Rodent Model of Gender-Affirming Hormone Therapies as Specific Tool for Identifying Susceptibility and Vulnerability of Transgender People and Future Applications for Risk Assessment
Abstract
:1. Introduction
2. Gender-Affirming Hormone Therapy
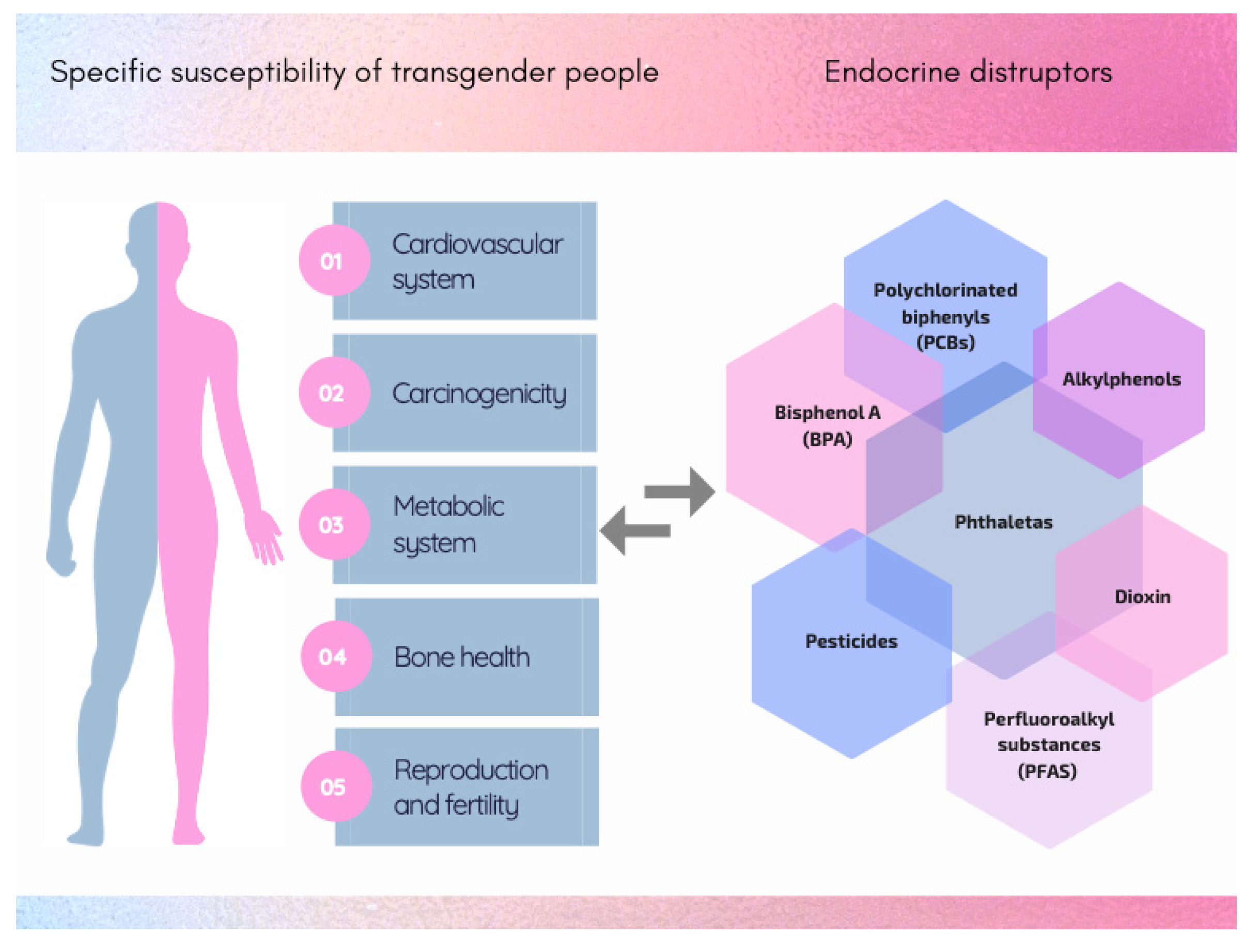
3. Specific Susceptibility and Vulnerability of Transgender People
3.1. Cardiovascular System
3.2. Carcinogenicity
3.3. Metabolic System
3.4. Bone Health
3.5. Reproduction and Fertility
3.6. Vulnerability Linked to Modified Behaviour (Lifestyles, Food Habits)
4. Rodent Models
4.1. State of the Art
4.2. New Perspectives
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Scandurra, C.; Carbone, A.; Baiocco, R.; Mezzalira, S.; Maldonato, N.M.; Bochicchio, V. Gender Identity Milestones, Minority Stress and Mental Health in Three Generational Cohorts of Italian Binary and Nonbinary Transgender People. Int. J. Environ. Res. Public Health 2021, 18, 9057. [Google Scholar] [CrossRef] [PubMed]
- T’Sjoen, G.; Arcelus, J.; De Vries, A.L.C.; Fisher, A.D.; Nieder, T.O.; Ozer, M.; Motmans, J. European Society for Sexual Medicine Position Statement Assessment and Hormonal Management in Adolescent and Adult Trans People, with Attention for Sexual Function and Satisfaction. J. Sex. Med. 2020, 17, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.; Bockting, W.; Botzer, M.; Cohen-Kettenis, P.; DeCuypere, G.; Feldman, J.; Fraser, L.; Green, J.; Knudson, G.; Meyer, W.J.; et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int. J. Transgend. 2012, 13, 165–232. [Google Scholar] [CrossRef]
- Cooper, K.; Russell, A.; Mandy, W.; Butler, C. The phenomenology of gender dysphoria in adults: A systematic review and meta-synthesis. Clin. Psychol. Rev. 2020, 80, 101875. [Google Scholar] [CrossRef] [PubMed]
- Arcelus, J.; Bouman, W.P.; Van Den Noortgate, W.; Claes, L.; Witcomb, G.; Fernandez-Aranda, F. Systematic Re-view and Meta-Analysis of Prevalence Studies in Transsexualism. Eur. Psychiatry 2015, 30, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.; Adams, N.; Corneil, T.; Kreukels, B.; Motmans, J.; Coleman, E. Size and Distribution of Transgender and Gender Nonconforming Populations: A Narrative Review. Endocrinol. Metab. Clin. N. Am. 2019, 48, 303–321. [Google Scholar] [CrossRef]
- Spizzirri, G.; Eufrásio, R.; Lima, M.C.P.; Nunes, H.R.D.C.; Kreukels, B.P.C.; Steensma, T.D.; Abdo, C.H.N. Proportion of people identified as transgender and non-binary gender in Brazil. Sci. Rep. 2021, 11, 2240. [Google Scholar] [CrossRef] [PubMed]
- Åhs, J.W.; Dhejne, C.; Magnusson, C.; Dal, H.; Lundin, A.; Arver, S.; Dalman, C.; Kosidou, K. Proportion of adults in the general population of Stockholm County who want gender-affirming medical treatment. PLoS ONE 2018, 13, e0204606. [Google Scholar] [CrossRef] [Green Version]
