Diesel in Antarctica and a Bibliometric Study on Its Indigenous Microorganisms as Remediation Agent
Abstract
1. Introduction
2. Review Methodology
3. Diesel and Its Use in Antarctica
4. Diesel Pollution and Toxicity
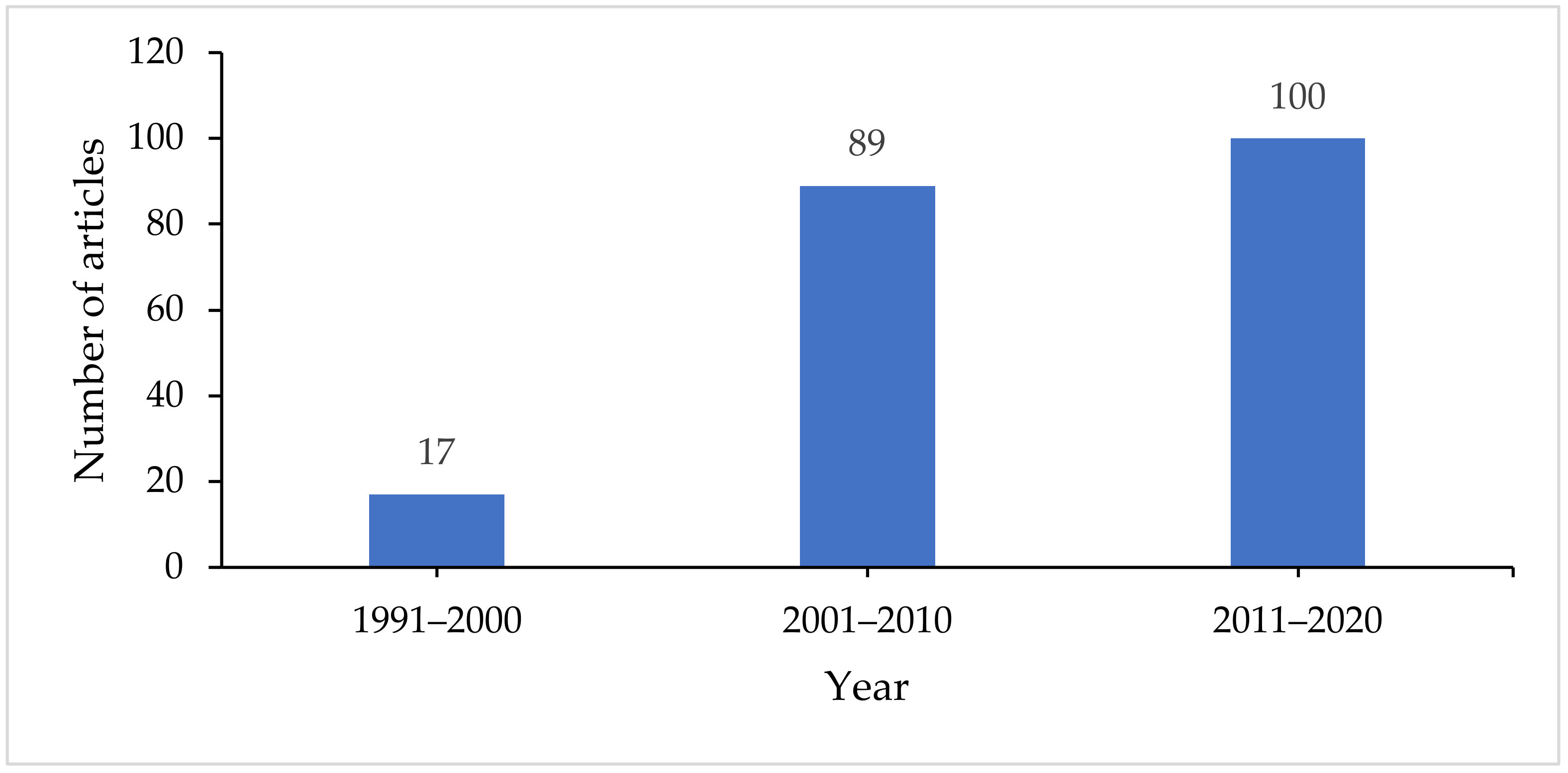
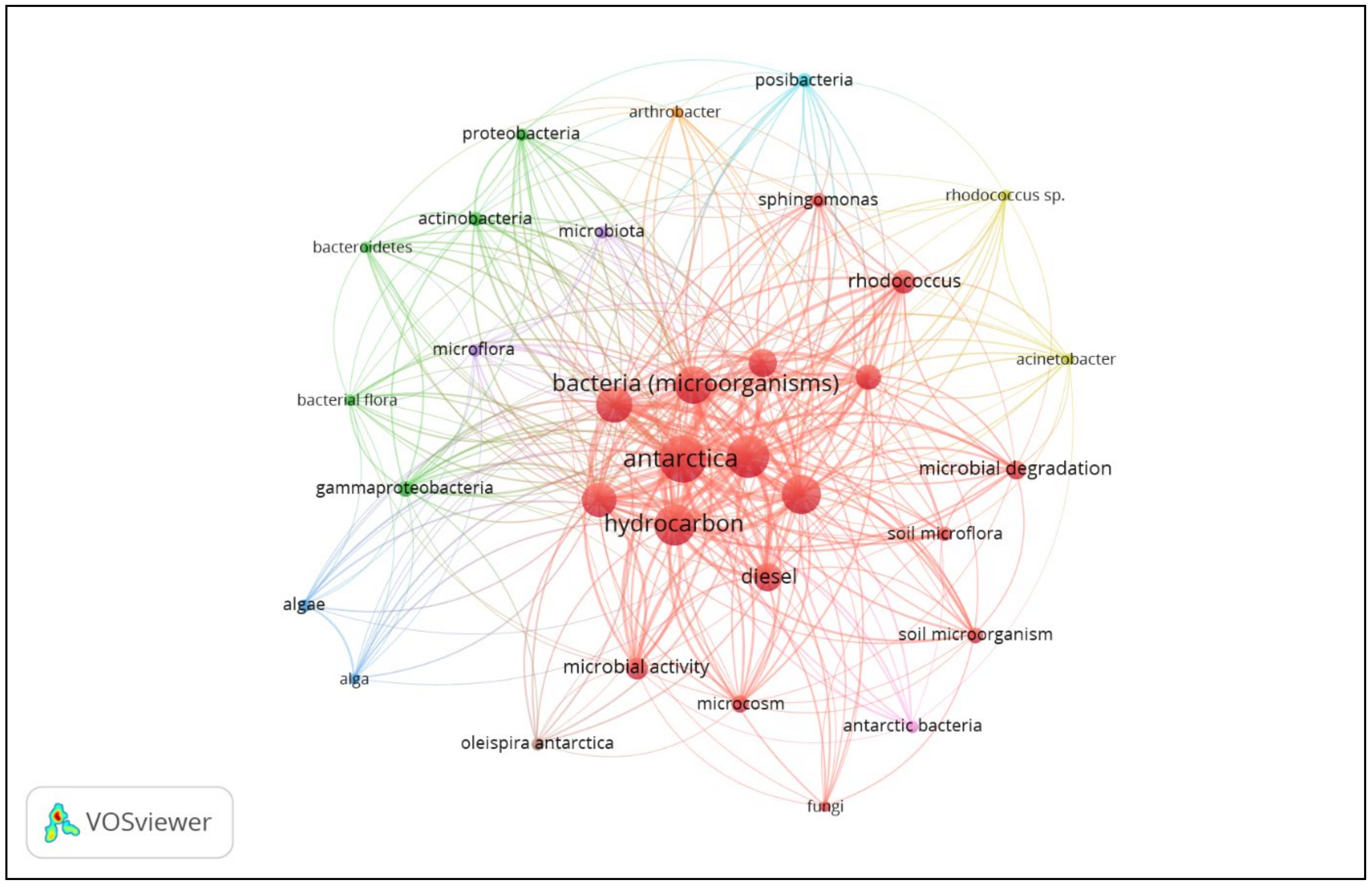
5. Role of Antarctic Microbes in Diesel Remediation—A Bibliometric Analysis
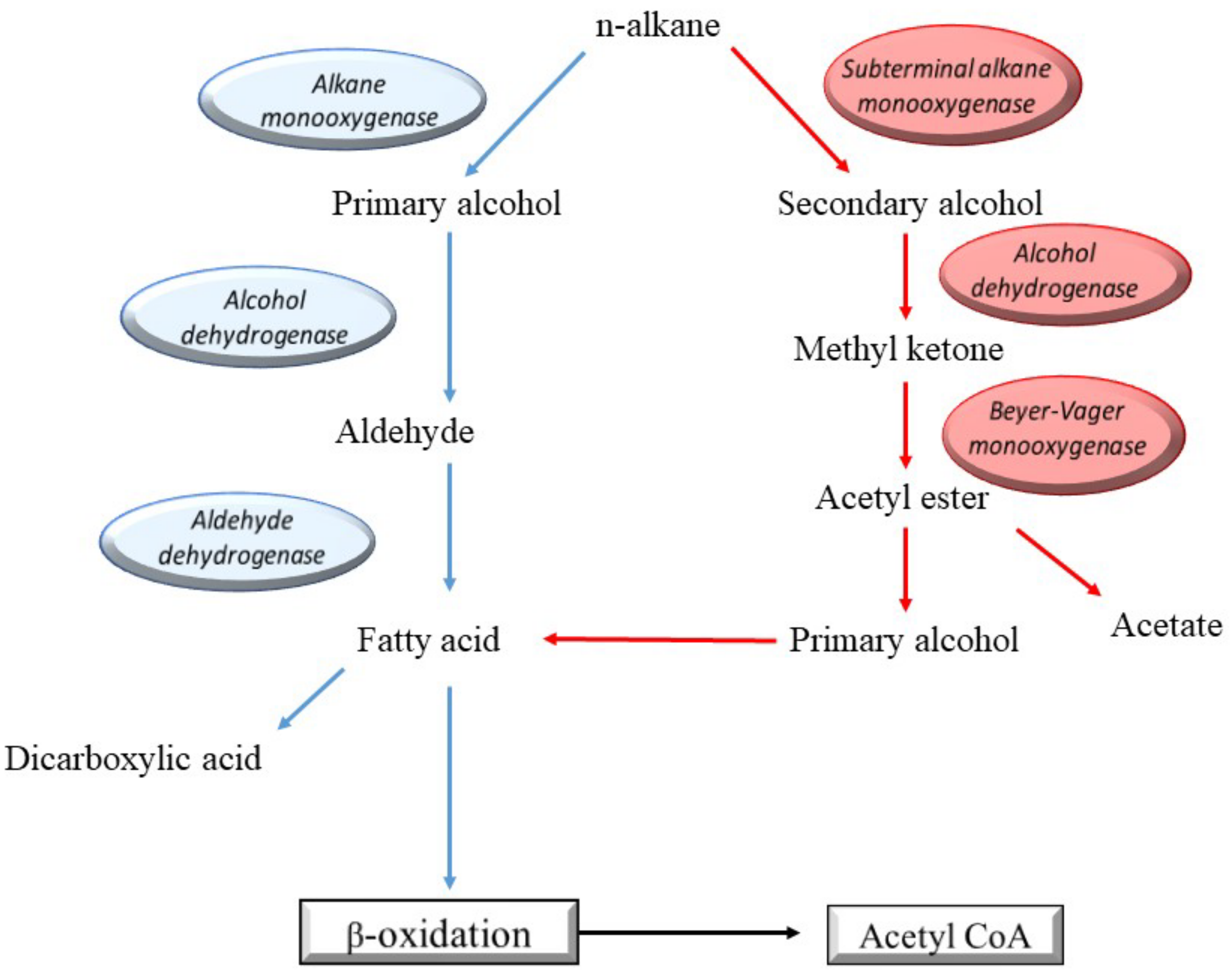
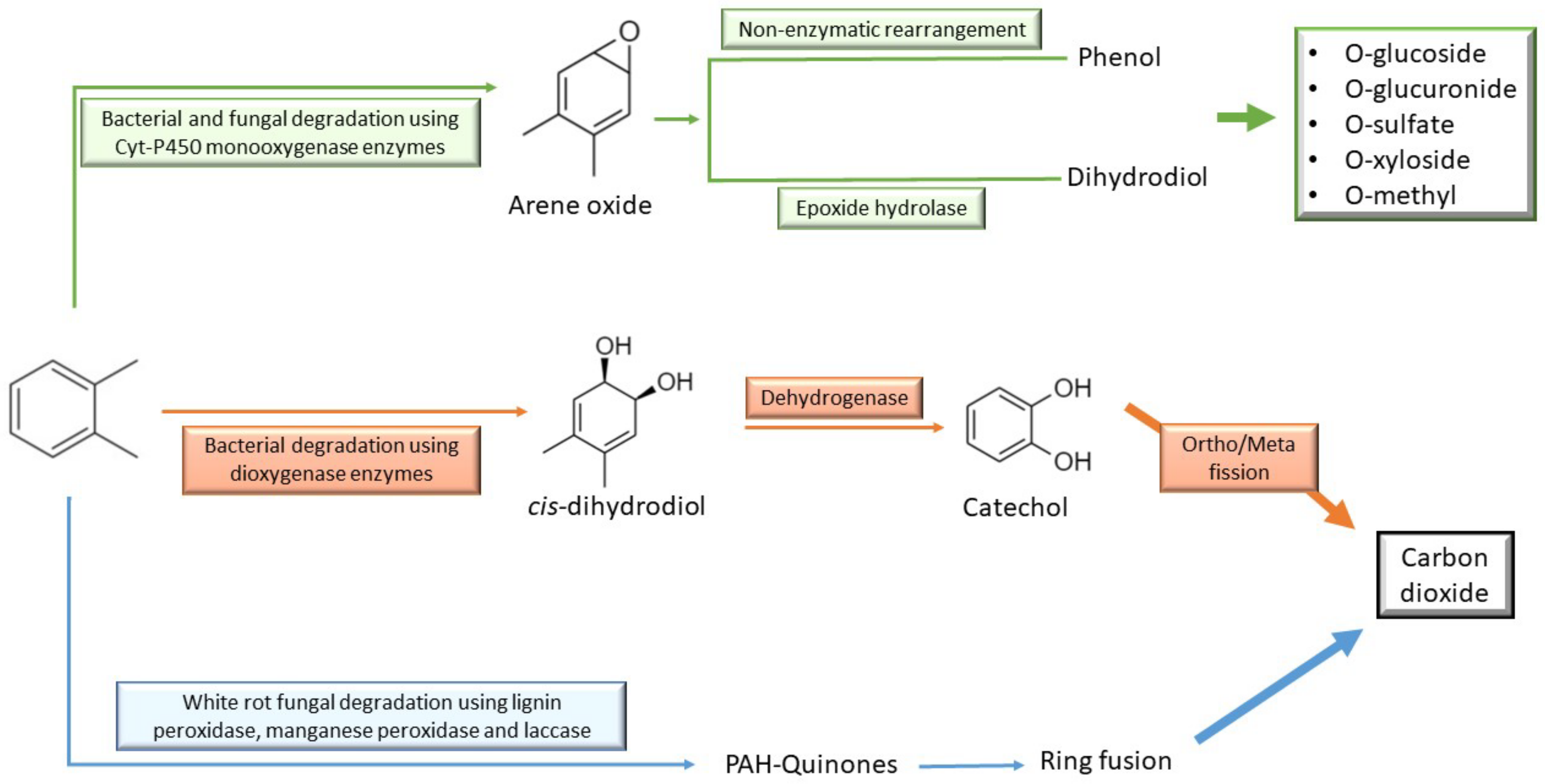
6. Microbial Diesel Degradation and Its Metabolic Pathways
7. Microbial Adaptation Mechanisms in Cold Region
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Errington, I.; King, C.K.; Wilkins, D.; Spedding, T.; Hose, G.C. Ecosystem effects and the management of petroleum-contaminated soils on sub-Antarctic islands. Chemosphere 2018, 194, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; King, C.K.; Kotzakoulakis, K.; George, S.C.; Harrison, P.L. Assessing fuel spill risks in polar waters: Temporal dynamics and behaviour of hydrocarbons from Antarctic diesel, marine gas oil and residual fuel oil. Mar. Pollut. Bull. 2016, 110, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Macoustra, G.K.; King, C.K.; Wasley, J.; Robinson, S.A.; Jolley, D.F. Impact of hydrocarbons from a diesel fuel on the germination and early growth of subantarctic plants. Environ. Sci. Process Impacts 2015, 17, 1238–1248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dorst, J.; Siciliano, S.D.; Winsley, T.; Snape, I.; Ferrari, B.C. Bacterial targets as potential indicators of diesel fuel toxicity in subantarctic soils. Appl. Environ. Microbiol. 2014, 80, 4021–4033. