Influence of Maternal Active and Secondhand Smoking during Pregnancy on Childhood Obesity at 3 Years of Age: A Nested Case–Control Study from the Japan Environment and Children’s Study (JECS)
Abstract
:1. Introduction
2. Materials and Methods
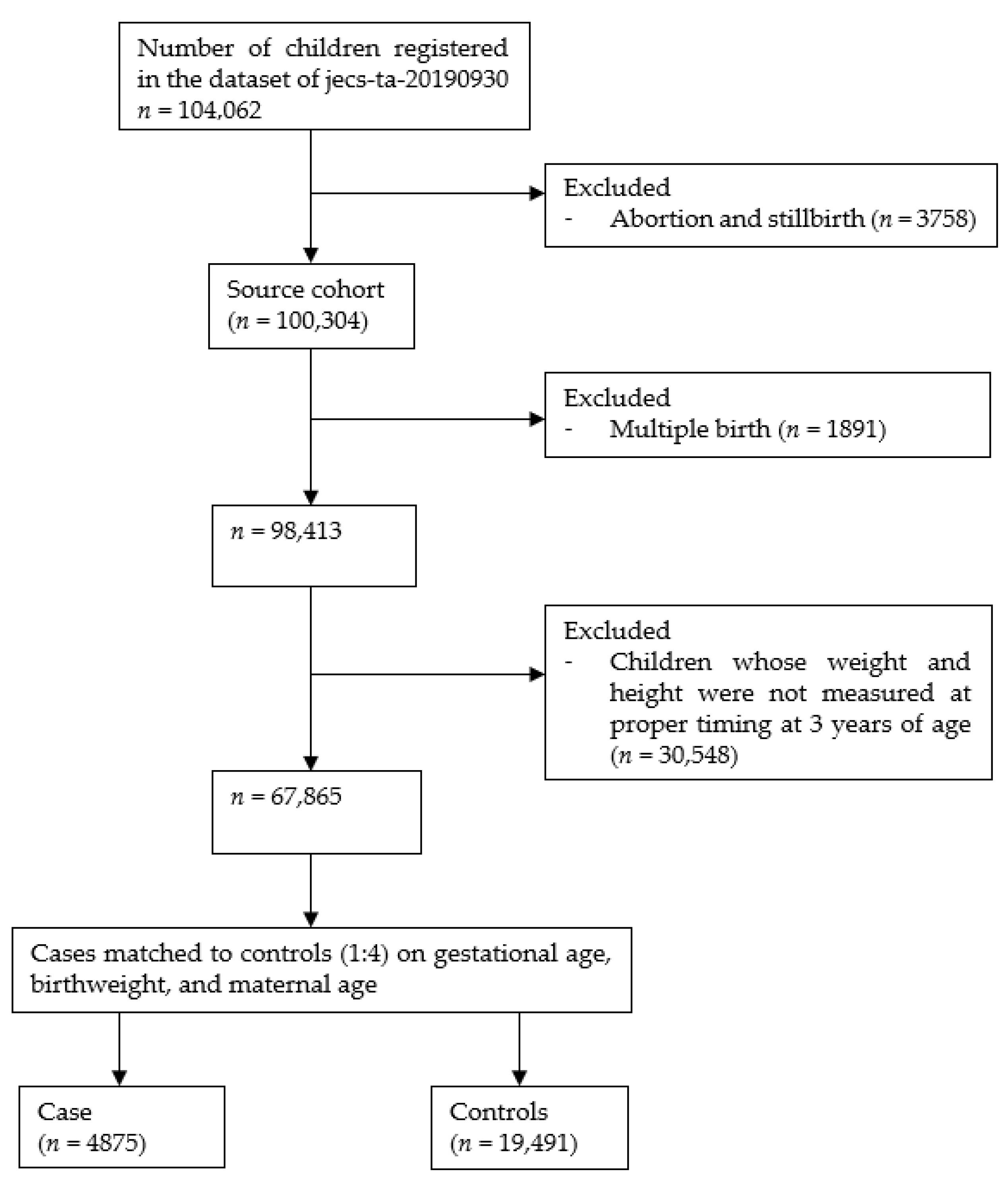
2.1. Study Setting and Population
2.2. Measure of Exposure
2.3. Outcome Definitions and Measurements
2.4. Measure of Covariates
2.5. Statistical Analyses
2.6. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Facts and Figures on Childhood Obesity. Available online: https://www.who.int/end-childhood-obesity/facts/en/ (accessed on 23 April 2020).
