Application of Capacitive-Resistive Electric Transfer in Physiotherapeutic Clinical Practice and Sports
Abstract
1. Introduction
Objectives
- (A)
- Areas of application within physiotherapy clinical practice;
- (B)
- Areas of application within the sports field;
- (C)
- Demonstrated effects of radiofrequency diathermy;
- (D)
- Study quality;
- (E)
- Identification of gaps in knowledge for future research.
2. Methods
2.1. Search Strategy
2.2. Article Selection
2.3. Data Analysis
2.4. Study Quality and Bias Assessment
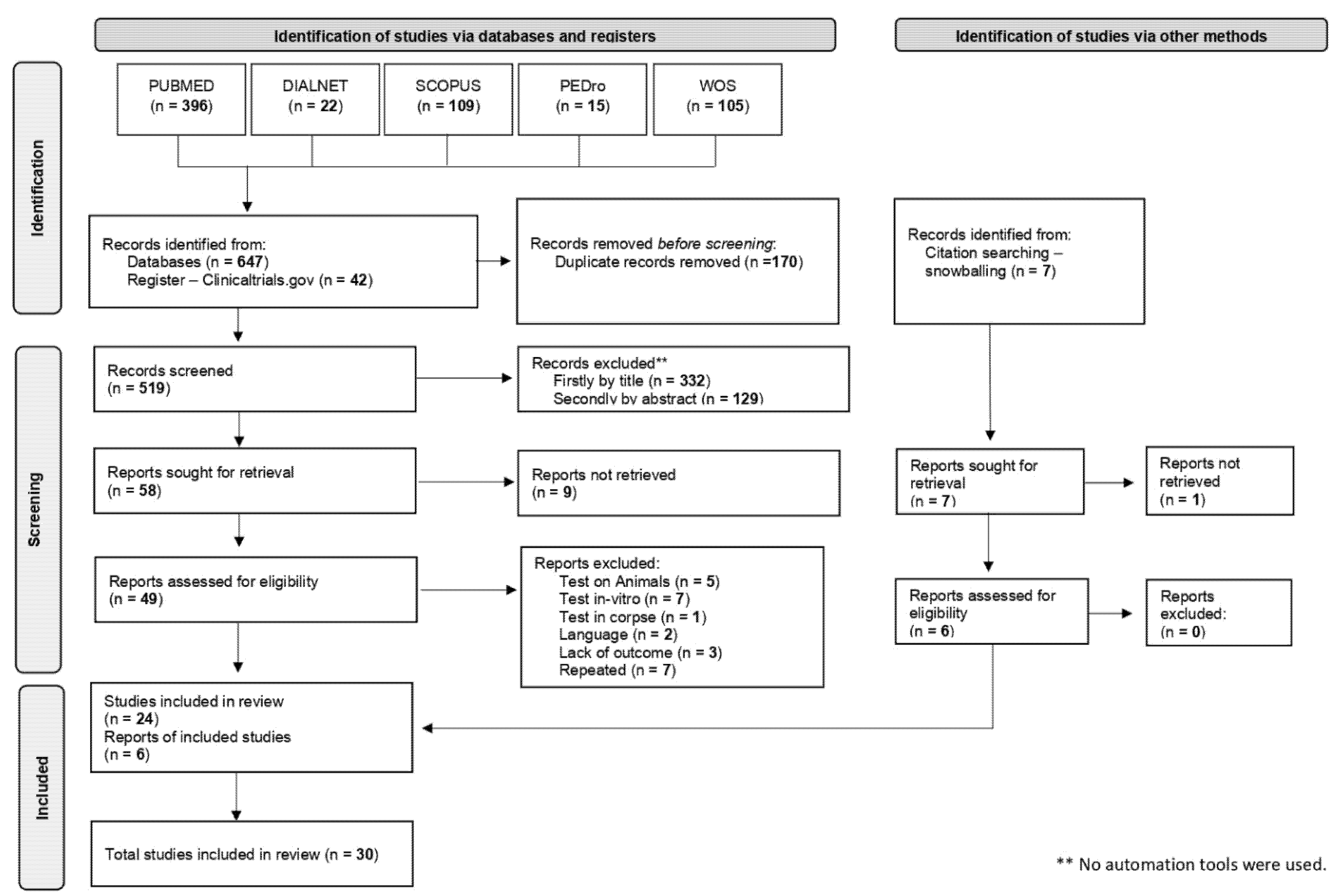
3. Results
3.1. Included Studies
3.2. Participants, Experimental Conditions, Apparatus and Frequencies
3.3. Methodological Quality of the Included Studies
4. Discussion
4.1. CRET Effects on Temperature and Irrigation
4.2. Applications in the Field of Sports
4.3. Applications in Musculoskeletal Conditions
4.4. Pelvic Floor Applications
4.5. Applications in Dermato-Functional Physiotherapy
4.6. Other Applications
4.7. Nomenclature
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Krusen, F.H. The present status of short wave diathermy. J. Am. Med. Assoc. 1938, 110, 1280. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Martínez-Botas, J.; Trillo, M.Á.; Paíno, C.L.; Úbeda, A. Antiadipogenic effects of subthermal electric stimulation at 448 kHz on differentiating human mesenchymal stem cells. Mol. Med. Rep. 2016, 13, 3895–3903. [Google Scholar] [CrossRef][Green Version]
- Hernández-Bule, M.L.; Paino, C.; Trillo, M.; Ángeles; Úbeda, A. Electric Stimulation at 448 kHz Promotes Proliferation of Human Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Medel, E.; Colastra, C.; Roldán, R.; Úbeda, A. Response of neuroblastoma cells to RF currents as a function of the signal frequency. BMC Cancer 2019, 19, 89. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Trillo, M.; Ángeles; Úbeda, A. Molecular Mechanisms Underlying Antiproliferative and Differentiating Responses of Hepatocarcinoma Cells to Subthermal Electric Stimulation. PLoS ONE 2014, 9, e84636. [Google Scholar] [CrossRef] [PubMed]
- Clijsen, R.; Leoni, D.; Schneebeli, A.; Cescon, C.; Soldini, E.; Li, L.; Barbero, M. Does the Application of Tecar Therapy Affect Temperature and Perfusion of Skin and Muscle Microcirculation? A Pilot Feasibility Study on Healthy Subjects. J. Altern. Complement. Med. 2020, 26, 147–153. [Google Scholar] [CrossRef]
- Coccetta, C.A.; Sale, P.; Ferrara, P.E.; Specchia, A.; Maccauro, G.; Ferriero, G.; Ronconi, G. Effects of capacitive and resistive electric transfer therapy in patients with knee osteoarthritis: A randomized controlled trial. Int. J. Rehabil. Res. 2019, 42, 106–111. [Google Scholar] [CrossRef]
- Notarnicola, A.; Maccagnano, G.; Gallone, M.F.; Covelli, I.; Tafuri, S.; Moretti, B. Short term efficacy of capacitive-resistive diathermy therapy in patients with low back pain: A prospective randomized controlled trial. J. Boil. Regul. Homeost. Agents 2017, 31, 509–515. [Google Scholar]
- Sanguedolce, G.; Venza, C.; Cataldo, P.; Mauro, G.L. TECAR-Terapia nelle tendinopatie della cuffia dei rotatori:nostra esperienza [TECAR-Therapy in rotator cuff tendinopathies: Our experience]. Eur. J. Phys. Rehabil. Med. 2009, 45, 1–4. (In Italian) [Google Scholar]