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; King, C.K.; Harrison, P.L. Lethal and behavioral impacts of diesel and fuel oil on the Antarctic amphipod Paramoera walkeri. Environ. Toxicol. Chem. 2017, 36, 2444–2455. [Google Scholar] [CrossRef]
- Alexander, F.J.; King, C.K.; Reichelt-Brushett, A.J.; Harrison, P.L. Fuel oil and dispersant toxicity to the Antarctic sea urchin (Sterechinus neumayeri). Environ. Toxicol. Chem. 2017, 36, 1563–1571. [Google Scholar] [CrossRef]
- Hughes, K.A.; Constable, A.; Frenot, Y.; López-Martínez, J.; McIvor, E.; Njåstad, B.; Terauds, A.; Liggett, D.; Roldan, G.; Wilmotte, A.; et al. Antarctic environmental protection: Strengthening the links between science and governance. Environ. Sci. Policy 2018, 83, 86–95. [Google Scholar] [CrossRef]
- Antarctica: The Madrid Protocol 25 Years on—Australian Institute of International Affairs. Available online: https://www.internationalaffairs.org.au (accessed on 26 September 2020).
- Lyons, W.B.; Saelens, E.; Welch, K.A. The impact of fossil fuel burning related to scientific activities in the McMurdo Dry Valleys, Antarctica: Revisited. Elementa 2018, 6. [Google Scholar] [CrossRef]
- Antarctic Governance: From ATCM to a Permanent Antarctic Organization? Available online: https://digitalcommons.law.ggu.edu (accessed on 26 December 2020).
- Bennett, J.R.; Shaw, J.D.; Terauds, A.; Smol, J.P.; Aerts, R.; Bergstrom, D.M.; Blais, J.M.; Cheung, W.W.; Chown, S.L.; Lea, M.A.; et al. Polar lessons learned: Long-term management based on shared threats in Arctic and Antarctic environments. Front. Ecol. Environ. 2015, 13, 316–324. [Google Scholar] [CrossRef]
