan, H.; Lim, J.M.; Lee, S.W.; Cho, M.; Park, Y.J.; Seralathan, K.K.; Oh, B.T. V2O5/RGO/Pt nanocomposite on oxytetracycline degradation and pharmaceutical effluent detoxification. J. Chem. Technol. Biotechnol. 2019, 95, 297–307. [Google Scholar] [CrossRef]
- Dittrich, H.; Stadler, A.; Topa, D.; Schimper, H.-J.; Basch, A. Progress in sulfosalt research. Phys. Status Solidi A 2009, 206, 1034–1041. [Google Scholar] [CrossRef]
- Che, W.; Luo, Y.; Deng, F.; Zhang, A.; Zhao, L.; Luo, X.; Ruan, Q. Facile solvothermal fabrication of cubic-like reduced graphene oxide/AgIn5S8 nanocomposites with anti-photocorrosion and high visible-light photocatalytic performance for highly-efficient treatment of nitrophenols and real pharmaceutical wastewater. Appl. Catal. A Gen. 2018, 565, 170–180. [Google Scholar] [CrossRef]
- Fu, K.; Pan, Y.; Ding, C.; Shi, J.; Deng, H. Reduced Graphene Oxide/ZnIn2S4 Nanocomposite Photocatalyst with Enhanced Photocatalytic Performance for the Degradation of Naproxen under Visible Light Irradiation. Catalysts 2020, 10, 710. [Google Scholar] [CrossRef]
- Rajendran, R.; Shrestha, L.K.; Minami, K.; Subramanian, M.; Jayavel, R.; Ariga, K. Dimensionally integrated nanoarchitectonics for a novel composite from 0D, 1D, and 2D nanomaterials: RGO/CNT/CeO2 ternary nanocomposites with electrochemical performance. J. Mater. Chem. A 2014, 2, 18480–18487. [Google Scholar] [CrossRef]
- Arumugam, M.; Lee, S.J.; Begildayeva, T.; Naik, S.S.; Yu, Y.; Lee, H.; Theerthagiri, J.; Choi, M.Y. Enhanced photocatalytic activity at multidimensional interface of 1D-Bi2S3@2D-GO/3D-BiOI ternary nanocomposites for tetracycline degradation under visible-light. J. Hazard. Mater. 2021, 404, 123868. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, A.; Sharma, G.; Du, B.; Naushad, M.; Stadler, F.J. Carbon quantum dots and reduced graphene oxide modified self-assembled S@C3N4/B@C3N4 metal-free nano-photocatalyst for high performance degradation of chloramphenicol. J. Mol. Liq. 2020, 300. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Al-Muhtaseb, A.H.; Kumar, A.; Hira, I.; Ahamad, T.; Ghfar, A.A.; Stadler, F.J. Visible photodegradation of ibuprofen and 2,4-D in simulated waste water using sustainable metal free-hybrids based on carbon nitride and biochar. J. Environ. Manag. 2019, 231, 1164–1175. [Google Scholar] [CrossRef]
- Donga, C.; Mishra, S.B.; Abd-El-Aziz, A.S.; Mishra, A.K. Advances in Graphene-Based Magnetic and Graphene-Based/TiO2 Nanoparticles in the Removal of Heavy Metals and Organic Pollutants from Industrial Wastewater. J. Inorg. Organomet. Polym. Mater. 2020. [Google Scholar] [CrossRef]
- El-Fawal, E.M.; Younis, S.A.; Zaki, T. Designing AgFeO2-graphene/Cu2(BTC)3 MOF heterojunction photocatalysts for enhanced treatment of pharmaceutical wastewater under sunlight. J. Photochem. Photobiol. A Chem. 2020, 401, 112746. [Google Scholar] [CrossRef]
- El-Maraghy, C.M.; El-Borady, O.M.; El-Naem, O.A. Effective Removal of Levofloxacin from Pharmaceutical Wastewater Using Synthesized Zinc Oxide, Graphen Oxide Nanoparticles Compared with their Combination. Sci. Rep. 2020, 10, 5914. [Google Scholar] [CrossRef] [PubMed]
- Mohan, H.; Selvaraj, D.; Kuppusamy, S.; Venkatachalam, J.; Park, Y.J.; Seralathan, K.K.; Oh, B.T. E-Waste Based V2O5/RGO/Pt Nanocomposite for Photocatalytic Degradation of Oxytetracycline. Environ. Prog. Sustain. Energy 2018, 38, 13123. [Google Scholar] [CrossRef]
- Qureshi, K.; Ahmad, M.Z.; Bhatti, I.A.; Zahid, M.; Nisar, J.; Iqbal, M. Graphene oxide decorated ZnWO4 architecture synthesis, characterization and photocatalytic activity evaluation. J. Mol. Liq. 2019, 285, 778–789. [Google Scholar] [CrossRef]
- Entezami, N.; Farhadian, M.; Davari, N. Removal of metronidazole antibiotic pharmaceutical from aqueous solution using TiO2/Fe2O3/GO photocatalyst: Experimental study on the effects of mineral salts. Adv. Environ. Technol. 2019, 5, 55–65. [Google Scholar] [CrossRef]