- Daniels, S.R. Complications of obesity in children and adolescents. Int. J. Obes. 2009, 33, S60–S65. [Google Scholar] [CrossRef] [Green Version]
- Oken, E.; Levitan, E.B.; Gillman, M.W. Maternal smoking during pregnancy and child overweight: Systematic review and meta-analysis. Int. J. Obes. 2008, 32, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Rayfield, S.; Plugge, E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J. Epidemiol. Community Health 2016, 71, 162–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedel, C.; Fenske, N.; Müller, M.J.; Plachta-Danielzik, S.; Keil, T.; Grabenhenrich, L.; von Kries, R. Differences in BMI z-scores between offspring of smoking and nonsmoking mothers: A longitudinal study of German children from birth through 14 years of age. Environ. Health Perspect. 2014, 122, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, R.C. Predicting preschooler obesity at birth: The role of maternal obesity in early pregnancy. Pediatrics 2004, 114, e29–e36. [Google Scholar] [CrossRef] [Green Version]
- Adams, A.K.; Harvey, H.E.; Prince, R.J. Association of maternal smoking with overweight at age 3 y in American Indian children. Am. J. Clin. Nutr. 2005, 82, 393–398. [Google Scholar] [CrossRef]
- Oken, E.; Huh, S.Y.; Taveras, E.M.; Rich-Edwards, J.W.; Gillman, M.W. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes. Res. 2005, 13, 2021–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Ando, D.; Sato, M.; Tanaka, T.; Kondo, N.; Yamagata, Z. The association between maternal smoking during pregnancy and childhood obesity persists to the age of 9–10 years. J. Epidemiol. 2009, 19, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Sato, M.; Zheng, W.; Shinohara, R.; Yokomichi, H.; Yamagata, Z. Childhood Growth Trajectories According to Combinations of Pregestational Weight Status and Maternal Smoking during Pregnancy: A Multilevel Analysis. PLoS ONE 2015, 10, e0118538. [Google Scholar]
- Mizutani, T.; Suzuki, K.; Kondo, N.; Yamagata, Z. Association of maternal lifestyles including smoking during pregnancy with childhood obesity. Obesity 2007, 15, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, R.; Jadotte, Y.; Zha, P.; Porter, S.A.; Holly, C.; Salmond, S.; Watkins, E.A. The association between prenatal exposure to environmental tobacco smoke and childhood obesity: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 1643–1662. [Google Scholar] [CrossRef]
- Review Meeting Members. Mid-Term Review of the Healthy Parents and Children 21 (2nd Phase) (Japanese only). 2019. Available online: https://www.mhlw.go.jp/content/11901000/000614300.pdf (accessed on 28 January 2020).
- Sansone, G.; Fong, G.T.; Meng, G.; Craig, L.V.; Xu, S.S.; Quah, A.C.; Ouimet, J.; Mochizuki, Y.; Yoshimi, I.; Tabuchi, T. Secondhand Smoke Exposure in Public Places and Support for Smoke-Free Laws in Japan: Findings from the 2018 ITC Japan Survey. Int. J. Environ. Res. Public Health 2020, 17, 979. [Google Scholar] [CrossRef] [Green Version]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, S.F.; Michikawa, T.; Sekiyama, M.; Kobayashi, Y.; Nishihama, Y. Japan environment and children’s study (JECS): Concept, protocol and current status. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 720–728. [Google Scholar]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Ono, M.; Yonemoto, J.; Tamura, K.; Suda, E.; Ito, H.; Takeuchi, A.; Kawamoto, T. The Japan Environment and Children’s Study (JECS): A preliminary report on selected characteristics of approximately 10,000 pregnant women recruited during the first year of the study. J. Epidemiol. 2015, 25, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline profile of participants in the Japan environment and children’s study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Siervo, M.; Horta, B.L.; Stephan, B.C.M.; Victora, C.G.; Wells, J.C. First-Borns Carry a Higher Metabolic Risk in Early Adulthood: Evidence from a Prospective Cohort Study. PLoS ONE 2010, 5, e13907. [Google Scholar] [CrossRef]
- Wells, J.C.; Hallal, P.C.; Reichert, F.F.; Dumith, S.C.; Menezes, A.M.; Victora, C.G. Associations of Birth Order With Early Growth and Adolescent Height, Body Composition, and Blood Pressure: Prospective Birth Cohort From Brazil. Am. J. Epidemiol. 2011, 174, 1028–1035. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Body Mass Index-for-Age. World Health Organization. 2014. Available online: https://www.who.int/childgrowth/standards/bmi_for_age/en/ (accessed on 24 September 2020).