- Martinez-Varela, A.; Casas, G.; Piña, B.; Dachs, J.; Vila-Costa, M. Large enrichment of anthropogenic organic matter degrading bacteria in the sea-surface microlayer at coastal Livingston Island (Antarctica). Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Malavenda, R.; Rizzo, C.; Michaud, L.; Gerçe, B.; Bruni, V.; Syldatk, C.; Hausmann, R.; Lo Giudice, A. Biosurfactant production by Arctic and Antarctic bacteria growing on hydrocarbons. Polar Biol. 2015, 38, 1565–1574. [Google Scholar] [CrossRef]
- Speight, J.G. Diesel Fuel. In Handbook of Petroleum Product Analysis; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 155–168. [Google Scholar]
- Li, Z.; Liu, G.; Cui, X.; Sun, X.; Li, S.; Qian, Y.; Jiang, C.; Lu, X. Effects of the variation in diesel fuel components on the particulate matter and unregulated gaseous emissions from a common rail diesel engine. Fuel 2018, 232, 279–289. [Google Scholar] [CrossRef]
- Dupuis, A.; Ucan-Marin, F. A Literature Review on the Aquatic Toxicology of Petroleum Oil: An Overview of Oil Properties and Effects to Aquatic Biota; Research Document; Canadian Science Advisory Secretiat (CSAS): Ottawa, ON, Canada, 2015; No. 007: 52. [Google Scholar]
- Brown, K.E. Impacts of Diesel and Residual Fuel Oil on Antarctic Marine Invertebrates. Ph.D. Thesis, Southern Cross University, Lismore, Australia, 2017. [Google Scholar]
- Amendments to the Annex of the Protocol of 1978 Relating to the International Convention for the Prevention of Pollution from Ships. 1973. Available online: http://www.imo.org (accessed on 21 September 2020).