- Beltrame, R.; Ronconi, G.; Ferrara, P.E.; Salgovic, L.; Vercelli, S.; Solaro, C.; Ferriero, G. Capacitive and resistive electric transfer therapy in rehabilitation. Int. J. Rehabil. Res. 2020, 43, 291–298. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Elkins, M.; Moseley, A.M. Intention-to-treat analysis. J. Physiother. 2015, 61, 165–167. [Google Scholar] [CrossRef]
- Armstrong, R.; Hall, B.J.; Doyle, J.; Waters, E. ‘Scoping the scope’ of a cochrane review. J. Public Heal. 2011, 33, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Albornoz-Cabello, M.; Ibáñez-Vera, A.; Aguilar-Ferrándiz, M.; Espejo-Antúnez, L. Monopolar dielectric diathermy by emission of radiofrequency in Patellofemoral pain. A single-blind-randomized clinical trial. Electromagn. Biol. Med. 2020, 39, 282–289. [Google Scholar] [CrossRef]
- Diego, I.M.A.; Fernández-Carnero, J.; Val, S.L.; Cano-De-La-Cuerda, R.; Calvo-Lobo, C.; Piédrola, R.M.; Oliva, L.C.L.; Rueda, F.M. Analgesic effects of a capacitive-resistive monopolar radiofrequency in patients with myofascial chronic neck pain: A pilot randomized controlled trial. Rev. Assoc. Médica Bras. 2019, 65, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, B.; Watson, T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: A prospective randomised crossover study in healthy adults. Int. J. Hyperth. 2015, 31, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Bretelle, F.; Fabre, C.; Golka, M.; Pauly, V.; Roth, B.; Bechadergue, V.; Blanc, J. Capacitive-resistive radiofrequency therapy to treat postpartum perineal pain: A randomized study. PLoS ONE 2020, 15, e0231869. [Google Scholar] [CrossRef] [PubMed]
- Devran, S. Efficacy of Capacitive-Resistive Therapy on the Treatment of Myofascial Pain; Istanbul University: Istanbul, Turkey, 2020. [Google Scholar]
- Kumaran, B.; Watson, T. Treatment using 448 kHz capacitive resistive monopolar radiofrequency improves pain and function in patients with osteoarthritis of the knee joint: A randomised controlled trial. Physiotheraphy 2019, 105, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, B.; Watson, T. Skin thermophysiological effects of 448 kHz capacitive resistive monopolar radiofrequency in healthy adults: A randomised crossover study and comparison with pulsed shortwave therapy. Electromagn. Biol. Med. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- Pavone, C.; Romeo, S.; D’Amato, F.; Usala, M.; Mauro, G.L.; Caruana, G. Does Transfer Capacitive Resistive Energy Has a Therapeutic Effect on Peyronie’s Disease? Randomized, Single-Blind, Sham-Controlled Study on 96 Patients: Fast Pain Relief. Urol. Int. 2017, 99, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Noites, A.; Vale, A.L.; Pereira, A.S.; Morais, A.; Vilarinho, R.; Carvalho, P.; Amorim, M.; Moreira, T.; Mendonça, A.; Mendonça, A. Effect of an aerobic exercise session combined with abdominal radiofrequency on lipolytic activity in women: Randomized control trial. J. Cosmet. Dermatol. 2019, 19, 638–645. [Google Scholar] [CrossRef]
- Vale, A.L.; Pereira, A.S.; Morais, A.; de Carvalho, P.; Vilarinho, R.; Mendonça, A.; Noites, A. Effect of four sessions of aerobic exercise with abdominal radiofrequency in adipose tissue in healthy women: Randomized control trial. J. Cosmet. Dermatol. 2020, 19, 359–367. [Google Scholar] [CrossRef]
