Effectiveness of Pre-Hospital Tourniquet in Emergency Patients with Major Trauma and Uncontrolled Haemorrhage: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Outcome Measures and Follow-Up Assessment
2.3. Search Strategy
2.4. Study Selection and Data Extraction
2.5. Internal Validity
2.6. Data Synthesis
2.7. Quality of Evidence
3. Results
3.1. General Characteristics
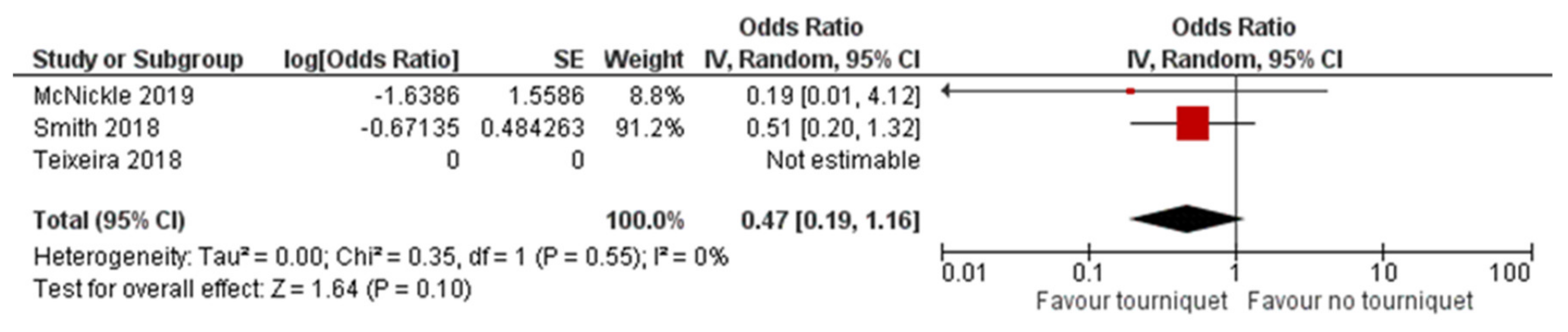
3.2. Primary Outcomes
3.2.1. Mortality at 24 h, 30 Days, and 12 Months
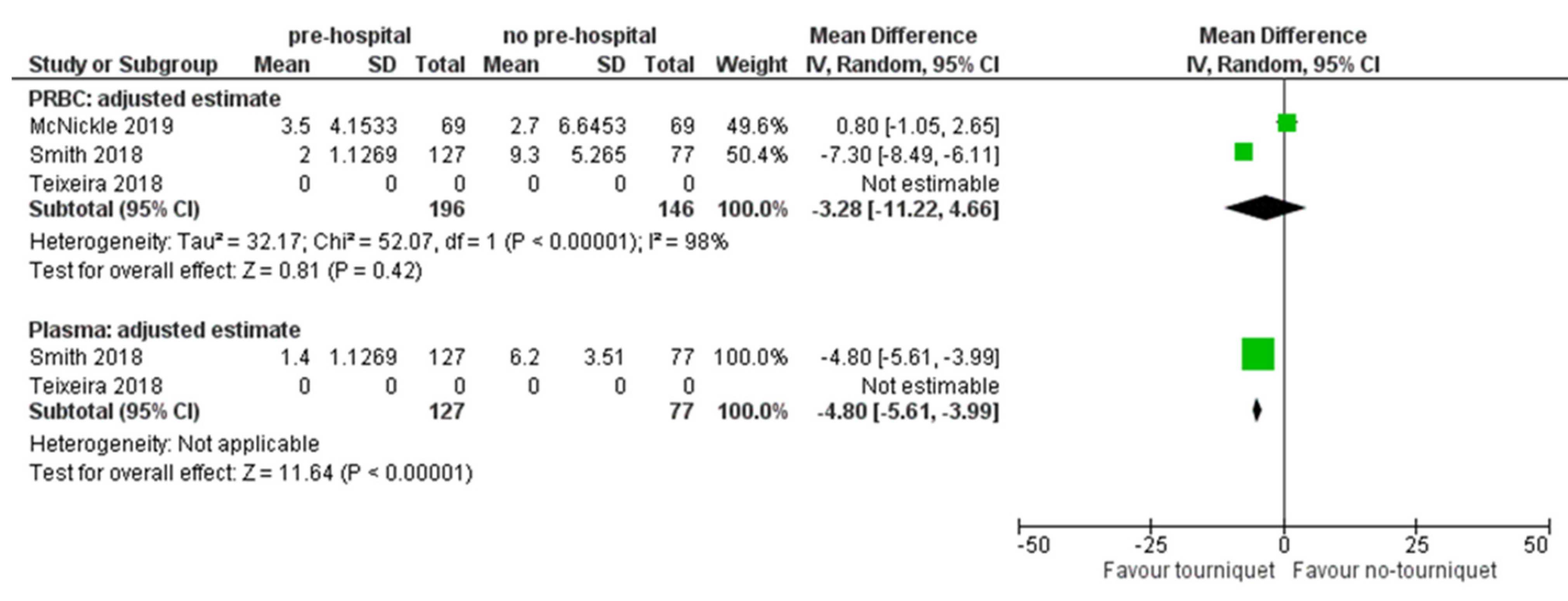
3.2.2. Packed of Infused Blood Components