- Lim, Y.K.; Kim, J.Y.; Kim, J.R.; Ha, J.H. Study of alternative fuel suitability for special Antarctic blend diesel. Appl. Chem. Eng. 2017, 28, 460–466. [Google Scholar] [CrossRef]
- De Christo, T.M.; Fardin, J.F.; Simonetti, D.S.L.; Encarnação, L.F.; de Alvarez, C.E. Design and analysis of hybrid energy systems: The Brazilian Antarctic Station case. Renew. Energy 2016, 88, 236–246. [Google Scholar] [CrossRef]
- Ayodele, T.R.; Ogunjuyigbe, A.S.O. Wind energy potential of Vesleskarvet and the feasibility of meeting the South Africans SANAE IV energy demand. Renew. Sustain. Energy Rev. 2016, 56, 226–234. [Google Scholar] [CrossRef]
- Dou, Y.; Zuo, G.; Chang, X.; Chen, Y. A study of a standalone renewable energy system of the Chinese Zhongshan Station in Antarctica. Appl. Sci. 2019, 9, 1968. [Google Scholar] [CrossRef]
- Cheek, J.; Huyge, B.; De Pomereu, J. Princess Elisabeth Antarctica: An international polar year outreach and media success story. Polar Res. 2011, 30 (Suppl. 1). [Google Scholar] [CrossRef]
- Tidal Energy Resource Assessment for McMurdo Station, Antarctica Tidal Energy Resource Assessment for McMurdo Station, Antarctica. 2016. Available online: https://www.semanticscholar.org (accessed on 26 December 2020).
- The Antarctic Sun: News about Antarctica—Wind Farm. Available online: https://antarcticsun.usap.gov/features/1527 (accessed on 26 December 2020).
- Klekociuk, A.; Wienecke, B. Antarctic environment: Australian Antarctic program’s station environment: Operation indicators. In Australia State of the Environment 2016; Australian Government Department of the Environment and Energy: Canberra, Australia, 2016. [Google Scholar] [CrossRef]
- Tin, T.; Sovacool, B.K.; Blake, D.; Magill, P.; El Naggar, S.; Lidstrom, S.; Lidstrom, S.; Ishizawa, K.; Berte, J. Energy efficiency and renewable energy under extreme conditions: Case studies from Antarctica. Renew. Energy 2010, 35, 1715–1723. [Google Scholar] [CrossRef]
- Assessment of Green Power Production in Antarctica. 2013. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-341702 (accessed on 26 December 2020).
- Baring-Gould, I.; Robichaud, R.; Mclain, K. Analysis of the Use of Wind Energy to Supplement the Power Needs at McMurdo Station and Amundsen-Scott South Pole Station, Antarctica. In National Renewable Energy Laboratory 2005; United States Department of Energy: Golden, CO, USA, 2005. [Google Scholar]
- Kanai, T.; Obara, S.; Hamanaka, R.; Ishizawa, K.; Ouchi, T. Research on reduction of fuel consumption in the Showa Base microgrid by using distributed diesel generators. In Proceedings of the International Conference on Renewable Energy Research and Applications, Palermo, Italy, 22–25 November 2015; pp. 505–509. [Google Scholar] [CrossRef]
- Report of the Inspection Program Carried Out by Argentina and Chile under Article VII of the Antarctic Treaty and Article 14 of the Protocol on Environmental Protection. 2016. Available online: https://ats.aq (accessed on 20 November 2020).
- Klee, R.J. Industrial metabolism on ice: A case study of industrial materials flows and environmental management alternatives for Scott Base, New Zealand’s Antarctic research station. J. R. Soc. N. Z. 2001, 31, 393–409. [Google Scholar] [CrossRef]
- Arnold, M. The Potential for Solar Power at Scott Base; Supervised Project; University of Canterbury: Christchurch, New Zealand, 2015. [Google Scholar]
- Rodríguez, C.; Iglesias, K.; Bícego, M.C.; Taniguchi, S.; Sasaki, S.T.; Kandratavicius, N.; Bueno, C.; Brugnoli, E.; Venturini, N. Hydrocarbons in soil and meltwater stream sediments near Artigas Antarctic Research Station: Origin, sources and levels. Antarct. Sci. 2018, 30, 170–182. [Google Scholar] [CrossRef]
- Cabrerizo, A.; Tejedo, P.; Dachs, J.; Benayas, J. Anthropogenic and biogenic hydrocarbons in soils and vegetation from the South Shetland Islands (Antarctica). Sci. Total Environ. 2016, 569–570, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Prus, W.; Fabiańska, M.J.; Łabno, R. Geochemical markers of soil anthropogenic contaminants in polar scientific stations nearby (Antarctica, King George Island). Sci. Total Environ. 2015, 518–519, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.M. Environmental effects of human activities on King George Island, South Shetland Islands, Antarctica. Polar Rec. 1991, 27, 193–204. [Google Scholar] [CrossRef]
- Braun, C.; Mustafa, O.; Nordt, A.; Pfeiffer, S.; Peter, H.U. Environmental monitoring and management proposals for the Fildes Region, King George Island, Antarctica. Polar Res. 2012, 31. [Google Scholar] [CrossRef]
- McWatters, R.S.; Rowe, R.K.; Wilkins, D.; Spedding, T.; Hince, G.; Richardson, J.; Snape, I. Modelling of vapour intrusion into a building impacted by a fuel spill in Antarctica. J. Environ. Manag. 2019, 231, 467–482. [Google Scholar] [CrossRef]
- Lim, Z.S.; Wong, R.R.; Wong, C.; Zulkharnain, A.; Shaharuddin, N.A.; Ahmad, S.A. Bibliometric analysis of research on diesel pollution in Antarctica and a review on remediation techniques. Appl. Sci. 2021, 11, 1123. [Google Scholar] [CrossRef]
- Sweet, S.T.; Kennicutt, M.C.; Klein, A.G. The Grounding of the Bahia Paraiso, Arthur Harbor, Antarctica. In Handbook of Oil Spill Science and Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 547–556. [Google Scholar]
- Liggett, D.; Frame, B.; Gilbert, N.; Morgan, F. Is it all going south? Four future scenarios for Antarctica. Polar Rec. 2017, 53, 459–478. [Google Scholar] [CrossRef]
- Ship Accidents in Antarctica Raise Ecological and Safety Concerns: Travel Weekly. 2009. Available online: https://www.travelweekly.com/Cruise-Travel/Ship-accidents-in-Antarctica-raise-ecological-and-safety-concerns (accessed on 26 December 2020).