- Kong, L.; Nilsson, I.A.K.; Gissler, M.; Lavebratt, C. Associations of Maternal Diabetes and Body Mass Index with Offspring Birth Weight and Prematurity. JAMA Pediatr. 2019, 173, 371–378. [Google Scholar] [CrossRef]
- Longmore, D.K.; Barr, E.L.M.; Lee, I.L.; Barzi, F.; Kirkwood, M.; Whitbread, C.; Hampton, V.; Graham, S.; van Dokkum, P.; Connors, C.; et al. Maternal body mass index, excess gestational weight gain, and diabetes are positively associated with neonatal adiposity in the Pregnancy and Neonatal Diabetes Outcomes in Remote Australia (PANDORA) study. Pediatr. Obes. 2019, 14, e12490. [Google Scholar] [CrossRef]
- Andres, A.; Hull, H.R.; Shankar, K.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Longitudinal body composition of children born to mothers with normal weight, overweight, and obesity. Obesity 2015, 23, 1252–1258. [Google Scholar] [CrossRef]
- Riaz, M.; Lewis, S.; Naughton, F.; Ussher, M. Predictors of smoking cessation during pregnancy: A systematic review and meta-analysis. Addiction 2018, 113, 610–622. [Google Scholar] [CrossRef]
- Chen, A.; Machiorlatti, M.; Krebs, N.M.; Muscat, J.E. Socioeconomic differences in nicotine exposure and dependence in adult daily smokers 14 Economics 1402 Applied Economics. BMC Public Health 2019, 19, 375. [Google Scholar]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar]
- Newton, S.; Braithwaite, D.; Akinyemiju, T.F. Socio-economic status over the life course and obesity: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0177151. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.J.; Von Dem Knesebeck, O. Income and obesity: What is the direction of the relationship? A systematic review and meta-analysis. BMJ Open 2018, 8, e019862. [Google Scholar]
- Rolland-Cachera, M.F.; Deheeger, M.; Bellisle, F.; Sempé, M.; Guilloud-Bataille, M.; Patois, E. Adiposity rebound in children: A simple indicator for predicting obesity. Am. J. Clin. Nutr. 1984, 39, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Boeke, C.E.; Mantzoros, C.S.; Hughes, M.D.; Rifas-Shiman, S.; Villamor, E.; Zera, C.A.; Gillman, M.W. Differential associations of leptin with adiposity across early childhood. Obesity 2013, 21, 1430–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arisaka, O.; Sairenchi, T.; Ichikawa, G.; Koyama, S. Increase of body mass index (BMI) from 1.5 to 3 years of age augments the degree of insulin resistance corresponding to BMI at 12 years of age. J. Pediatr. Endocrinol. Metab. 2017, 30, 455–457. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 November 2021).
- Ministry of Health Labour and Welfare. National Health and Nutrition Survey 2019. Available online: https://www.mhlw.go.jp/content/10900000/000687163.pdf (accessed on 13 November 2021).