- Maretti, C.; Canale, D. New Therapeutical Procedures of Peyronie’s Disease: Transfer Capacitive Resistive Energy in Association with Hydroelectrophoresis with Verapamil. Int. J. Pharm. Res. Allied Sci. 2020, 9, 16–23. [Google Scholar]
- Tashiro, Y.; Hasegawa, S.; Yokota, Y.; Nishiguchi, S.; Fukutani, N.; Shirooka, H.; Tasaka, S.; Matsushita, T.; Matsubara, K.; Nakayama, Y.; et al. Effect of Capacitive and Resistive electric transfer on haemoglobin saturation and tissue temperature. Int. J. Hyperth. 2017, 33, 696–702. [Google Scholar] [CrossRef] [PubMed]
- De la Casa Almeida, M. Eficacia Terapéutica de la Diatermia Capacitiva Monopolar Mediante Radiofrecuencia en la Disminución de la Paniculopatía Fibroedematoesclerótica y Perímetros Corporales [Efficacy of Capacitive Diathermy Using Radiofrequency in Reducing Cellulite and Body. Ph.D. Thesis, University of Sevilla, Sevilla, Spain, 2011. [Google Scholar]
- Fousekis, K.; Chrysanthopoulos, G.; Tsekoura, M.; Mandalidis, D.; Mylonas, K.; Angelopoulos, P.; Koumoundourou, D.; Billis, V.; Tsepis, E. Posterior thigh thermal skin adaptations to radiofrequency treatment at 448 kHz applied with or without Indiba® fascia treatment tools. J. Phys. Ther. Sci. 2020, 32, 292–296. [Google Scholar] [CrossRef][Green Version]
- Duñabeitia, I.; Arrieta, H.; Torres-Unda, J.; Gil, J.; Santos-Concejero, J.; Gil, S.M.; Irazusta, J.; Bidaurrazaga-Letona, I. Effects of a capacitive-resistive electric transfer therapy on physiological and biomechanical parameters in recreational runners: A randomized controlled crossover trial. Phys. Ther. Sport 2018, 32, 227–234. [Google Scholar] [CrossRef]
- Rodríguez Sanz, D.; Martínez Pascual, B.; Fernández Martínez, S.; de la Cueva Reguera, M.; Díez Vega, I.; Calvo Lobo, C. Effectiveness of diathermy in patients with low back and pelvic pain referred to lower limb: A pilot study. Eur. J. Pod. 2017, 3, 41–45. [Google Scholar] [CrossRef][Green Version]
- Cau, N.; Cimolin, V.; Aspesi, V.; Galli, M.; Postiglione, F.; Todisco, A.; Tacchini, E.; Darno, D.; Capodaglio, P. Preliminary evidence of effectiveness of TECAR in lymphedema. Lymphology 2019, 52, 35–43. [Google Scholar]
- Stagi, P.; Paoloni, M.; Ioppolo, F.; Palmerini, V.; Santilli, V. Studio clinico randomizzato in doppio cieco tecarterapia versus placebo nel trattamento della lombalgia [A randomised, double blind, placebo controlled clinical trial on Tecartherapy in the treatment of backpain]. Eur. J. Phys. Rehabil. Med. 2008, 44, 1–3. (In Italian) [Google Scholar]
- Takahashi, K.; Suyama, T.; Takakura, Y.; Hirabayashi, S.; Tsuzuki, N.; Li, Z.-S. Clinical Effects of Capacitive Electric Transfer Hyperthermia Therapy for Cervico-Omo-Brachial Pain. J. Phys. Ther. Sci. 2000, 12, 43–48. [Google Scholar] [CrossRef]
- Vera, A.J.I.; Puyalto, A.G.; Vivancos, M.A.I.; Diaz, D.C.; Achalandabaso, A.; Vega, R.L. Effects of Dielectric Monopolar Radiofrequency with Vacuumtherapy in the Treatment of Chronic Constipation in Patients with Intellectual Developmental Disorders. Neuropsychiatry 2019, 9, 2070–2075. [Google Scholar] [CrossRef]
- Ganzit, G.P.; Stefanini, L.; Stesina, G. Nuove methodice nei trattamento della patología muscolo-articolare dell’atleta: La tercaterapia [New methods in the treatment of athlete’s musculoskeletal pathology: Cret therapy]. Med. Sport 2000, 53, 361–367. (In Italian) [Google Scholar]