3.2.3. Length of Stay (LOS) in ICU
3.2.4. Health-Related Quality of Life
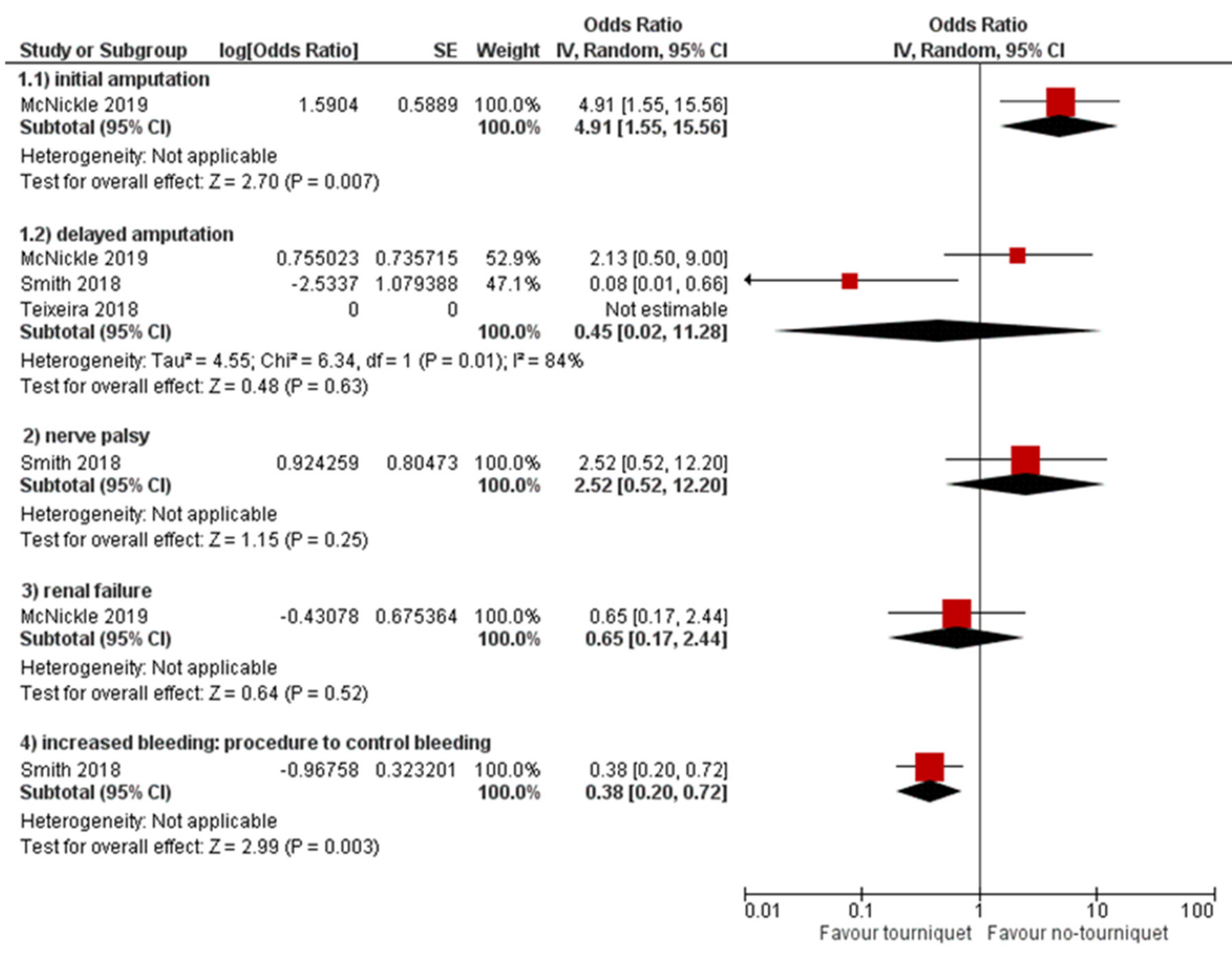
3.3. Secondary Outcomes
3.4. Internal Validity
3.5. Quality of Evidence
4. Discussion
4.1. Use of Tourniquet and Mortality
4.2. Blood Products Use and LOS in ICU
4.3. Secondary Outcomes
4.4. The Quality of Evidence
4.5. Implication for Clinical Practice
5. Limits and Strength
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Violence and Injury Prevention and Disability (VIP). 2021. Available online: https://www.who.int/violence_injury_prevention/en/ (accessed on 8 March 2021).