- Ino, T.; Shibuya, T.; Saito, K.; Ohtani, T. Effects of maternal smoking during pregnancy on body composition in offspring. Pediatr. Int. 2011, 53, 851–857. [Google Scholar] [CrossRef]
- Secker-Walker, R.H.; Vacek, P.M. Relationships between cigarette smoking during pregnancy, gestational age, maternal weight gain, and infant birthweight. Addict. Behav. 2003, 28, 55–66. [Google Scholar] [CrossRef]
- Jauniaux, E.; Burton, G.J. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum. Dev. 2007, 83, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. Obesity and early life. Obes. Rev. 2007, 8, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, C.; Wang, H.; Comstock, J.M.; Singh, P.; Wang, Z.; Locklear, B.A.; Goodwin, K.L.; Maschek, J.A.; Cox, J.E.; Baack, M.L.; et al. Maternal Tobacco Smoke Exposure Causes Sex-Divergent Changes in Placental Lipid Metabolism in the Rat. Reprod. Sci. 2020, 27, 631–643. [Google Scholar] [CrossRef]
- Riedel, C.; Schönberger, K.; Yang, S.; Koshy, G.; Chen, Y.C.; Gopinath, B.; Ziebarth, S.; von Kries, R. Parental smoking and childhood obesity: Higher effect estimates for maternal smoking in pregnancy compared with paternal smoking—A meta-analysis. Int. J. Epidemiol. 2014, 43, 1593–1606. [Google Scholar] [CrossRef] [Green Version]
- Shipton, D.; Tappin, D.M.; Vadiveloo, T.; Crossley, J.A.; Aitken, D.A.; Chalmers, J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. BMJ 2009, 339, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, V.T.; Althabe, F.; Alemán, A.; Johnson, C.C.; Dietz, P.M.; Berrueta, M.; Morello, P.; Colomar, M.; Buekens, P.; Sosnoff, C.S.; et al. Accuracy of self-reported smoking cessation during pregnancy. Acta. Obstet. Gynecol. Scand. 2015, 94, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.R.; Gearhardt, A.N.; Miller, A.L.; Hyde, L.W.; Lumeng, J.C. Maternal nicotine dependence is associated with longitudinal increases in child obesogenic eating behaviors. Pediatr. Obes. 2019, 14, e12541. [Google Scholar] [CrossRef]
| Variable | Not Measured (n = 30,548) | Measured (n = 67,865) | p-Value (chi-Square) |
|---|---|---|---|
| Maternal smoking during pregnancy | |||
| Never or quit smoking before pregnancy | 21,300 (69.7) | 57,039 (84.1) | <0.001 |
| Quit smoking after pregnancy | 5249 (17.2) | 7885 (11.6) | |
| Continue smoking | 2241 (7.3) | 2180 (3.2) | |
| Missing data | 1758 (5.8) | 761 (1.1) | |
| Paternal smoking during pregnancy | |||
| Never or quit smoking before pregnancy | 12,079 (39.5) | 35,708 (52.6) | <0.001 |
| Quit smoking after pregnancy | 872 (2.9) | 1894 (2.8) | |
| Continue smoking | 15,368 (50.3) | 28,974 (42.7) | |
| Missing data | 2229 (7.3) | 1289 (1.9) | |
| Exposure to secondhand smoking before pregnancy | |||
| Rare | 12,865 (42.1) | 35,384 (52.1) | <0.001 |
| Several times per week | 9435 (30.9) | 21,360 (31.5) | |
| Everyday | 7077 (23.2) | 10,547 (15.5) | |
| Missing data | 1171 (3.8) | 574 (0.9) | |
| Exposure to secondhand smoking during pregnancy | |||
| Rare | 16,207 (53.1) | 43,748 (64.5) | <0.001 |