- Sodré, D.S.M.; Sodré, P.R.S.; Brasil, C.; Teles, A.; Dória, M.; Café, L.E.; Lordelo, P. New concept for treating urinary incontinence after radical prostatectomy with radiofrequency: Phase 1 clinical trial. Lasers Med. Sci. 2019, 34, 1865–1871. [Google Scholar] [CrossRef]
- Raffaetà, G.; Menconi, A.; Togo, R. Studio sperimentale: Applicazione terapeutica della “tecarterapia” nelle sindromi algiche cervicali [Experimental study: Therapeutic application of “tecar therapy” in cervical pain syndromes]. Eur. J. Phys. Rehabil. Med. 2007, 43, 1–4. (In Italian) [Google Scholar]
- Takahashi, K.; Suyama, T.; Onodera, M.; Hirabayashi, S.; Tsuzuki, N.; Zhong-Shi, L. Clinical Effects of Capacitive Electric Transfer Hyperthermia Therapy for Lumbago. J. Phys. Ther. Sci. 1999, 11, 45–51. [Google Scholar] [CrossRef]
- Osti, R.; Pari, C.; Salvatori, G.; Massari, L. Tri-length laser therapy associated to tecar therapy in the treatment of low-back pain in adults: A preliminary report of a prospective case series. Lasers Med. Sci. 2015, 30, 407–412. [Google Scholar] [CrossRef]
- Fernández-Cuadros, M.; Kazlauskas, S.; Albaladejo-Florin, M.; Robles-López, M.; Laborda-Delgado, A.; De La Cal-Alvarez, C.; Pérez-Moro, O. Effectiveness of multimodal rehabilitation (biofeedback plus capacitive-resistive radiofrequency) on chronic pelvic pain and dyspareunia: Prospective study and literature review. Rehabilitación 2020, 54, 154–161. [Google Scholar] [CrossRef]
- Edmond, S.N.; Turk, D.C.; Williams, D.A.; Kerns, R.D. Considerations of trial design and conduct in behavioral interventions for the management of chronic pain in adults. PAIN Rep. 2019, 4, e655. [Google Scholar] [CrossRef] [PubMed]
- Opara, J.; Kucio, C.; Małecki, A.; Pilch, J. Methods of blinding clinical trials in physiotherapy. Physiotherapy 2013, 21. [Google Scholar] [CrossRef]
- Schulz, K.F.; A Grimes, D. Blinding in randomised trials: Hiding who got what. Lancet 2002, 359, 696–700. [Google Scholar] [CrossRef]
- López-Olmos, J. Dispareunia: Investigación de causa física y de causa infecciosa crónica (estudio prospectivo de 4 años). Clínica Investig. Ginecol. Obstet. 2008, 35, 152–159. (In Spanish) [Google Scholar] [CrossRef]
| Conducted | 28 October 2020 | |
|---|---|---|
| Search Strategy | Natural, MeSH Terms and Applied Equations | Results Obtained |
| # 1 | Diathermy [MeSH Terms] | |
| # 2 | “Physical therapy modalities” [MeSH Terms] OR “Sports Medicine” [MeSH Terms] | |
| # 3 | “Treatment Outcome” [MeSH Terms] | |
| # 4 | # 1 AND # 2 AND # 3 | 358 |
| # 5 | Wintecare [tiab] OR Winback [tiab] OR Vossman [tiab] OR Indiba [tiab] OR Lavatron [tiab] OR Quilmed [tiab] OR Capenergy [tiab] OR Erbalaser [tiab] OR Tecar [tiab] | 23 |
| # 6 | Capacitive-Resistive [Title/Abstract] | |
| # 7 | Therapeutics [MeSH Terms] OR “Sports Medicine” [MeSH Terms] | |
| # 8 | # 6 AND # 7 | 15 |
| TOTAL, sum of blocks # 4 OR # 5 OR # 8 | 396 |
| Author, Year, PEDro Score | Participants, Sample Size | Experimental Conditions | Outcome Measures | Measurement Time Points | Main Results |
|---|---|---|---|---|---|