- Shinde, Y.; Wadhai, S.; Ponkshe, A.; Kapoor, S.; Thakur, P. Decoration of Pt on the metal free RGO-TiO2 composite photocatalyst for the enhanced photocatalytic hydrogen evolution and photocatalytic degradation of pharmaceutical pollutant β blocker. Int. J. Hydrogen Energy 2018, 43, 4015–4027. [Google Scholar] [CrossRef]
- Esmaeili, A.; Entezari, M.H. Cubic Ag/AgBr-graphene oxide nanocomposite: Sono-synthesis and use as a solar photocatalyst for the degradation of DCF as a pharmaceutical pollutant. RSC Adv. 2015, 5, 97027–97035. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martinez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Graphene oxide-P25 photocatalysts for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Surf. Sci. 2013, 275, 361–368. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M.T. Advanced nanostructured photocatalysts based on reduced graphene oxide–TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Xu, P.; Wang, P.; Wang, Q.; Wei, R.; Li, Y.; Xin, Y.; Zheng, T.; Hu, L.; Wang, X.; Zhang, G. Facile synthesis of Ag2O/ZnO/rGO heterojunction with enhanced photocatalytic activity under simulated solar light: Kinetics and mechanism. J. Hazard. Mater. 2021, 403, 124011. [Google Scholar] [CrossRef]
- Ali, A.; Shoeb, M.; Li, B.; Khan, M.A. Enhanced Photcatalytic Degradation of Antibiotic Drug and Dye Pollutants by graphene Ordered Mesoporous Silica (SBA15)/TiO2 Nanocomposite Under Visible-Light Irradiation. J. Mol. Liq. 2020, 114696. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Zhang, J.; Jin, S.; Zheng, H. Efficient adsorption and full spectrum photocatalytic degradation of low concentration PPCPs promoted by graphene/TiO2 nanowires hybrid structure in 3D hydrogel networks. RSC Adv. 2020, 10, 27050–27057. [Google Scholar] [CrossRef]
- Zhao, W.; Duan, J.; Ji, B.; Ma, L.; Yang, Z. Novel formation of large area N-TiO2/graphene layered materials and enhanced photocatalytic degradation of antibiotics. J. Environ. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Nourieh, N.; Nabizadeh, R.; Faramarzi, M.A.; Nasseri, S.; Yaghmaeian, K.; Mahmoudi, B.; Alimohammadi, M.; Khoobi, M. Photocatalytic degradation of ketoconazole by Z-scheme Ag3PO4/graphene oxide: Response surface modeling and optimization. Environ. Sci. Pollut. Res. Int. 2020, 27, 250–263. [Google Scholar] [CrossRef]
- Liu, X.; Ji, H.; Li, S.; Liu, W. Graphene modified anatase/titanate nanosheets with enhanced photocatalytic activity for efficient degradation of sulfamethazine under simulated solar light. Chemosphere 2019, 233, 198–206. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Sobhani, S.; Shekari, H. Photocatalytic degradation of azithromycin using GO@Fe3O4/ ZnO/ SnO2 nanocomposites. J. Clean. Prod. 2019, 232, 127–136. [Google Scholar] [CrossRef]
- Moztahida, M.; Jang, J.; Nawaz, M.; Lim, S.R.; Lee, D.S. Effect of rGO loading on Fe3O4: A visible light assisted catalyst material for carbamazepine degradation. Sci. Total Environ. 2019, 667, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Soltani, T.; Tayyebi, A.; Lee, B.K. Photolysis and photocatalysis of tetracycline by sonochemically heterojunctioned BiVO4/reduced graphene oxide under visible-light irradiation. J. Environ. Manag. 2019, 232, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Cheshme Khavar, A.H.; Moussavi, G.; Mahjoub, A.; Yaghmaeian, K.; Srivastava, V.; Sillanpää, M.; Satari, M. Novel magnetic Fe3O4@rGO@ZnO onion-like microspheres decorated with Ag nanoparticles for the efficient photocatalytic oxidation of metformin: Toxicity evaluation and insights into the mechanisms. Catal. Sci. Technol. 2019, 9, 5819–5837. [Google Scholar] [CrossRef]
- Rostami, M.; Zamani, R.M.; Aghajanzadeh, K.M.; Danafar, H. Sol–gel synthesis and characterization of zinc ferrite–graphene nano-hybrids for photo-catalytic degradation of the paracetamol. J. Pharm. Investig. 2017, 48, 657–664. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Rodrigo, M.A.; Sires, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Divyapriya, G.; Srinivasan, R.; Nambi, I.M.; Senthilnathan, J. Highly active and stable ferrocene functionalized graphene encapsulated carbon felt array—A novel rotating disc electrode for electro-Fenton oxidation of pharmaceutical compounds. Electrochim. Acta 2018, 283, 858–870. [Google Scholar] [CrossRef]