- Rana, J.S.; Khan, S.S.; Lloyd-Jones, D.M.; Sidney, S. Changes in Mortality in Top 10 Causes of Death from 2011 to 2018. J. Gen. Intern. Med. 2020, 36, 2517–2518. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.G.R.; Inaba, K.; Hadjizacharia, P.; Brown, C.; Salim, A.; Rhee, P.; Browder, T.; Noguchi, T.T.; Demetriades, D. Preventable or Potentially Preventable Mortality at a Mature Trauma Center. J. Trauma Inj. Infect. Crit. Care 2007, 63, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- ISTAT. Incidenti Stradali in Italia. Available online: https://www.istat.it/it/archivio/245757 (accessed on 2 September 2021).
- Trauma, I.C.M.M. Progetto RITG. Available online: http://pprg.infoteca.it/ritg/ (accessed on 2 September 2021).
- Beaucreux, C.; Vivien, B.; Miles, E.; Ausset, S.; Pasquier, P. Application of tourniquet in civilian trauma: Systematic review of the literature. Anaesth. Crit. Care Pain Med. 2018, 37, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Algarabel, M.; Esteban-Sebastià, X.; Santillán-García, A.; Vila-Candel, R. Tourniquet use in out-of-hospital emergency care: A systematic review. Emerg. Rev. Soc. Esp. Med. Emerg. 2019, 31, 47–54. [Google Scholar]
- Cornelissen, M.P.; Brandwijk, A.; Schoonmade, L.; Giannakopoulos, G.; Van Oostendorp, S.; Geeraedts, L. The safety and efficacy of improvised tourniquets in life-threatening hemorrhage: A systematic review. Eur. J. Trauma Emerg. Surg. 2019, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Oyeniyi, B.T.; Fox, E.E.; Scerbo, M.; Tomasek, J.S.; Wade, C.E.; Holcomb, J.B. Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury 2017, 48, 5–12. [Google Scholar] [CrossRef]
- Bulger, E.M.; Snyder, D.; Schoelles, K.; Gotschall, C.; Dawson, D.; Lang, E.; Sanddal, N.D.; Butler, F.K.; Fallat, M.; Taillac, P.; et al. An Evidence-based Prehospital Guideline for External Hemorrhage Control: American College of Surgeons Committee on Trauma. Prehospital Emerg. Care 2014, 18, 163–173. [Google Scholar] [CrossRef]
- CNEC, Istituto Superiore di Sanità. Linea Guida sulla Gestione Integrata del Trauma Maggiore dalla Scena dell’Evento alla Cura Definitiva. 2021. Available online: https://snlg.iss.it/?p=2533 (accessed on 6 September 2021).