- Ruoppolo, V.; Woehler, E.J.; Morgan, K.; Clumpner, C.J. Wildlife and oil in the Antarctic: A recipe for cold disaster. Polar Rec. 2013, 49, 97–109. [Google Scholar] [CrossRef]
- Andres, B. Health of Antarctic Wildlife: A Challenge for Science and Policy; Knowles, K.R., Riddle, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; p. 470. [Google Scholar] [CrossRef]
- Cruise Boat Runs Aground Off Antarctica. The New York Times. Available online: https://www.nytimes.com/2008/12/05/world/05cruise.html (accessed on 15 October 2020).
- Nordblom, U. Cruise Tourism in the Arctic-Sustainability Issues and Protection of the Marine Environment in International Law. Ph.D. Thesis, University of Akureyri, Akureyri, Iceland, 2016. [Google Scholar]
- Stewart, E.J.; Draper, D. The sinking of the M.S. Explorer: Implications for cruise tourism in Arctic Canada. ARCTIC 2009, 61, 224–228. [Google Scholar] [CrossRef]
- Tin, T.; Fleming, Z.L.; Hughes, K.A.; Ainley, D.G.; Convey, P.; Moreno, C.A.; Pfeiffer, S.; Scott, J.; Snape, I. Impacts of local human activities on the Antarctic environment. Antarct. Sci. 2009, 21, 3. [Google Scholar] [CrossRef]
- Roslee, A.F.A.; Zakaria, N.N.; Convey, P.; Zulkharnain, A.; Lee, G.L.Y.; Gomez-Fuentes, C.; Ahmad, S.A. Statistical optimisation of growth conditions and diesel degradation by the Antarctic bacterium, Rhodococcus sp. strain AQ5-07. Extremophiles 2020, 24, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Tengku-Mazuki, T.A.; Subramanian, K.; Zakaria, N.N.; Convey, P.; Abdul Khalil, K.; Lee, G.L.Y.; Zulkharnain, A.; Shaharuddin, N.A.; Ahmad, S.A. Optimization of phenol degradation by Antarctic bacterium Rhodococcus sp. Antarct. Sci. 2020, 1–10. [Google Scholar] [CrossRef]
- Okere, U.V.; Cabrerizo, A.; Dachs, J.; Jones, K.C.; Semple, K.T. Biodegradation of phenanthrene by indigenous microorganisms in soils from Livingstone Island, Antarctica. FEMS Microbiol. Lett. 2012, 329, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.W.; Silvaraj, S.; Supramaniam, Y.; Snape, I.; Tan, I.K.P. Effect of temperature on bacterial community in petroleum hydrocarbon-contaminated and uncontaminated Antarctic soil. Polar Biol. 2018, 41, 1763–1775. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Kim, J. New insights into bioremediation strategies for oil-contaminated soil in cold environments. Int. Biodeterior. Biodegrad. 2019, 142, 58–72. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Caruso, G.; Rizzo, C.; Papale, M.; Azzaro, M. Bacterial communities versus anthropogenic disturbances in the Antarctic coastal marine environment. Environ. Sustain. 2019, 2, 297–310. [Google Scholar] [CrossRef]
- Vázquez, S.; Monien, P.; Pepino Minetti, R.; Jürgens, J.; Curtosi, A.; Villalba Primitz, J.; Frickenhaus, S.; Abele, D.; Mac Cormack, W.; Helmke, E. Bacterial communities and chemical parameters in soils and coastal sediments in response to diesel spills at Carlini Station, Antarctica. Sci. Total Environ. 2017, 605–606, 26–37. [Google Scholar] [CrossRef]
- Rizzo, C.; Malavenda, R.; Gerçe, B.; Papale, M.; Syldatk, C.; Hausmann, R.; Bruni, V.; Michaud, L.; Lo Giudice, A.; Amalfitano, S. Effects of a Simulated Acute Oil Spillage on Bacterial Communities from Arctic and Antarctic Marine Sediments. Microorganisms 2019, 7, 632. [Google Scholar] [CrossRef]
- Kennicutt, M.C. Oil spillage in Antarctica: Initial report of the National Science Foundation-sponsored Quick Response Team on the grounding of the Bahia paraiso. Environ. Sci. Technol. 1990, 24, 620–624. [Google Scholar] [CrossRef]
- Eppley, Z.A. Assessing indirect effects of oil in the presence of natural variation: The problem of reproductive failure in South Polar Skuas during the Bahia paraiso oil spill. Mar. Pollut. Bull. 1992, 25, 307–312. [Google Scholar] [CrossRef]
- Nielsen, U.N.; King, C.K. Abundance and diversity of soil invertebrates in the Windmill Islands region, East Antarctica. Polar Biol. 2015, 38, 1391–1400. [Google Scholar] [CrossRef]
- Simpson, R.D.; Smith, S.D.A.; Pople, A.R. The effects of a spillage of diesel fuel on a rocky shore in the sub-Antarctic region (Macquarie Island). Mar. Pollut. Bull. 1995, 31, 367–371. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, Y.; Lin, Z.; Bhatt, P.; Chen, S. New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ. Res. 2020, 182, 109138. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Zahri, K.N.M.; Convey, P.; Abdul Khalil, K.; Gomez-Fuentes, C.; Zulkarnain, A.; Sabri, S.; Alias, S.A.; González-Rocha, G.; Ahmad, S.A. Optimisation of biodegradation conditions for waste canola oil by cold-adapted Rhodococcus sp. AQ5-07 from Antarctica. Electron. J. Biotechnol. 2020, 48, 1–12. [Google Scholar] [CrossRef]
- De Jesus, H.E.; Peixoto, R.S. Bioremediation in Antarctic soils. J. Pet. Environ. Biotechnol. 2015, 6. [Google Scholar] [CrossRef]
- Delille, D. Potential bioremediation of Antarctic sea-ice contaminated by diesel fuel and crude oil. Indian J. Microbiol. 2006, 46, 179–190. [Google Scholar]
- Abed, R.M.M.; Al-Kharusi, S.; Al-Hinai, M. Effect of biostimulation, temperature and salinity on respiration activities and bacterial community composition in an oil polluted desert soil. Int. Biodeterior. Biodegrad. 2015, 98, 43–45. [Google Scholar] [CrossRef]
- Camenzuli, D.; Fryirs, K.A.; Gore, D.B.; Freidman, B.L. Managing legacy waste in the presence of cultural heritage at Wilkes Station, East Antarctica. Polar Rec. 2015, 51, 151–159. [Google Scholar] [CrossRef]
- Ruberto, L.; Dias, R.; Lo Balbo, A.; Vazquez, S.C.; Hernandez, E.A.; Mac Cormack, W.P. Influence of nutrients addition and bioaugmentation on the hydrocarbon biodegradation of a chronically contaminated Antarctic soil. J. Appl. Microbiol. 2009, 106, 1101–1110. [Google Scholar] [CrossRef]
- Rayner, J.L.; Snape, I.; Walworth, J.L.; Harvey, P.M.; Ferguson, S.H. Petroleum-hydrocarbon contamination and remediation by microbioventing at sub-Antarctic Macquarie Island. Cold Reg. Sci. Technol. 2007, 48, 139–153. [Google Scholar] [CrossRef]