| Albornoz-Cabello et al. 2020 [15], 9/10 | W (n: 52) and M (n: 32), Adults with patellofemoral pain | EC1: CRET-840 kHz (n: 42) EC2: Control (n: 42) | 1. Pain 2. Neuropathic Pain 3. Patellofemoral pain scale 4. lower limb functional scale 5. Range of movement | T0 Baseline T1 After intervention | Pain: lower in EC1 vs. EC2 (6 ± 12.8 vs. 59 ± 16.1 mm). Neuropathic Pain: reduced in EC1 vs. EC2 (0.2 ± 0.52 vs. 3.5 ± 1.83 mm). Patellofemoral pain scale: improved EC1 vs. EC2 (74 ± 13.77 vs. 53 ± 13.49 pts). Lower limb functional scale: improvement in EC1 vs. EC2 (74 ± 14.77 vs. 52 ± 15.84 pts). Range of movement: flexion enhanced in EC1 vs. EC2 (133 ± 7.86 vs. 113 ± 12.58°). |
| Alguacil-Diego et al. 2019 [16], 8/10 | W (n: 14) and M (n: 10) Adults with myofascial chronic neck pain | EC1: CRET-448 kHz (n: 14) EC2: Placebo (n: 10) | 1. Pain 2. Neck Disability Index 3. Range of movement | T0 Baseline T1 After first intervention T2 After last intervention | Pain: intragroup in EC1, greater in T0 vs. T1 mean 4.9 cm with a Q1–Q3 dispersion measure of (2.8–6.4) vs. 2.0 cm (1.0–3.0), T0 vs. T2 a mean of 4.9 cm (2.8–6.4) vs. 0.5 cm (0–2.0). Neck Disability Index: in EC1 decrease in T2 vs. T1, 3.0 pts. (2.0–9.0) vs. 10.0 pts. (8.0–12.0) and in EC2 7.0 pts. (6.0–14.5) vs. 14.0 pts. (10.3–20.5). |
| Kumaran et al. 2015 [17], 8/10 | W (n: 9) and M (n: 6), Healthy adults | EC1: CRET-RES448 kHz (n: 15) EC2: CRET-CAP448 kHz (n: 15) | 1. Skin temperature | T0 Baseline T1 After intervention T2 45 min After intervention | Higher skin temperature in EC1 (T0–T2) vs. EC2 (T0–T2) (33.2 ± 1.4 vs. 31.5 ± 1.4 °C), within EC1 an increase in T1 vs. T0 (35.0 ± 1.2 vs. 31.1 ± 1.0 °C) and T2 vs. T0 (33.2 ± 1.4 vs. 31.1 ± 1.0 °C); within EC2 an increase in T1 vs. T0 (34.3 ± 1.6 vs. 30.9 ± 1.1 °C) and T2 vs. T0 (33.2 ± 1.4 vs. 30.9 ± 1.1 °C). |
| Bretelle et al. 2020 [18], 8/10 | W (n: 60), Adults with postpartum perineal pain | EC1: CRET-500/300 kHz (n: 29) EC2: Placebo (n: 31) | 1. Pain 2. Pain kind 3. Discomfort (Sitting/walking) 4. Paracetamol daily dose | T0 Baseline T1 2 days After intervention T2 30 days After intervention | Discomfort in sitting: decrease in EC1 vs. EC2 (12 ± 41.4 vs. 20 ± 64.5%) when comparing T1. |
| Coccetta et al. 2019 [7], 8/10 P | W (n: 47) and M (n: 6), Adults with knee osteoarthritis | EC1: CRET-448 kHz (n: 31) EC2: Placebo (n: 22) | 1. Osteoarthritis Index 2. Muscle strength scale 3. Pain | T0 Baseline T1 After intervention T2 1 month after intervention T3 3 months after intervention | Osteoarthritis Index: without significant differences, the minimal clinically important difference is exceeded in EC1 vs. EC2 (32.2 vs. 49.4 pts) in T1, (27 vs. 45.9 pts) T2 and (21.4 vs. 43.9 pts) T3. Pain: decrease in EC1 vs. EC2 in T1 (3.4 * vs. 6 mm), T2 (2.8 * vs. 6.4 mm) and T3 (2.6 * vs. 5.9 mm). Quadriceps strength scale: increase in EC1 vs. EC2 in T2 (4.48 * vs. 3.63 pts) and in T3 (4.58 * vs. 3.72 pts) (* significant compared to T0). |
| Devran et al. 2020 [19], 7/10 | W (n: 33) and M (n: 3), Adults with Trapezius myofascial pain | EC1: CRET-N/A + exercise (n: 18) EC2: Placebo + exercise (n: 18) | 1. Pain 2. Neck Disability Index 3. Range of movement 4. Trigger points. | T0 Baseline T1 After intervention | No significant differences between experimental conditions. |