- Nolan, I.T.; Kuhner, C.J.; Dy, G.W. Demographic and temporal trends in transgender identities and gender confirming surgery. Transl. Androl. Urol. 2019, 8, 184–190. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Rich, A.L.; Phipps, L.M.; Tiwari, S.; Rudraraju, H.; Dokpesi, P.O. The Increasing Prevalence in Intersex Variation from Toxicological Dysregulation in Fetal Reproductive Tissue Differentiation and Development by Endo-crine-Disrupting Chemicals. Environ. Health Insights 2016, 10, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Fausto-Sterling, A. The Dynamic Development of Gender Variability. J. Homosex. 2012, 59, 398–421. [Google Scholar] [CrossRef]
- Laino, L.; Majore, S.; Preziosi, N.; Grammatico, B.; De Bernardo, C.; Scommegna, S.; Rapone, A.M.; Marrocco, G.; Bottillo, I.; Grammatico, P. Disorders of sex development: A genetic study of patients in a multidisciplinary clinic. Endocr. Connect. 2014, 3, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Fernandez, R.; Collet, S.; Kiyar, M.; Delgado-Zayas, E.; Gomez-Gil, E.; Van Den Eynde, T.; T’Sjoen, G.; Guillamon, A.; Mueller, S.C.; et al. Epigenetics is Implicated in the Basis of Gender Incongruence: An Epigenome-Wide Association Analysis. Front. Neurosci. 2021, 15, 701017. [Google Scholar] [CrossRef] [PubMed]
- T’Sjoen, G.; Arcelus, J.; Gooren, L.; Klink, D.T.; Tangpricha, V. Endocrinology of Transgender Medicine. Endocr. Rev. 2018, 40, 97–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Promotion of Chemical Safety Unit, International Programme on Chemical Safety, United Nations Environment Programme & Inter-Organization Programme for the Sound Management of Chemicals. Chemical Risk Assessment: Human Risk Assessment, Environmental Risk Assessment and Ecological Risk Assessment; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Mantovani, A.; Maranghi, F. Risk assessment of chemicals potentially affecting male fertility. Contraception 2005, 72, 308–313. [Google Scholar] [CrossRef]
- You, H.H.; Song, G. Review of endocrine disruptors on male and female reproductive systems. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2021, 244, 109002. [Google Scholar] [CrossRef]
- Van Larebeke, N.; Fucic, A. Chapter 5 Sex-specific Actions of Endocrine Disruptors. In Challenges in Endocrine Disruptor Toxicology and Risk Assessment, Anonymous; The Royal Society of Chemistry: London, UK, 2021; pp. 121–154. [Google Scholar]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid. Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hembree, W.C.; Cohen-Kettenis, P.T.; Gooren, L.; E Hannema, S.; Meyer, W.J.; Murad, M.H.; Rosenthal, S.M.; Safer, J.D.; Tangpricha, V.; T’Sjoen, G.G. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 3869–3903. [Google Scholar] [CrossRef]
- Glintborg, D.; T’Sjoen, G.; Ravn, P.; Andersen, M.S. Management of Endocrine Disease: Optimal Femi-nizing Hormone Treatment in Transgender People. Eur. J. Endocrinol. 2021, 185, R49–R63. [Google Scholar] [CrossRef]