- Deng, F.X.; Garcia-Rodriguez, O.; Olvera-Vargas, H.; Qiu, S.; Lefebvre, O.; Yang, J.X. Iron-foam as a heterogeneous catalyst in the presence of tripolyphosphate electrolyte for improving electro-Fenton oxidation capability. Electrochim. Acta 2018, 272, 176–183. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Garrido, J.A.; Rodriguez, R.M.; Cabot, P.L.; Centellas, F.; Arias, C.; Brillas, E. Mineralization of flumequine in acidic medium by electro-Fenton and photoelectro-Fenton processes. Water Res. 2012, 46, 2067–2076. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Chai, S.; Zhao, H.; Wang, Y. Three-dimensional homogeneous ferrite-carbon aerogel: One pot fabrication and enhanced electro-Fenton reactivity. ACS Appl. Mater. Interfaces 2013, 5, 842–852. [Google Scholar] [CrossRef]
- Dong, P.; Liu, W.J.; Wang, S.J.; Wang, H.L.; Wang, Y.Q.; Zhao, C.C. In suit synthesis of Fe3O4 on carbon fiber paper@polyaniline substrate as novel self-supported electrode for heterogeneous electro-Fenton oxidation. Electrochim. Acta 2019, 308, 54–63. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, Y.J.; Wang, Y.B.; Cao, T.C.; Zhao, G.H. Electro-Fenton oxidation of pesticides with a novel Fe3O4@Fe2O3/activated carbon aerogel cathode: High activity, wide pH range and catalytic mechanism. Appl. Catal. B 2012, 125, 120–127. [Google Scholar] [CrossRef]
- Fernández-Sáez, N.; Villela-Martinez, D.E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Pastrana-Martínez, L.M. Heteroatom-doped graphene aerogels and carbon-magnetite catalysts for the heterogeneous electro-Fenton degradation of acetaminophen in aqueous solution. J. Catal. 2019, 378, 68–79. [Google Scholar] [CrossRef]
- Görmez, F.; Görmez, Ö.; Gözmen, B.; Kalderis, D. Degradation of chloramphenicol and metronidazole by electro-Fenton process using graphene oxide-Fe3O4 as heterogeneous catalyst. J. Environ. Chem. Eng. 2019, 7, 102990. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Li, B.; Yu, M.; Zhao, R.; Xu, X.; Cai, L. γ-FeOOH graphene polyacrylamide carbonized aerogel as air-cathode in electro-Fenton process for enhanced degradation of sulfamethoxazole. Chem. Eng. J. 2019, 359, 914–923. [Google Scholar] [CrossRef]
- Mi, X.; Han, J.; Sun, Y.; Li, Y.; Hu, W.; Zhan, S. Enhanced catalytic degradation by using RGO-Ce/WO3 nanosheets modified CF as electro-Fenton cathode: Influence factors, reaction mechanism and pathways. J. Hazard. Mater. 2019, 367, 365–374. [Google Scholar] [CrossRef]
- Yu, S.; Gao, Y.; Khan, R.; Liang, P.; Zhang, X.; Huang, X. Electrospun PAN-based graphene/SnO2 carbon nanofibers as anodic electrocatalysis microfiltration membrane for sulfamethoxazole degradation. J. Membr. Sci. 2020, 614, 118368. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Li, W.; Fan, W.; Song, S.; Yang, J. Adsorption and degradation of sulfadiazine over nanoscale zero-valent iron encapsulated in three-dimensional graphene network through oxygen-driven heterogeneous Fenton-like reactions. Appl. Catal. B 2019, 259, 118057. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nambi, I.; Senthilnathan, J. Ferrocene functionalized graphene based electrode for the electro−Fenton oxidation of ciprofloxacin. Chemosphere 2018, 209, 113–123. [Google Scholar] [CrossRef]
- Zarei, M.; Beheshti Nahand, F.; Khataee, A.; Hasanzadeh, A. Removal of nalidixic acid from aqueous solutions using a cathode containing three-dimensional graphene. J. Water Process Eng. 2019, 32, 100978. [Google Scholar] [CrossRef]
- Kumar, A.; Omar, R.A.; Verma, N. Efficient electro-oxidation of diclofenac persistent organic pollutant in wastewater using carbon film-supported Cu-rGO electrode. Chemosphere 2020, 248, 126030. [Google Scholar] [CrossRef]