- McWatters, R.S.; Rowe, R.K.; Wilkins, D.; Spedding, T.; Jones, D.; Wise, L.; Mets, J.; Terry, D.; Hince, G.; Gates, W.P.; et al. Geosynthetics in Antarctica: Performance of a composite barrier system to contain hydrocarbon-contaminated soil after three years in the field. Geotext. Geomembr. 2016, 44, 673–685. [Google Scholar] [CrossRef]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for treatment of VOCs and odours—A review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef] [PubMed]
- Sanscartier, D.; Reimer, K.; Zeeb, B.; Koch, I. The effect of temperature and aeration rate on bioremediation of diesel-contaminated soil in solid-phase bench-scale bioreactors. Soil Sediment Contam. 2011, 20, 353–369. [Google Scholar] [CrossRef]
- Snape, I.; Morris, C.E.; Cole, C.M. The use of permeable reactive barriers to control contaminant dispersal during site remediation in Antarctica. Cold Reg. Sci. Technol. 2001, 32, 157–174. [Google Scholar] [CrossRef]
- The Protocol on Environmental Protection to the Antarctic Treaty. Available online: https://www.ats.aq/e/protocol (accessed on 20 November 2020).
- Abdulrasheed, M.; Zulkharnain, A.; Zakaria, N.N.; Roslee, A.F.A.; Abdul Khalil, K.; Napis, S.; Convey, P.; Gomez-Fuentes, C.; Ahmad, S.A. Response surface methodology optimization and kinetics of diesel degradation by a cold-adapted Antarctic bacterium, Arthrobacter sp. strain AQ5-05. Sustainability 2020, 12, 6966. [Google Scholar] [CrossRef]
- Pandey, P.; Pathak, H.; Dave, S. Microbial ecology of hydrocarbon degradation in the soil: A review. Res. J. Environ. Toxicol. 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Abdulrasheed, M.; Zakaria, N.N.; Roslee, A.F.A.; Shukor, M.Y.; Zulkharnain, A.; Napis, S.; Convey, P.; Alias, S.A.; Gonzalez-Rocha, G.; Ahmad, S.A. Biodegradation of diesel oil by cold-adapted bacterial strains of Arthrobacter spp. from Antarctica. Antarc. Sci. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liang, Q.; He, B.J.; Miao, J.L. Low-temperature degradation mechanism analysis of petroleum hydrocarbon-degrading Antarctic psychrophilic strains. J. Pure Appl. Microbiol. 2014, 8, 47–53. [Google Scholar]
- Baraniecki, C.A.; Aislabie, J.; Foght, J.M. Characterization of Sphingomonas sp. Ant 17, an aromatic hydrocarbon-degrading bacterium isolated from Antarctic soil. Microb. Ecol. 2002, 43, 44–54. [Google Scholar] [CrossRef]
- Stallwood, B.; Shears, J.; Williams, P.A.; Hughes, K.A. Low temperature bioremediation of oil-contaminated soil using biostimulation and bioaugmentation with a Pseudomonas sp. from maritime Antarctica. J. Appl. Microbiol. 2005, 99, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Gran-Scheuch, A.; Fuentes, E.; Bravo, D.M.; Jiménez, J.C.; Pérez-Donoso, J.M. Isolation and characterization of phenanthrene degrading bacteria from diesel fuel-contaminated Antarctic soils. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Deka, S. A cost-effective and environmentally sustainable process for phycoremediation of oil field formation water for its safe disposal and reuse. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xaaldi Kalhor, A.; Movafeghi, A.; Mohammadi-Nassab, A.D.; Abedi, E.; Bahrami, A. Potential of the green alga Chlorella vulgaris for biodegradation of crude oil hydrocarbons. Mar. Pollut. Bull. 2017, 123, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Nydahl, A.C.; King, C.K.; Wasley, J.; Jolley, D.F.; Robinson, S.A. Toxicity of fuel-contaminated soil to Antarctic moss and terrestrial algae. Environ. Toxicol. Chem. 2015, 34, 2004–2012. [Google Scholar] [CrossRef]
- Martorell, M.M.; Ruberto, L.A.M.; de Castellanos, L.I.F.; Cormack, W.P.C. Bioremediation abilities of Antarctic fungi. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Springer International Publishing: Basel, Switzerland, 2019; pp. 517–534. [Google Scholar]
- Joshi-Navare, K.; Singh, P.K.; Prabhune, A.A. New yeast isolate Pichia caribbica synthesizes xylolipid biosurfactant with enhanced functionality. Eur. J. Lipid Sci. Technol. 2014, 116, 1070–1079. [Google Scholar] [CrossRef]
- Ferrari, B.C.; Zhang, C.; Van Dorst, J. Recovering greater fungal diversity from pristine and diesel fuel contaminated sub-Antarctic soil through cultivation using both a high and a low nutrient media approach. Front. Microbiol. 2011, 2, 217. [Google Scholar] [CrossRef]
- Hughes, K.A.; Bridge, P.; Clark, M.S. Tolerance of Antarctic soil fungi to hydrocarbons. Sci. Total Environ. 2007, 372, 539–548. [Google Scholar] [CrossRef]
- Govarthanan, M.; Fuzisawa, S.; Hosogai, T.; Chang, Y. Biodegradation of aliphatic and aromatic hydrocarbons using the filamentous fungus Penicillium sp. CHY-2 and characterization of its manganese peroxidase activity. RSC Adv. 2017, 7, 20716–20723. [Google Scholar] [CrossRef]
- Zhang, C.; Sirijovski, N.; Adler, L.; Ferrari, B.C. Exophiala macquariensis sp. nov., a cold adapted black yeast species recovered from a hydrocarbon contaminated sub-Antarctic soil. Fungal Biol. 2019, 123, 151–158. [Google Scholar] [CrossRef]
- Shahsavari, E.; Adetutu, E.M.; Ball, A.S. Phytoremediation and necrophytoremediation of petrogenic hydrocarbon-contaminated soils. Phytoremediation 2015, 2, 321–334. [Google Scholar] [CrossRef]
- Yang, S.; Jin, H.; Wei, Z.; He, R.; Ji, Y.; Li, X.; Yu, P. Bioremediation of oil spills in cold environments: A review. Pedosphere 2009, 19, 371–381. [Google Scholar] [CrossRef]
- Fritsche, W.; Hofrichter, M. Aerobic degradation by microorganisms. In Biotechnology: Environmental Processes II, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008; pp. 144–167. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Imron, M.F.; Kurniawan, S.B.; Ismail, N.I.; Abdullah, S.R.S. Future challenges in diesel biodegradation by bacteria isolates: A review. J. Clean. Prod. 2020, 251. [Google Scholar] [CrossRef]