- Kuijpers, S.M.E.; Wiepjes, C.M.; Conemans, E.B.; Fisher, A.D.; T’Sjoen, G.; den Heijer, M. Toward a Lowest Effective Dose of Cyproterone Acetate in Trans Women: Results from the ENIGI Study. J. Clin. Endocrinol. Metab. 2021, 106, e3936–e3945. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.; Brown, G.R.; Deutsch, M.B.; Hembree, W.; Meyer, W.; Meyer-Bahlburg, H.F.; Tangpricha, V.; T’Sjoen, G.; Safer, J.D. Priorities for transgender medical and healthcare research. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender Differences in Cardiovascular Disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M.C. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, Y.; Dong, M.; Song, W.; Belcher, S.M.; Wang, H.S. Bisphenol A and 17beta-Estradiol Promote Ar-rhythmia in the Female Heart Via Alteration of Calcium Handling. PLoS ONE 2011, 6, e25455. [Google Scholar] [CrossRef] [Green Version]
- Aekplakorn, W.; Chailurkit, L.-O.; Ongphiphadhanakul, B. Association of Serum Bisphenol A with Hypertension in Thai Population. Int. J. Hypertens. 2015, 2015, 594189. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, J.H.; Lim, Y.H.; Park, H.Y.; Hong, Y.C. Associations of Bisphenol A Exposure with Heart Rate Varia-bility and Blood Pressure. Hypertension 2012, 60, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S. Urinary Bisphenol A and Hypertension in a Multiethnic Sample of US Adults. J. Environ. Public Health 2012, 2012, 481641. [Google Scholar] [CrossRef]
- Bergkvist, C.; Berglund, M.; Glynn, A.; Wolk, A.; Åkesson, A. Dietary exposure to polychlorinated biphenyls and risk of myocardial infarction—A population-based prospective cohort study. Int. J. Cardiol. 2015, 183, 242–248. [Google Scholar] [CrossRef]
- de Blok, C.J.; Dreijerink, K.; Heijer, M.D. Cancer Risk in Transgender People. Endocrinol. Metab. Clin. N. Am. 2019, 48, 441–452. [Google Scholar] [CrossRef]
- Sterling, J.; Garcia, M.M. Cancer screening in the transgender population: A review of current guidelines, best practices, and a proposed care model. Transl. Androl. Urol. 2020, 9, 2771–2785. [Google Scholar] [CrossRef] [PubMed]
- Del Pup, L.; Mantovani, A.; Cavaliere, C.; Facchini, G.; Luce, A.; Sperlongano, P.; Caraglia, M.; Berretta, M. Carcinogenetic mechanisms of endocrine disruptors in female cancers (Review). Oncol. Rep. 2016, 36, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Park, M.A.; Hwang, K.A.; Choi, K.C. Diverse Animal Models to Examine Potential Role(s) and Mechanism of Endocrine Disrupting Chemicals on the Tumor Progression and Prevention: Do they have Tumorigenic Or An-ti-Tumorigenic Property? Lab. Anim. Res. 2011, 27, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprague, B.L.; Trentham-Dietz, A.; Hedman, C.J.; Wang, J.; Hemming, J.D.; Hampton, J.M.; Buist, D.S.; Bowles, E.J.A.; Sisney, G.S.; Burnside, E.S. Circulating serum xenoestrogens and mammographic breast density. Breast Cancer Res. 2013, 15, R45–R48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Wiik, A.; Andersson, D.P.; Brismar, T.; Chanpen, S.; Dhejne, C.; Ekström, T.J.; Flanagan, J.N.; Holmberg, M.; Kere, J.; Lilja, M.; et al. Metabolic and functional changes in transgender individuals following cross-sex hormone treatment: Design and methods of the GEnder Dysphoria Treatment in Sweden (GETS) study. Contemp. Clin. Trials Commun. 2018, 10, 148–153. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Janesick, A.; Blumberg, B. Endocrine Disrupting Chemicals and the Developmental Programming of Adipogene-sis and Obesity. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Biemann, R.; Bluher, M.; Isermann, B. Exposure to Endocrine-Disrupting Compounds such as Phthalates and Bi-sphenol A is Associated with an Increased Risk for Obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101546. [Google Scholar] [CrossRef] [PubMed]