| Several times per week | 7622 (25.0) | 16,403 (24.2) | |
| Everyday | 5233 (17.1) | 7202 (10.6) | |
| Missing data | 1486 (4.9) | 512 (0.8) | |
| Child sex | |||
| Male | 15,740 (51.5) | 34,694 (51.1) | |
| Female | 14,790 (48.4) | 33,171 (48.9) | |
| Unknown | 3 (0.01) | - | |
| Missing | 15 (0.1) | - | |
| Birthweight (g) | 2997.8 [469.2] | 3034.5 [394.9] | <0.001 * |
| Gestational week at birth (week) | 38.6 [2.0] | 38.9 [1.4] | <0.001 * |
| Breastfeeding during first 6 months | |||
| Exclusive | 8257 (27.0) | 24,935 (36.7) | <0.001 |
| Mixed | 15,594 (51.1) | 40,841 (60.2) | |
| Formula | 808 (2.7) | 1275 (1.9) | |
| Missing data | 5889 (19.3) | 814 (1.2) | |
| Attendance to nursery school by 1 year of age | |||
| Yes | 5372 (17.6) | 18,382 (27.1) | <0.001 |
| No | 16,720 (54.7) | 47,973 (70.7) | |
| Missing data | 8456 (27.7) | 1510 (2.2) | |
| Maternal age at entry (year) | 29.8 [5.3] | 31.2 [4.9] | <0.001 * |
| Maternal job type at conception | |||
| Employed | 17,392 (56.9) | 43,743 (64.5) | <0.001 |
| Housewife | 8974 (29.4) | 18,777 (27.7) | |
| Students, unemployed or others | 2121 (6.9) | 3367 (5.0) | |
| Missing data | 2061 (6.8) | 1978 (2.9) | |
| Maternal educational attainment | |||
| Elementary to junior high-school | 13,766 (45.1) | 22,756 (33.5) | <0.001 |
| Under-graduate | 14,876 (48.7) | 43,341 (63.9) | |
| Post-graduate | 279 (0.9) | 1131 (1.7) | |
| Missing data | 1627 (5.3) | 637 (0.9) | |
| Income (million yen) | |||
| <4 | 12,306 (40.3) | 23,843 (35.1) | <0.001 |
| 4–6 | 8242 (27.0) | 21,430 (31.6) | |
| 6–8 | 3463 (11.3) | 10,832 (16.0) | |
| ≥8 | 2310 (7.6) | 7384 (10.9) | |
| Missing data | 4227 (13.8) | 4376 (6.5) | |
| Marital status at registration | |||
| Married | 27,627 (90.4) | 64,892 (95.6) | <0.001 |
| Single, divorced or widowed | 1802 (5.9) | 2408 (3.6) | |
| Missing data | 1119 (3.7) | 565 (0.8) | |
| Maternal BMI before pregnancy (kg/m2) | 21.5 [3.6] | 21.1 [3.2] | |
| <18.5 (underweight) | 5002 (16.4) | 10,911 (16.1) | <0.001 |
| 18.5–25 (normal) | 21,530 (70.5) | 50,371 (74.2) | |
| 25–30 (overweight) | 2912 (9.5) | 5076 (7.5) | |
| ≥30 (obesity) | 1017 (3.3) | 1465 (2.2) | |
| Missing data | 87 (0.3) | 42 (0.1) | |
| Maternal drinking during pregnancy | |||
| No | 9512 (31.1) | 22,656 (33.4) | <0.001 |
| Quit | 18,415 (60.3) | 42,610 (62.8) | |
| Continue | 904 (3.0) | 1802 (2.7) | |
| Missing data | 1717 (5.6) | 797 (1.2) | |
| Maternal diabetes or gestational diabetes | |||
| No | 29,291 (95.9) | 65,756 (96.9) | 0.448 |
| Yes | 968 (3.2) | 2109 (3.1) | |
| Missing data | 289 (0.9) | - |
| Variable | Cases (n = 4875) | Controls (n = 19,491) |
|---|---|---|
| Maternal smoking during pregnancy | ||
| Never or quit smoking before pregnancy | 3971 (81.5) | 16,458 (84.4) |
| Quit smoking after pregnancy | 669 (13.7) | 2290 (11.8) |
| Continue smoking | 188 (3.9) | 565 (2.9) |
| Missing data | 47 (1.0) | 178 (0.9) |
| Paternal smoking during pregnancy | ||
| Never or quit smoking before pregnancy | 2417 (49.6) | 10,260 (52.6) |
| Quit smoking after pregnancy | 128 (2.6) | 550 (2.8) |