- Presentato, A.; Cappelletti, M.; Sansone, A.; Ferreri, C.; Piacenza, E.; Demeter, M.A.; Crognale, S.; Petruccioli, M.; Milazzo, G.; Fedi, S.; et al. Aerobic growth of Rhodococcus aetherivorans BCP1 using selected naphthenic acids as the sole carbon and energy sources. Front. Microbiol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, D.; Engesser, K.H. Isolation and characterization of two novel strains capable of using cyclohexane as carbon source. Environ. Sci. Pollut. Res. 2014, 21, 12757–12766. [Google Scholar] [CrossRef]
- Kothari, V.; Panchal, M.; Srivastava, N. Microbial Degradation of Hydrocarbons; Institute of Science, Nirma University: Ahmedabad, Gujarat, India, 2013. [Google Scholar]
- Haddock, J.D. Aerobic degradation of aromatic hydrocarbons: Enzyme structures and catalytic mechanisms. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1057–1069. [Google Scholar] [CrossRef]
- Bhatt, P.; Gangola, S.; Bhandari, G.; Zhang, W.; Maithani, D.; Mishra, S.; Chen, S. New insights into the degradation of synthetic pollutants in contaminated environments. Chemosphere 2020, 128827. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and hot extremozymes: Industrial relevance and current trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef]
- Poursat, B.A.J.; van Spanning, R.J.M.; de Voogt, P.; Parsons, J.R. Implications of microbial adaptation for the assessment of environmental persistence of chemicals. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2220–2255. [Google Scholar] [CrossRef]
- Zucconi, L.; Canini, F.; Temporiti, M.E.; Tosi, S. Extracellular enzymes and bioactive compounds from antarctic terrestrial fungi for bioprospecting. Int. J. Environ. Res. Public Health 2020, 17, 6459. [Google Scholar] [CrossRef]
- Yao, Y.; Tang, H.; Su, F.; Xu, P. Comparative genome analysis reveals the molecular basis of nicotine degradation and survival capacities of Arthrobacter. Sci. Rep. 2015, 5, 8642. [Google Scholar] [CrossRef] [PubMed]
- Králová, S. Role of fatty acids in cold adaptation of Antarctic psychrophilic Flavobacterium spp. Syst. Appl. Microbiol. 2017, 40, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.P.; Pellizari, V.H.; Whyte, L.G.; Greer, C.W. A survey of indigenous microbial hydrocarbon degradation genes in soils from Antarctica and Brazil. Can. J. Microbiol. 2004, 50, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Whyte, L.G.; Schultz, A.; van Beilen, J.B.; Luz, A.P.; Pellizari, V.; Labbe, D. Prevalence of alkane monooxygenase genes in Arctic and Antarctic hydrocarbon-contamianed and pristine soils. FEMS Microbiol. Ecol. 2002, 41, 141–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guibert, L.M.; Loviso, C.L.; Marcos, M.S.; Commendatore, M.G.; Dionisi, H.M.; Lozada, M. Alkane biodegradation genes from chronically polluted subantarctic coastal sediments and their shifts in response to oil exposure. Microb. Ecol. 2018, 64, 605–616. [Google Scholar] [CrossRef]
- Flocco, C.G.; Gomes, N.C.M.; Cormack, W.M.; Smalla, K. Occurrence and diversity of naphthalene dioxygenase genes in soil microbial communities from the Maritime Antarctic. Environ. Microbiol. 2009, 11, 700–714. [Google Scholar] [CrossRef]
- Rafiq, M.; Hassan, N.; Rehman, M.; Hasan, F. Adaptation mechanisms and applications of psychrophilic fungi. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Springer: Cham, Switzerland, 2019; pp. 157–174. [Google Scholar] [CrossRef]
- Buzzini, P.; Branda, E.; Goretti, M.; Turchetti, B. Psychrophilic yeasts from worldwide glacial habitats: Diversity, adaptation strategies and biotechnological potential. FEMS Microbiol. Ecol. 2012, 82, 217–241. [Google Scholar] [CrossRef]
- Margesin, R. Bioremediation and biodegradation of hydrocarbons by cold-adapted yeasts. In Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzino, P., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 465–480. [Google Scholar] [CrossRef]
- Singh, H. Mycoremediation: Fungal Bioremediation; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Martorell, M.M.; Ruberto, L.A.M.; Fernández, P.M.; Castellanos de Figueroa, L.I.; Mac Cormack, W.P. Bioprospection of cold-adapted yeasts with biotechnological potential from Antarctica. J. Basic Microbiol. 2017, 57, 504–516. [Google Scholar] [CrossRef]
- Routaboul, J.M.; Fischer, S.F.; Browse, J. Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol. 2000, 124, 1697–1705. [Google Scholar] [CrossRef]
- Morgan-Kiss, R.M.; Priscu, J.C.; Pocock, T.; Gudynaite-Savitch, L.; Huner, N.P.A. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006, 70, 222–252. [Google Scholar] [CrossRef] [PubMed]
- Mock, T.; Otillar, R.P.; Strauss, J.; McMullan, M.; Paajanen, P.; Schmutz, J.; Salamov, A.; Sanges, R.; Toseland, A.; Ward, B.J.; et al. Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 2017, 541, 536–540. [Google Scholar] [CrossRef] [PubMed]

| Research Station | Country | Annual Fuel Consumption (L) 1 | Alternative Energy Source (s) | Reference (s) |
|---|---|---|---|---|
| McMurdo Station | United States of America | 5,000,000 |
| [24,25] |
| Casey Station; Mawson Station; Davis Station; Macquarie Island Station | Australia | 1,933,000 |
| [26,27] |
| Amundsen-Scott South Pole Station | United States of America | 1,916,448 |
| [28,29] |
| Syowa Station | Japan | 607,000 |
| [27,30] |
| King Sejong Station | Republic of Korea | 380,000 |
| [31] |
| Comandante Ferraz Antarctic Station | Brazil | 358,985 |
| [32] |
| Scott Base | New Zealand | 320,000 |
| [21,25,33] |
| SANAE IV Station | South Africa | 297,872 |
| [21] |
| Zhongshan Station | China | 272,689 |
| [22] |
| Great Wall Station | China | 169,492 |
| [31] |
| Bellingshausen Station | Russia | 150,000 |
| [31] |
| Bioremediation Method | Feasibility and Limitations | Reference (s) |
|---|---|---|