| Kumaran et al. 2019 [20], 7/10 P | W (n: 27) and M (n: 18), Adults with knee osteoarthritis | EC1: CRET-448 kHz + ST (n: 15) EC2: Placebo + ST (n: 15) EC3: ST (n: 15) | 1. Pain 2. Osteoarthritis Index 3. Gait evaluation 4. Range of movement 5. physiological variables | T0 Baseline T1 After intervention T2 8 Weeks after intervention T3 3 months after intervention | Pain: an improvement on EC1 vs. EC3, both T0-T1 and T0-T2 (MD 0.82 cm 95% CI 0.32–1.3) and (MD 0.68 cm 95% CI 0.10–1.3) respectively, as well as an improvement in EC1 vs. EC2 (T0-T1) (MD 0.79 cm 95% CI 0.29–1.3). Osteoarthritis Index: improvement in CS1 vs. EC3 (T0-T1) (MD 1.3 pts. 95% CI 0.02–2.6). |
| Clijsen et al. 2020 [6], 7/10 | W (n: 4) and M (n: 6), Healthy adults | EC1: CRET-RES448 kHz (n: 10) EC2: CRET-CAP448 kHz (n: 10) EC3: Placebo (n: 10) | 1. Skin perfusion 2. Intramuscular blood flow (distal/proximal) 3. Heart rate 4. Blood pressure 5. Skin temperature | T0 Baseline T1 After intervention T2 2 min after intervention T3 10 min after intervention | Skin perfusion: lower in EC3 vs. EC2 (−24.8 (16.8) vs. −3.97 (22.01) %) in turn, lower in EC2 vs. EC1 (−3.97 (22.01) vs. 23.1 (54.4) %). Distal intramuscular blood flow: greater difference in EC1 (T0-T3) vs. EC3 (T0-T1) (2.06 (3.3) vs. 0.01 (0.7) %) as well as in EC1 (T0-T3) vs. EC2 (T0-T2) (2.06 (3.3) vs. 0.79 (3.3) %), higher skin temperature in EC1 (T0-T3) vs. EC3 (T0-T3) (2.8 (2) vs. −2.3 (1.5) °C). (Average (Q1–Q3)). |
| Kumaran et al. 2018 [21], 7/10 | W (n: 10) and M (n: 7), Healthy adults | EC1: CRET-448 kHz (n: 17) EC2: Placebo (n: 17) EC3: Control (n: 17) CE4: PSWT (n: 15) | 1. Skin temperature 2. Skin perfusion 3. Neural conduction velocity | T0 Baseline T1 After intervention T2 20 min after intervention | Skin temperature: significant increase in EC1 vs. the rest of the groups. Skin irrigation: significant increase in EC1 vs. EC2, EC1 vs. EC3, and EC1 vs. CE4. (unspecified results). |
| Pavone et al. 2017 [22], 7/10 | M, Adults with Peyronie’s disease (n: 96) | EC1: CRET-250/500 kHz (n: 64) EC2: Placebo (n: 32) | 1. Pain 2. Degree of penile curvature 3. Erectile dysfunction questionnaire | T0 Baseline T1 After intervention T2 3 months after intervention | Pain: improvement in EC1 from T0 to T1 (2 points on the VAS scale in 58% of subjects) (Results not specified). |
| Noites et al. 2019 [23], 7/10 | W, Healthy adults (n:30) | EC1: CRET-480/500 kHz + exercise (n: 15) EC2: Placebo + exercise (n: 15) | 1. Lipid profile | T0 Baseline T1 After intervention | Lipid profile without differences between EC1 vs. EC2, changes only in glycerin within EC1 and in EC2 separately, comparing T0 vs. T1 (0.06 ± 0.03 vs. 0.10 ± 0.06 mmol/L and 0.04 ± 0.01 0.07 ± 0.03 mmol/L, respectively). |
| Vale et al. 2020 [24], 7/10 | W, Healthy adults (n: 28) | EC1: CRET-480/500 kHz + exercise (n: 14) EC2: Placebo + exercise (n: 14) | 1. Bodyweight 2. Body mass index 3. Waist circumference 4. Subcutaneous adipose tissue thickness 5. Abdominal fold | T0 Baseline T1 After intervention | Body mass index: within EC1 lower in T1 vs. T0 (22.70 ± 2.57 vs. 23.00 ± 2.55 kg/cm3). Waist circumference: in EC1 decreases in T1 vs. T0 (78.39 ± 9.52 vs. 80.21 ± 10.21 cm); this difference is greater vs. difference in EC2. Adipose tissue thickness: within EC1 decreases in T1 vs. T0 (12.51 ± 6.40 vs. 10.26 ± 5.44 mm) greater difference vs. difference in EC2. Abdominal fold: decreased in EC1 vs. EC2 (16.32 ± 4.98 vs. 18.01 ± 5 mm). |