- Schünemann, H.J.; Wiercioch, W.; Brozek, J.; Etxeandia-Ikobaltzeta, I.; Mustafa, R.A.; Manja, V.; Brignardello-Petersen, R.; Neumann, I.; Falavigna, M.; Alhazzani, W.; et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J. Clin. Epidemiol. 2016, 81, 101–110. [Google Scholar] [CrossRef]
- Iannone, P.; Coclite, D.; Napoletano, A.; Fauci, A.; Graziano, G.; Iacorossi, L.; D’Angelo, D. The new National Guidelines System in Italy: A first evaluation. G. Ital. Nefrol. 2019, 36. [Google Scholar]
- Kanani, A.N.; Hartshorn, S. NICE clinical guideline NG39: Major trauma: Assessment and initial management. Arch. Dis. Child.-Educ. Pr. Ed. 2016, 102, 20–23. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’connell, D.; Robertson, J.; Peterson, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Department of Epidemiology and Community Medicine, University of Ottawa: Ottawa, ON, Canada, 2010. [Google Scholar]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.L. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.G.; Brown, C.V.; Emigh, B.; Long, M.; Foreman, M.; Eastridge, B.; Gale, S.; Truitt, M.S.; Dissanaike, S.; Duane, T.; et al. Civilian Prehospital Tourniquet Use Is Associated with Improved Survival in Patients with Peripheral Vascular Injury. J. Am. Coll. Surg. 2018, 226, 769–776.e1. [Google Scholar] [CrossRef] [PubMed]
- McNickle, A.G.; Fraser, D.R.; Chestovich, P.J.; Kuhls, D.A.; Fildes, J.J. Effect of prehospital tourniquets on resuscitation in extremity arterial trauma. Trauma Surg. Acute Care Open 2019, 4, e000267. [Google Scholar] [CrossRef] [PubMed]
- Scerbo, M.H.; Holcomb, J.B.; Taub, E.; Gates, K.; Love, J.D.; Wade, C.E.; Cotton, B.A. The trauma center is too late: Major limb trauma without a pre-hospital tourniquet has increased death from hemorrhagic shock. J. Trauma Acute Care Surg. 2017, 83, 1165–1172. [Google Scholar] [CrossRef]
- Smith, A.A.; Ochoa, J.E.; Wong, S.; Beatty, S.; Elder, J.; Guidry, C.; McGrew, P.; McGinness, C.; Duchesne, J.; Schroll, R. Prehospital tourniquet use in penetrating extremity trauma: Decreased blood transfusions and limb complications. J. Trauma Acute Care Surg. 2019, 86, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Scerbo, M.H.; Mumm, J.P.; Gates, K.; Love, J.D.; Wade, C.E.; Holcomb, J.B.; Cotton, B.A. Safety and Appropriateness of Tourniquets in 105 Civilians. Prehospital Emerg. Care 2016, 20, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Schroll, R.; Smith, A.; McSwain, N.E.; Myers, J.; Rocchi, K.; Inaba, K.; Siboni, S.; Vercruysse, G.A.; Ibrahim-Zada, I.; Sperry, J.L.; et al. A multi-institutional analysis of prehospital tourniquet use. J. Trauma Acute Care Surg. 2015, 79, 10–14. [Google Scholar] [CrossRef]
- Kragh, J.F.; Littrel, M.L.; Jones, J.A.; Walters, J.; Baer, D.G.; Wade, C.E.; Holcomb, J.B. Battle Casualty Survival with Emergency Tourniquet Use to Stop Limb Bleeding. J. Emerg. Med. 2011, 41, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.P.; Swain, J.M.; Brozek, J.L.; Ludwikowska, M.; Singletary, E.; Zideman, D.; Epstein, J.; Darzi, A.; Bak, A.; Karam, S.; et al. Control of Severe, Life-Threatening External Bleeding in the Out-of-Hospital Setting: A Systematic Review. Prehospital Emerg. Care 2020, 25, 235–267. [Google Scholar] [CrossRef] [PubMed]
- Aberle, S.J.; Dennis, A.J.; Landry, J.M.; Sztajnkrycer, M.D. Hemorrhage Control by Law Enforcement Personnel: A Survey of Knowledge Translation from the Military Combat Experience. Mil. Med. 2015, 180, 615–620. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Study (First Author, Year) | Country (State) | Study Design | Setting | Patients (Total Sample) | Intervention | Comparison | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|