| Biostimulation (nutrient amendment) | As the success of bioremediation heavily depends on its biotic and abiotic parameters, biostimulation enhances degradation by balancing the C:N:P. The addition of ad hoc nutrients will amplify the removal of hydrocarbon contaminants, therefore minimising the toxicity effect of diesel on the environment. Notwithstanding, this in-situ method might lead to the dominance of bacterial classes with the highest diesel tolerance in a community, which might affect the soil diversity in due course. | [66,67,68] |
| Bioaugmentation | Bioaugmentation includes the addition of pre-adapted strain to polluted site, which has been found to significantly shorten remediation period. This in-situ technique aims to maintain a high microbial biomass. Optimisation is usually performed before bioaugmentation, where actual environmental conditions, soil features, predation and competitive effects are not inferred. Moreover, the Antarctic Treaty legislates forbid any introduction or release of nonindigenous species to the area; therefore, the use of commercially available bioremediation non-Antarctic bacteria to help in cleaning up pollutants is not an option. | [65,69] |
| Aeration | This in-situ method was pioneered in Macquarie Island by enhancing aeration through microbioventing. This trial was conducted on the postulation that the buried oxygen sensor array would be uniform throughout the subsurface. However, buried organic and beach cobble horizons were found to limit the distribution of oxygen and nutrients in the system. In addition, a diesel-generated air compressor was required to run constantly to supply air, which hold its own environmental risks. | [1,70] |
| Biopile | A large-scale biopile system was successfully carried out at Casey Station and reckoned to be replicable across the Antarctic region. Biopile operation encompasses the combination of aeration via mechanical turning, fertiliser application and leachate to increase soil moisture. While this in-situ technique is promising, large-scale biopile operation is costly and requires a large amount of energy, which reduces its feasibility in remote regions. Disturbance on soil profile should also be considered. | [2,68,71] |
| Biocells or bioreactors | This ex-situ technique involves the containerisation of contaminated soil or water in an enclosed system with monitored biotic and abiotic parameters, thus expediting the biodegradation rate. The application of biocells or bioreactors in Antarctica is yet to be reported despite its proven practicality in several studies. However, extensive site excavation is required to relocate the contaminants to the degradation system, risking more damage to the sensitive environment. | [1,40,72,73] |
| Permeable reactive barriers (PRBs) | PRBs are an in-situ containment technique that is mainly used to remediate water that passes through them. It is viable in remote and cold regions due to the passive low-power system required to operate by allowing the adsorption of contaminants from flowing water to the barrier. Additionally, PRBs have the least impact on the environment since a complete removal of the system can be done at the end of site operation. Nonetheless, the viability of PRBs is limited as they do not remediate contaminated source soils. | [1,68,74] |
| Microbial Species | Isolation Place | Substrate Degraded | Research Summary | Reference (s) |
|---|---|---|---|---|
| Arthrobacter spp. AQ5-05 | King George Island | Diesel |
| [76,78] |
| Planococcus sp. NJ41 | Antarctic Ocean | Diesel |
| [79] |
| Shewanella sp. NJ49 | Antarctic Ocean | Diesel |
| [79] |
| Rhodococcus sp. AQ5-07 | King George Island | Diesel |
| [50] |
| Waste canola oil |
| [64] | ||
| Sphingomonas sp. Ant 17 | Scott Base, Ross Island | Aromatic fraction of crude oil, jet fuel and diesel |
| [80] |
| Pseudomonas sp. ST41 | Signy Island | Hydrocarbons |
| [81] |
| Sphingobium xenophagum D43FB | King George Island | Phenanthrene |
| [82] |
| Fungal Species | Isolation Place | Substrate Degraded | Research Summary | Reference |
|---|---|---|---|---|
| P. caribbica | Carlini, Potter Cove, King George Island | n-alkanes and diesel fuel |
| [86,87] |
| Pseudeurotium bakeri | Macquarie Island | SAB Diesel |
| [88] |
| Mortierella sp. | Rothera Point, Adelaide Island | Dodecane |
| [89] |
| Penicillium sp. CHY-2 | Unspecified Antarctic soil | Hydrocarbons |
| [90] |
| Exophiala macquariensis | Macquarie Island | Toluene |
| [91] |
| Phenoliferia glacialis | Carlini, Potter Cove, King George Island | n-hexadecane |
| [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, R.R.; Lim, Z.S.; Shaharuddin, N.A.; Zulkharnain, A.; Gomez-Fuentes, C.; Ahmad, S.A. Diesel in Antarctica and a Bibliometric Study on Its Indigenous Microorganisms as Remediation Agent. Int. J. Environ. Res. Public Health 2021, 18, 1512. https://doi.org/10.3390/ijerph18041512
Wong RR, Lim ZS, Shaharuddin NA, Zulkharnain A, Gomez-Fuentes C, Ahmad SA. Diesel in Antarctica and a Bibliometric Study on Its Indigenous Microorganisms as Remediation Agent. International Journal of Environmental Research and Public Health. 2021; 18(4):1512. https://doi.org/10.3390/ijerph18041512
Chicago/Turabian StyleWong, Rasidnie Razin, Zheng Syuen Lim, Noor Azmi Shaharuddin, Azham Zulkharnain, Claudio Gomez-Fuentes, and Siti Aqlima Ahmad. 2021. "Diesel in Antarctica and a Bibliometric Study on Its Indigenous Microorganisms as Remediation Agent" International Journal of Environmental Research and Public Health 18, no. 4: 1512. https://doi.org/10.3390/ijerph18041512
APA StyleWong, R. R., Lim, Z. S., Shaharuddin, N. A., Zulkharnain, A., Gomez-Fuentes, C., & Ahmad, S. A. (2021). Diesel in Antarctica and a Bibliometric Study on Its Indigenous Microorganisms as Remediation Agent. International Journal of Environmental Research and Public Health, 18(4), 1512. https://doi.org/10.3390/ijerph18041512