- Dag, Z.O.; Dilbaz, B. Impact of obesity on infertility in women. J. Turk. Gynecol. Assoc. 2015, 16, 111–117. [Google Scholar] [CrossRef]
- Katib, A. Mechanisms linking obesity with male infertility. Cent. Eur. J. Urol. 2015, 68, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Kothmann, K.H.; Jacobsen, V.; Laffitte, E.; Bromfield, C.; Grizzaffi, M.; Jarboe, M.; Braundmeier-Fleming, A.G.; Bahr, J.M.; Nowak, R.A.; Newell-Fugate, A.E. Virilizing doses of testosterone decrease circulating insulin levels and differentially regulate insulin signaling in liver and adipose tissue of females. Am. J. Physiol. Metab. 2021, 320, E1107–E1118. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S.; Lolli, F.; Ciana, P.; Maggi, A. Sexual Dimorphism and Estrogen Action in Mouse Liver. Sex. Gend. Factors Affect. Metab. Homeost. Diabetes Obes. 2017, 1043, 141–151. [Google Scholar] [CrossRef]
- Maggi, A.; Della Torre, S. Sex, Metabolism and Health. Mol. Metab. 2018, 15, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Agas, D.; Lacava, G.; Sabbieti, M.G. Bone and bone marrow disruption by endocrine-active substances. J. Cell. Physiol. 2018, 234, 192–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turan, S. Endocrine disrupting chemicals and bone. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101495. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Shah, S.; Mamoulakis, C.; Docea, A.; Kalantzi, O.-I.; Zachariou, A.; Calina, D.; Carvalho, F.; Sofikitis, N.; Makrigiannakis, A.; et al. Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int. J. Environ. Res. Public Health 2021, 18, 1464. [Google Scholar] [CrossRef]
- Della Torre, S.; Maggi, A. Sex Differences: A Resultant of an Evolutionary Pressure? Cell Metab. 2017, 25, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Cardona, O.; van Aalst, M.K.; Birkmann, J.; Fordham, M.; McGregor, G.; Perez, R.; Pulwarty, R.S.; Schipper, E.L.F.; Sinh, B.T.; Décamps, H.; et al. Determinants of Risk: Exposure and Vulnerability. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V., Stocker, T.F., Dahe, Q., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 65–108. [Google Scholar]
- Gonzales, G.; Young, C.; Masters, E.; de Mola, E.L. Health Disparities among Lesbian, Gay, Bisexual, and Transgender (LGBT) Adults in Nashville, Tennessee. South. Med. J. 2021, 114, 299–304. [Google Scholar] [CrossRef]