| McNickle et al. (2019) | Retrospective cohort study | Level I trauma centre (Pre-hospital data) | 192 | Pre-hospital tourniquet application | No pre-hospital tourniquet application | Blood transfusions within the first 24 h |
| |
| Smith et al. (2019) | New Orleans | Retrospective cohort study | Level I trauma centre (Pre-hospital data) | 238 | Pre-hospital commercial tourniquet application for extremity injuries | No pre-hospital tourniquet application | Blood product utilization |
|
| Teixeira et al. (2018) | Texas | Multicentre retrospective cohort study | 11 level I trauma centres (Pre-hospital data) | 1026 | Pre-hospital tourniquet application | No pre-hospital tourniquet application | In-hospital mortality |
|
| Scerbo et al. (2017) | Texas | Retrospective cohort study | Memorial Hermann Hospital | 306 | Pre-hospital tourniquet application | Trauma centre tourniquet application | Death from haemorrhagic shock |
|
| The outcomes were determined by a combination of Trauma Registry data and electronic health record review. | ||||||||
| Study | Sample | Age (years) | Sex (Male) | ISS | Extremity AIS | GCS | HR | SBP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SE) | N(%) | Mean (SE) | Mean (SE) | Median (IRQ Range) | Mean (SE) | Mean (SE) | |||||||||
| PH-T | NPH-T | PH-T | NPH-T | PH-T | NPH-T | PH-T | NPH-T | PH-T | NPH-T | PH-T | NPH-T | PH-T | NPH-T | PH-T | NPH-T | |
| McNickleet al., 2019 | 69 | 69 | 35 (±1.5) | 36.3 (±1.6) | 56 (88.9) | 53 (84.1) | 13.1 (±0.8) | 12.3 (±0.9) | 3.2 (±0.1) | 3 (±0.1) | - | - | 110 (±4) | 100 (±3) | 126 (±4) | 130 (±3) |
| Smith et al., 2018 | 127 | 77 | 31.3 (±0.7) | 31.2 (±1.6) | 111 (87.4) | 68 (88.3) | 9 (±0.5) | 10.1 (±0.6) | 2.8 (±0.2) | 2.7 (±0.2) | - | - | 100 (±2) | 104 (±5) | 114 (±2) | 98 (±4) |
| Scerbo et al., 2017 * | 252 | 29 | 33 (25.46) (1) | 34 (24.50) (1) | 212 (84.1) | 27 (93.1) | 9 (5.17) (1) | 20 (9.27) (1) | 3 (2.3) (1) | 3 (3.4) (1) | 15 (14.15) (1) | 14 (3.15) (1) | 100 (84.120) (1) | 122 (87.135) (1) | 119 (92.139) (1) | 100 (83.113) (1) |
| Teixeira et al., 2018 | 181 | 845 | 34.4 (±1.1) (2) | 35.9 (±0.5) (2) | 157 (86.7) | 708 (83.7) | 13.2 (±0.8) (2) | 11.3 (±0.3) (2) | 36 (180) (3) | 77 (9.1) (3) | 28 (178) (4) | 91 (838) (4) | 105.9 (±2.1) (2) | 92.6 (±0.9) (2) | 125.3 (±7) (2) | 121.7 (±1.2) (2) |
| Study | Pre-Hospital Tourniquet | No Pre-Hospital Tourniquet | p-Value | Time Point | Adjustment | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | N Events | % | Sample | N Events | % | ||||
| McNickle et al. (2019) | 69 | 0 | 0 | 69 | 2 | 2.9 | NS | NR | Variable matching by patient demographics and injured artery, ISS, and mechanism of injury. |
| Smith et al. (2018) | 127 | 9 | 7.1 | 77 | 10 | 13 | 0.21 | NR | Variable matching by patient demographics and injury severity. |
| Teixeira et al. (2018) | 181 | 7 | 3.9 | 845 | 44 | 5.2 | 0.45 | NR | ISS, presence of associated severe head or torso injury, presence of major vascular injury, and traumatic amputation. |
| Study | Pre-Hospital Tourniquet | Trauma Centre Tourniquet | p-value | Time point | Adjustment | ||||
| Sample | N events | % | Sample | N events | % | ||||
| Scerbo et al. (2017) | 252 | 13 | 5.2 | 29 | 4 | 13.8 | 0.07 | NR | Data not adjusted |
| (1) Packed Red Blood Cells Transfusion (pRBC) | |||||||
|---|---|---|---|---|---|---|---|
| Studies | Pre-Hospital Tourniquet | No Pre-Hospital Tourniquet | p-Value | Time Point (Hours) | Adjustment | ||
| Sample | Mean (SD) | Sample | Mean (SD) | ||||
| McNickle et al. (2019) | 69 | 3.5 (0.5) | 69 | 2.7 (0.8) | NS | within first 24 h | Variable matching by patient demographics and injured artery, and mechanism of injury. |
| Teixeira et al. (2018) | 181 | 5.0 (8.6) | 845 | 3.9 (14.5) | 0.380 | within first 24 h | Variable adjusted by age, sex, mechanism of injury, hypotension on admission, GCS, ISS, presence of associated severe head or torso injury, presence of major vascular injury, and traumatic amputation. |