| Maretti et al. 2020 [25], 6/10 | M, Adults with Peyronie’s disease (n = 66) | EC1: CRET-500 kHz (n: 32) EC2: CRET-500 kHz + hydro electrophoresis de Verapamil (n: 34) | 1. Pain 2. Degree of penile curvature 3. Erectile dysfunction questionnaire | T0 Baseline T1 After intervention T2 3 months after intervention | Pain: Improved EC2 vs. EC1 (0.74 ± 0.11 vs. 1.82 ± 0.11 cm) in T2. Erectile dysfunction questionaire: EC2 improvement vs. EC1 (27.83 ± 0.18 vs. 26.56 ± 0.25 pts) in T2. Degree of penile curvature: Less in EC2 vs. EC1 (9.4 ± 0.76 vs. 12.7 ± 0. 77°). All significant when comparing T2 vs. T0 (intragroup). |
| Tashiro et al. 2017 [26], 6/10 | M, Healthy adults (n: 13) | EC1: CRET-448 kHz (n: 13) EC2: Placebo (n: 13) EC3: Hot-Pack (n: 13) | 1. Hemoglobin 2. Temperature at 10/20 mm deep | T0 Baseline T1 periods of 5 min during half an hour | Hemoglobin: increase in EC1 vs. EC2 (98.1 umol/L (79.8–108.9) vs. 92.6 umol/L (78.3–99.5), Mean and ranges), as well as EC1 vs. EC3 (98.1 umol/L (79.8–108.9) vs. 92.3 umol/L (69.8–103.8)), temperature at 20 mm: increase of EC1 vs. EC2 (36.8 °C (35.6–37.5) vs. 35.0 °C (34.0–36.3)), as well as EC1 vs. EC3 (36.8 °C (35.6–37.5) vs. 36.5 °C (34.8–37.1)). |
| Casa-Almeida et al. 2011 [27], 5/10 | W, Adults with edematous fibro sclerotic Panniculopaty (n: 27) | EC1: local CRET-1 MHz (n: 27) EC2: segmented CRET-1 MHz (n: 27) | 1. Clinical classification of cellulite severity 2. Perimeters 3. Physiological variables | T0 Baseline T1 After 10th intervention T2 After 20th intervention | Severity classification: no differences between groups, total score improves within EC1 and EC2 when comparing T2 vs. T0 (4.32 ± 2.6 vs. 7.35 ± 2.4 pts and 3.96 ± 2.9 vs. 7.03 ± 2.7 pts, respectively). Perimeter: no differences between groups, within-group EC1 decreases T2 vs. T0 of the iliac crest (75.93 ± 1.1 vs. 77.87 ± 1.1 mm), of ASIS (88.63 ± 1.0 vs. 90.07 ± 1.1 mm) and greater trochanter (96.23 ± 1.2 vs. 97.68 ± 1.2 mm), in EC2 differences in the rest of the perimeters when comparing T2 vs. T0. Weight: decreases in T2 vs. T0 (Results not specified). |
| Fousekis et al. 2020 [28], 5/10 | M, Healthy adults (n: 10) | EC1: CRET-448 kHz (n: 10) EC2: Placebo (n: 10) EC3: CRET-448 kHz + fascia tool (n: 10) CE4: Placebo (n: 10) | 1. Skin temperature | T0 Baseline T1 After intervention | Skin temperature: within EC1 and EC3, a significant increase in temperature, Maintenance of this temperature: EC1 vs. EC2 significantly different (39.9 vs. 34.9 °C), EC1 vs. CE4 (39.9 vs. 35.7 °C), likewise EC3 vs. EC2 significantly different (39.7 vs. 34.9 °C) and EC3 vs. CE4 (39.7 vs. 35.7 °C). |
| Duñabeitia et al. 2018 [29], 5/10 | W, Recreational adult runners (n: 14) | EC1: CRET-0.8/1.2 MHz (n: 14) EC2: Control (n: 14) | 1. Physiological variables 2. Biomechanical variables (in four tests 10, 12, 14 and 16 km/h) | T0 24 h after excercise T1 72 h after excercise | Biomechanical variables: increase in stride length: in EC1 vs. EC2 (123.39 ± 5.81 vs. 121.91 ± 5.12 cm) at 12 km/h, (139.88 ± 6.47 vs. 138.31 ± 6.57 cm) at 14 km/h and (154.59 ± 8.12 vs. 152.35 ± 9.7 cm) at 16 km/h, stride angle: increase in EC1 vs. EC2 (0.94 ± 0.43 vs. 0.73 ± 0.39°) at 12 km/h, (139.88 ± 6.47 vs. 138.31 ± 6.57°) at 14 km/h (1.91 ± 0.50 vs. 2.01 ± 0.64°) at 16 km/h, stride height: increase in EC1 vs. EC2 (0.90 ± 0.34 vs. 0.85 ± 0.36 cm) at 14 km/h and (1.30 ± 0.38 vs. 1.36 ± 0.44 cm) at 16 km/h, Stride frequency: lower in EC1 vs. EC2 (2.78 ± 0.12 vs. 2.83 ± 0.14 Hz) at 14 km/h and (2.83 ± 0.23 vs. 2.89 ± 0.15 Hz), as well as an increase in these variables when comparing intragroup in EC1. |