- Gaither, T.W.; Williams, K.; Mann, C.; Weimer, A.; Ng, G.; Litwin, M.S. Initial Clinical Needs Among Transgender and Non-binary Individuals in a Large, Urban Gender Health Program. J. Gen. Intern. Med. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Flores, H.L.; Amiri, A. CE: Addressing Food Insecurity in Vulnerable Populations. AJN Am. J. Nurs. 2019, 119, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Fauk, N.; Merry, M.; Siri, T.; Mwanri, L.; Ward, P. Structural, Personal and Socioenvironmental Determinants of HIV Transmission among Transgender Women in Indonesia. Int. J. Environ. Res. Public Health 2021, 18, 5814. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Balbuena, S.; Belza, M.J.; Urdaneta, E.; Esteso, R.; Rosales-Statkus, M.E.; de la Fuente, L.; Rapid HIV Testing Group. Serving the Underserved: An HIV Testing Program for Populations Reluctant to Attend Conven-tional Settings. Int. J. Public Health 2015, 60, 121–126. [Google Scholar] [CrossRef]
- Acosta-Deprez, V.; Jou, J.; London, M.; Ai, M.; Chu, C.; Cermak, N.; Kozlovich, S. Tobacco Control as an LGBTQ+ Issue: Knowledge, Attitudes, and Recommendations from LGBTQ+ Community Leaders. Int. J. Environ. Res. Public Health 2021, 18, 5546. [Google Scholar] [CrossRef]
- Kumar, G.; Sethi, A.K.; Bagchi, A.; Rai, S.; Tamilselvan, P. Knowledge, attitudes and behaviour towards oral hygiene of transgenders in Bhubaneswar during COVID-19. J. Fam. Med. Prim. Care 2021, 10, 1353–1358. [Google Scholar] [CrossRef]
- Patel, S. Fragrance compounds: The wolves in sheep’s clothings. Med. Hypotheses 2017, 102, 106–111. [Google Scholar] [CrossRef]
- Sergi, F.D.; Wilson, E.C. Filler Use Among Trans Women: Correlates of Feminizing Subcutaneous Injections and Their Health Consequences. Transgend. Health 2021, 6, 82–90. [Google Scholar] [CrossRef]
- Goetz, T.; Mamillapalli, R.; Devlin, M.J.; Robbins, A.E.; Majidi-Zolbin, M.; Taylor, H.S. Cross-sex testosterone therapy in ovariectomized mice: Addition of low-dose estrogen preserves bone architecture. Am. J. Physiol. Metab. 2017, 313, E540–E551. [Google Scholar] [CrossRef] [Green Version]
- Goetz, T.G.; Mamillapalli, R.; Sahin, C.; Majidi-Zolbin, M.; Ge, G.; Mani, A.; Taylor, H.S. Addition of Estradiol to Cross-Sex Testosterone Therapy Reduces Atherosclerosis Plaque Formation in Female ApoE−/− Mice. Endocrinology 2018, 159, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Lichtenecker, D.C.K.; Argeri, R.; Castro, C.H.M.; Dias-da-Silva, M.R.; Gomes, G.N. Cross-Sex Testosterone Ther-apy Modifies the Renal Morphology and Function in Female Rats and might Underlie Increased Systolic Pressure. Clin. Exp. Pharmacol. Physiol. 2021, 48, 978–986. [Google Scholar] [CrossRef]
- Kinnear, H.M.; Constance, E.S.; David, A.; E Marsh, E.; Padmanabhan, V.; Shikanov, A.; Moravek, M.B. A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Hum. Reprod. 2019, 34, 2009–2017. [Google Scholar] [CrossRef]
- Bartels, C.B.; Uliasz, T.F.; Lestz, L.; Mehlmann, L.M. Short-Term Testosterone use in Female Mice does Not Im-pair Fertilizability of Eggs: Implications for the Fertility Care of Transgender Males. Hum. Reprod. 2021, 36, 189–198. [Google Scholar]
- Kinnear, H.M.; Hashim, P.H.; Cruz, C.D.; Rubenstein, G.; Chang, F.L.; Nimmagadda, L.; Brunette, M.A.; Padmanabhan, V.; Shikanov, A.; Moravek, M.B. Reversibility of testosterone-induced acyclicity after testosterone cessation in a transgender mouse model. FS Sci. 2021, 2, 116–123. [Google Scholar] [CrossRef]