| Smith et al. (2018) | 127 | 2.0 (0.1) (1) | 77 | 9.3 (0.6) (1) | <0.001 | within first 24 h | Variable matching by patient demographics and injury severity. |
| Study | Pre-Hospital Tourniquet | Trauma Centre Tourniquet | p-value | Time point (hours) | Adjustment | ||
| Sample | Median (IQR) | Sample | Median (IQR) | ||||
| Scerbo et al. (2017) | 252 | 3 (1.6) | 29 | 4 (2.9) | 0.10 | within first 24 h | |
| (2) Platelets transfusion | |||||||
| Study | Pre-Hospital Tourniquet | No Pre-Hospital Tourniquet | p-value | Time point (hours) | Adjustment | ||
| Sample | Mean (SD) | Sample | Mean (SD) | ||||
| Teixeira et al. (2018) | 181 | 0.8 (2.2) | 845 | 0.5 (2.4) | 0.237 | within first 24 h | Variable adjusted by age, sex, mechanism of injury, hypotension on admission, GCS, ISS, presence of associated severe head or torso injury, presence of major vascular injury, and traumatic amputation. |
| Study | Pre-Hospital Tourniquet | Trauma Centre Tourniquet | p-value | Time point (hours) | Adjustment | ||
| Sample | Median (IQR) | Sample | Median (IQR) | ||||
| Scerbo et al. (2017) | 252 | 1 (1.3) | 29 | 2 (1.6) | 0.11 | within first 24 h | |
| (3) Plasma transfusion | |||||||
| Studies | Pre-Hospital Tourniquet | No Pre-Hospital Tourniquet | p-value | Time point (hours) | Adjustment | ||
| Sample | Mean (SD) | Sample | Mean (SD) | ||||
| Teixeira et al. (2018) | 181 | 2.8 (6.8) | 845 | 1.8 (4.7) | 0.030 | within first 24 h | Variable adjusted by age, sex, mechanism of injury, hypotension on admission, GCS, ISS, presence of associated severe head or torso injury, presence of major vascular injury, and traumatic amputation.. |
| Smith et al. (2018) | 127 | 1.4 (0.1) (1) | 77 | 6.2 (0.4) (1) | <0.001 | within first 24 h | Variable matching for patient demographics and injury severity |
| Study | Pre-Hospital Tourniquet | Trauma Centre Tourniquet | p-value | Time point (hours) | Adjustment | ||
| Sample | Median (IQR) | Sample | Median (IQR) | ||||
| Scerbo et al. (2017) | 252 | 3 (2.5) | 29 | 5 (3, 10) | <0.01 | within first 24 h | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latina, R.; Iacorossi, L.; Fauci, A.J.; Biffi, A.; Castellini, G.; Coclite, D.; D’Angelo, D.; Gianola, S.; Mari, V.; Napoletano, A.; et al. Effectiveness of Pre-Hospital Tourniquet in Emergency Patients with Major Trauma and Uncontrolled Haemorrhage: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 12861. https://doi.org/10.3390/ijerph182312861
Latina R, Iacorossi L, Fauci AJ, Biffi A, Castellini G, Coclite D, D’Angelo D, Gianola S, Mari V, Napoletano A, et al. Effectiveness of Pre-Hospital Tourniquet in Emergency Patients with Major Trauma and Uncontrolled Haemorrhage: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(23):12861. https://doi.org/10.3390/ijerph182312861
Chicago/Turabian StyleLatina, Roberto, Laura Iacorossi, Alice Josephine Fauci, Annalisa Biffi, Greta Castellini, Daniela Coclite, Daniela D’Angelo, Silvia Gianola, Veronica Mari, Antonello Napoletano, and et al. 2021. "Effectiveness of Pre-Hospital Tourniquet in Emergency Patients with Major Trauma and Uncontrolled Haemorrhage: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 23: 12861. https://doi.org/10.3390/ijerph182312861
APA StyleLatina, R., Iacorossi, L., Fauci, A. J., Biffi, A., Castellini, G., Coclite, D., D’Angelo, D., Gianola, S., Mari, V., Napoletano, A., Porcu, G., Ruggeri, M., Iannone, P., Chiara, O., & on behalf of INIH—Major Trauma. (2021). Effectiveness of Pre-Hospital Tourniquet in Emergency Patients with Major Trauma and Uncontrolled Haemorrhage: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(23), 12861. https://doi.org/10.3390/ijerph182312861