- Mirzazadeh, H.; Lashanizadegan, M. Ag2O/CdO/CeO2/reduced graphene oxide: Synthesis, characterization and its application as catalyst for sulfasalazine, diclofenac and phenol degradation under ultrasonic irradiation. Mater. Res. Express 2019, 6, 065008. [Google Scholar] [CrossRef]
- Lee, G.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Heo, J.; Her, N.; Kim, D.H.; Yoon, Y. Fabrication of graphene-oxide/beta-Bi2O3/TiO2/Bi2Ti2O7 heterojuncted nanocomposite and its sonocatalytic degradation for selected pharmaceuticals. Chemosphere 2018, 212, 723–733. [Google Scholar] [CrossRef]
- Moradi, S.; Sobhgol, S.A.; Hayati, F.; Isari, A.A.; Kakavandi, B.; Bashardoust, P.; Anvaripour, B. Performance and reaction mechanism of MgO/ZnO/Graphene ternary nanocomposite in coupling with LED and ultrasound waves for the degradation of sulfamethoxazole and pharmaceutical wastewater. Sep. Purif. Technol. 2020, 251, ARTN 117373. [Google Scholar] [CrossRef]
- Akpotu, S.O.; Oseghe, E.O.; Ayanda, O.S.; Skelton, A.A.; Msagati, T.A.M.; Ofomaja, A.E. Photocatalysis and biodegradation of pharmaceuticals in wastewater: Effect of abiotic and biotic factors. Clean Technol. Environ. Policy 2019, 21, 1701–1721. [Google Scholar] [CrossRef]
- Tayeb, A.; Hussein, D. Synthesis of TiO2 Nanoparticles and Their Photocatalytic Activity for Methylene Blue. Am. J. Nanomater. 2015, 3, 57–63. [Google Scholar] [CrossRef]
- Kaur, H.; Dahake, R.; Maddigapu, P.R.; Hippargi, G.; Pophali, G.R.; Bansiwal, A. Enhanced photocatalytic degradation of antimicrobial triclosan using rGO–TiO2 composite under natural solar illumination. J. Mater. Sci. Mater. Electron. 2020, 31, 6045–6058. [Google Scholar] [CrossRef]
- Song, J.; Zhen, X.; Chang, C.-T. Hydrothermal Synthesis of Graphene and Titanium Dioxide Nanotubes by a One-Step Method for the Photocatalytic Degradation of Amoxicillin. Nanosci. Nanotechnol. Lett. 2016, 8, 113–119. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Choina, J.; Kosslick, H.; Fischer, C.; Flechsig, G.U.; Frunza, L.; Schulz, A. Photocatalytic decomposition of pharmaceutical ibuprofen pollutions in water over titania catalyst. Appl. Catal. B 2013, 129, 589–598. [Google Scholar] [CrossRef]
- Azim, M.B.; Nargis, F.; Dhar, S.; Qadir, M.; Gafur, M.; Asw, K.; Gulshan, F. Effect of Dissolved Oxygen Content on Photocatalytic Performance of Graphene Oxide; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lee, H.; Park, S.H.; Kim, B.H.; Kim, S.-J.; Kim, S.-C.; Seo, S.-G.; Jung, S.-C. Contribution of Dissolved Oxygen to Methylene Blue Decomposition by Hybrid Advanced Oxidation Processes System. Int. J. Photoenergy 2012, 2012, 305989. [Google Scholar] [CrossRef]
- Subramanian, M.; Kannan, A. Effect of dissolved oxygen concentration and light intensity on photocatalytic degradation of phenol. Korean J. Chem. Eng. 2008, 25, 1300–1308. [Google Scholar] [CrossRef]
- Malekshoar, G.; Pal, K.; He, Q.; Yu, A.; Ray, A.K. Enhanced Solar Photocatalytic Degradation of Phenol with Coupled Graphene-Based Titanium Dioxide and Zinc Oxide. Ind. Eng. Chem. Res. 2014, 53, 18824–18832. [Google Scholar] [CrossRef]
- Zanta, C.L.P.S.; Friedrich, L.C.; Machulek, A.; Higa, K.M.; Quina, F.H. Surfactant degradation by a catechol-driven Fenton reaction. J. Hazard. Mater. 2010, 178, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Araña, J.; Tello Rendón, E.; Doña Rodríguez, J.M.; Herrera Melián, J.A.; González Díaz, O.; Pérez Peña, J. Highly concentrated phenolic wastewater treatment by the Photo-Fenton reaction, mechanism study by FTIR-ATR. Chemosphere 2001, 44, 1017–1023. [Google Scholar] [CrossRef]
- Tokumura, M.; Sugawara, A.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Masunaga, S. Comprehensive study on effects of water matrices on removal of pharmaceuticals by three different kinds of advanced oxidation processes. Chemosphere 2016, 159, 317–325. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).