- Gómez, Á.; Cerdán, S.; Pérez-Laso, C.; Ortega, E.; Pásaro, E.; Fernández, R.; Gómez-Gil, E.; Mora, M.; Marcos, A.; del Cerro, M.C.R.; et al. Effects of adult male rat feminization treatments on brain morphology and metabolomic profile. Horm. Behav. 2020, 125, 104839. [Google Scholar] [CrossRef]
- Van Houten, E.L.; Visser, J.A. Mouse Models to Study Polycystic Ovary Syndrome: A Possible Link between Me-tabolism and Ovarian Function? Reprod. Biol. 2014, 14, 32–43. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Veiga-Lopez, A. Animal models of the polycystic ovary syndrome phenotype. Steroids 2013, 78, 734–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents; OECD Publishing: Paris, France, 2008. [Google Scholar]
- OECD. Test No. 421: Reproduction/Developmental Toxicity Screening Test; OECD Publishing: Paris, France, 2016; Volume 4. [Google Scholar]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, K.J.; Chiamolera, M.I.; Giannocco, G.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. Thyroid function disruptors: From nature to chemicals. J. Mol. Endocrinol. 2019, 62, R1–R19. [Google Scholar] [CrossRef] [PubMed]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Reisner, S.L.; Poteat, T.; Keatley, J.; Cabral, M.; Mothopeng, T.; Dunham, E.; E Holland, C.; Max, R.; Baral, S. Global health burden and needs of transgender populations: A review. Lancet 2016, 388, 412–436. [Google Scholar] [CrossRef]
- Lund, E.M.; Burgess, C.M. Sexual and Gender Minority Health Care Disparities: Barriers to Care and Strategies to Bridge the Gap. Prim. Care Clin. Off. Pract. 2021, 48, 179–189. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassinari, R.; Maranghi, F. Rodent Model of Gender-Affirming Hormone Therapies as Specific Tool for Identifying Susceptibility and Vulnerability of Transgender People and Future Applications for Risk Assessment. Int. J. Environ. Res. Public Health 2021, 18, 12640. https://doi.org/10.3390/ijerph182312640
Tassinari R, Maranghi F. Rodent Model of Gender-Affirming Hormone Therapies as Specific Tool for Identifying Susceptibility and Vulnerability of Transgender People and Future Applications for Risk Assessment. International Journal of Environmental Research and Public Health. 2021; 18(23):12640. https://doi.org/10.3390/ijerph182312640
Chicago/Turabian StyleTassinari, Roberta, and Francesca Maranghi. 2021. "Rodent Model of Gender-Affirming Hormone Therapies as Specific Tool for Identifying Susceptibility and Vulnerability of Transgender People and Future Applications for Risk Assessment" International Journal of Environmental Research and Public Health 18, no. 23: 12640. https://doi.org/10.3390/ijerph182312640
APA StyleTassinari, R., & Maranghi, F. (2021). Rodent Model of Gender-Affirming Hormone Therapies as Specific Tool for Identifying Susceptibility and Vulnerability of Transgender People and Future Applications for Risk Assessment. International Journal of Environmental Research and Public Health, 18(23), 12640. https://doi.org/10.3390/ijerph182312640