| Continue smoking | 2245 (46.1) | 8338 (42.8) |
| Missing data | 85 (1.7) | 343 (1.8) |
| Exposure to secondhand smoking before pregnancy | ||
| Rare | 2385 (48.9) | 10,250 (52.6) |
| Several times per week | 1601 (32.8) | 6188 (31.8) |
| Everyday | 875 (18.0) | 2988 (15.3) |
| Missing data | 14 (0.3) | 65 (0.3) |
| Exposure to secondhand smoking during pregnancy | ||
| Rare | 2942 (60.4) | 12,587 (64.6) |
| Several times per week | 1272 (26.1) | 4677 (24.0) |
| Everyday | 633 (13.0) | 2119 (10.8) |
| Missing data | 28 (0.6) | 108 (0.6) |
| Child sex | ||
| Male | 2562 (52.6) | 10,309 (52.9) |
| Female | 2313 (47.5) | 9182 (47.1) |
| Birthweight (g) 1 | 3182.5 [391.8] | 3123.6 [389.0] |
| Gestational week at birth (week) 1 | 38.9 [1.4] | 39.0 [1.3] |
| Breastfeeding during first 6 months | ||
| Exclusive | 1689 (34.7) | 7238 (37.1) |
| Mixed | 3006 (61.7) | 11,662 (59.8) |
| Formula | 124 (2.5) | 365 (1.9) |
| Missing data | 56 (1.2) | 226 (1.2) |
| Attendance to nursery school by 1 year of age | ||
| Yes | 1441 (29.6) | 5290 (27.1) |
| No | 3316 (68.0) | 13,780 (70.7) |
| Missing data | 118 (2.4) | 421 (2.2) |
| Adiposity rebound before 3 years of age | ||
| No | 2030 (41.6) | 13,242 (67.9) |
| Yes | 2645 (54.3) | 5519 (28.3) |
| Missing data | 200 (4.1) | 730 (3.8) |
| Maternal age at entry (year) 1 | 31.1 [4.9] | 31.2 [4.8] |
| Maternal job type at conception | ||
| Employed | 3213 (65.9) | 12,633 (64.8) |
| Housewife | 1305 (26.8) | 5530 (28.4) |
| Students, unemployed or others | 225 (4.6) | 893 (4.6) |
| Missing data | 132 (2.7) | 435 (2.2) |
| Maternal educational level | ||
| Elementary to junior high-school | 1772 (36.4) | 6506 (33.4) |
| Under-graduate | 2984 (61.2) | 12,501 (64.1) |
| Post-graduate | 81 (1.7) | 341 (1.8) |
| Missing data | 38 (0.8) | 143 (0.7) |
| Income (million yen) | ||
| <4 | 1743 (35.8) | 6831 (35.1) |
| 4–6 | 1513 (31.0) | 6145 (31.5) |
| 6–8 | 784 (16.1) | 3123 (16.0) |
| ≥8 | 519 (10.6) | 2177 (11.0) |
| Missing data | 316 (6.5) | 1215 (6.2) |
| Maternal BMI before pregnancy (kg/m2) | 22.2 (3.8) | 21.2 (3.2) |
| <18.5 (underweight) | 434 (8.9) | 3002 (15.4) |
| 18.5–25 (normal) | 3629 (74.4) | 14,541 (74.6) |
| 25–30 (overweight) | 583 (12.0) | 1511 (7.8) |
| ≥30 (obesity) | 227 (4.7) | 431 (2.2) |
| Missing data | 2 (0.1) | 6 (0.1) |
| Maternal drinking during pregnancy | ||
| No | 1584 (32.5) | 6499 (33.3) |
| Quit | 3131 (64.2) | 12,260 (62.9) |
| Continue | 121 (2.5) | 535 (2.7) |
| Missing data | 39 (0.8) | 197 (1.0) |
| Maternal fish intake during pregnancy | ||
| Less than once a month | 1005 (20.6) | 3827 (19.6) |
| Several times a month | 1071 (22.0) | 4663 (23.9) |
| Once a week | 1186 (24.3) | 4781 (24.5) |
| More than once a week | 1559 (32.0) | 6014 (30.9) |
| Missing data | 54 (1.1) | 206 (1.1) |
| Smoking Status during Pregnancy | Crude OR (95% CI) | Adjusted OR a (95% CI) n = 17,788 |
|---|---|---|
| Maternal smoking during pregnancy | ||
| Never or quit smoking before pregnancy | 1.00 | 1.00 |
| Quit smoking after pregnancy | 1.22 (1.11–1.35) | 1.14 (0.95–1.37) |
| Continue smoking | 1.40 (1.18–1.65) | 1.39 (1.01–1.92) |
| Paternal smoking during pregnancy | ||
| Never or quit smoking before pregnancy | 1.00 | 1.00 |
| Quit smoking after pregnancy | 0.98 (0.81–1.20) | 0.86 (0.61–1.21) |
| Continue smoking | 1.15 (1.08–1.23) | 1.03 (0.91–1.17) |
| Exposure to secondhand smoking before pregnancy | ||
| Seldom | 1.00 | - b |
| Several times per week | 1.12 (1.04–1.20) | - |
| Everyday | 1.27 (1.16–1.38) | - |
| Exposure to secondhand smoking during pregnancy | ||
| Seldom | 1.00 | 1.00 |
| Several times per week | 1.17 (1.09–1.26) | 1.14 (0.99–1.30) |
| Everyday | 1.29 (1.17–1.42) | 1.23 (1.01–1.50) |
| Maternal Smoking during Pregnancy | Exposure to Secondhand Smoking during Pregnancy | ||
|---|---|---|---|
| Seldom | Several Times per Week | Everyday | |
| Never or quit smoking before pregnancy | 1.00 | 1.11 (0.97–1.27) | 1.23 (0.98–1.55) |
| Quit smoking after pregnancy | 1.11 (0.97–1.27) | 1.30 (0.98–1.73) | 1.59 (1.17–2.15) |
| Continue smoking | 1.23 (0.98–1.55) | 1.59 (1.17–2.15) | 1.60 (1.06–2.39) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiuchi, S.; Shinohara, R.; Otawa, S.; Kushima, M.; Akiyama, Y.; Ooka, T.; Kojima, R.; Yokomichi, H.; Miyake, K.; Hirai, H.; et al. Influence of Maternal Active and Secondhand Smoking during Pregnancy on Childhood Obesity at 3 Years of Age: A Nested Case–Control Study from the Japan Environment and Children’s Study (JECS). Int. J. Environ. Res. Public Health 2021, 18, 12506. https://doi.org/10.3390/ijerph182312506
Horiuchi S, Shinohara R, Otawa S, Kushima M, Akiyama Y, Ooka T, Kojima R, Yokomichi H, Miyake K, Hirai H, et al. Influence of Maternal Active and Secondhand Smoking during Pregnancy on Childhood Obesity at 3 Years of Age: A Nested Case–Control Study from the Japan Environment and Children’s Study (JECS). International Journal of Environmental Research and Public Health. 2021; 18(23):12506. https://doi.org/10.3390/ijerph182312506
Chicago/Turabian StyleHoriuchi, Sayaka, Ryoji Shinohara, Sanae Otawa, Megumi Kushima, Yuka Akiyama, Tadao Ooka, Reiji Kojima, Hiroshi Yokomichi, Kunio Miyake, Hiroyuki Hirai, and et al. 2021. "Influence of Maternal Active and Secondhand Smoking during Pregnancy on Childhood Obesity at 3 Years of Age: A Nested Case–Control Study from the Japan Environment and Children’s Study (JECS)" International Journal of Environmental Research and Public Health 18, no. 23: 12506. https://doi.org/10.3390/ijerph182312506
APA StyleHoriuchi, S., Shinohara, R., Otawa, S., Kushima, M., Akiyama, Y., Ooka, T., Kojima, R., Yokomichi, H., Miyake, K., Hirai, H., Hashimoto, K., Shimabukuro, M., Yamagata, Z., & Japan Environment and Children’s Study Group. (2021). Influence of Maternal Active and Secondhand Smoking during Pregnancy on Childhood Obesity at 3 Years of Age: A Nested Case–Control Study from the Japan Environment and Children’s Study (JECS). International Journal of Environmental Research and Public Health, 18(23), 12506. https://doi.org/10.3390/ijerph182312506