| Rodriguez-Sanz et al. 2017 [30], 4/10 | N/A (n:20) Adults with low back and pelvic Pain | EC1: CRET-N/A (n: 10) EC2: Placebo (n: 10) | 1. Pain 2. Pain threshold by pressure 3. Physical disability test due to low back pain | T0 Baseline T1 After intervention T2 4 weeks after intervention | There are no significant differences between the groups; in EC1, there is a difference in Pain between T2-T0 greater than the clinically relevant one (2.1 ± 2.1 cm), while in EC2, it is less (0.84 ± 0.7. 2 cm). |
| Cau et al. 2019 [31], 4/10 P | W, Adults with obesity and lymphedema (n: 48) | EC1: CRET-0.8/1.2 MHz + ST (n:12) EC2: Pressotherapy + ST (n: 12) EC3: LD+ ST (n: 12) CE4: ST (n: 12) | 1. Pain 2. Gait evaluation 3. Volume of lower limb | T0 Baseline T1 After intervention | Pain: EC1 improvement in T1 vs. T0 (47.2 ± 24.2 vs. 73.9 ± 21.7 mm). Gait evaluation: decreased EC1 in T1 vs. T0 (13.3 ± 8.1 vs. 21.8 ± 19.7 s). Lower limb volume: decrease in total volume within EC1 in T1 vs. T0 (9.4 ± 2.8 vs. 9.7 ± 2.8 cubic dm) as well as the thigh volume (3.3 ± 1.2 vs. 3.5 ± 1.3 cubic dm), Thigh circumference: decrease in EC1 between T1 vs. T0 (712.8 ± 106.9 vs. 722.35 ± 191.2 mm). |
| Notarnicola et al. 2017 [8], 4/10 | W (n: 43) and M (n: 17), Adults with low back pain | EC1: CRET-450/600 kHz (n: 30) EC2: Laser (n: 30) | 1. Pain 2. Disability index 3. Disability questionnaire | T0 Baseline T1 After intervention T2 1 month after intervention T3 2 months after intervention | Pain: decrease in EC1 vs. EC2 (1.6 ± 0.9 vs. 3.5 ± 2.5 mm) in T2 and (0.8 ± 0.7 vs. 4.2 ± 2.4 mm) in T3, disability index: lower in EC1 vs. EC2 (9.4 ± 14.6 vs. 26.8 ± 19.8 pts) in T2 and (6.0 ± 2.7 vs. 26.0 ± 18.6 pts) T3, disability questionnaire: decrease in EC1 vs. EC2 (4.7 ± 2.5 vs. 8.8 ± 6.3 pts) in T1, (2.0 ± 1.9 vs. 9.1 ± 7.0 pts) in T2 and (1.5 ± 1.4 vs. 8.6 ± 6.5 pts) in T3. |
| Stagi et al. 2008 [32], 3/10 | N/A (n: 30) Adults with low back pain | EC1: CRET-485 kHz + massotherapy (n: 15) EC2: Placebo + massotherapy (n: 15) | 1. Pain 2. Physical disability test due to low back pain 3. Quality of life questionnaire 4. Medicine intake | T0 Baseline T1 After 4 th intervention T2 After 8 th intervention T3 3 month after intervention T4 6 month after intervention | Pain: difference in its perception and management, improvement within EC1 when comparing T5 vs. T0 (unspecified data). No significant difference between groups. |
| Sanguedolce et al. 2019 [9], 3/10 | W (n: 14) and M (n: 16), Adults with rotator cuff tendinitis | EC1: TENS + iontophoresis + laser + ultrasound + reeducation (n:15) EC2: CRET-N/A + reeducation (n: 15) | 1. Pain 2. Specific shoulder test 3. Functional Scale | T0 Baseline T1 4 weeks after intervention T2 8 weeks after intervention | Pain: unspecified statistical contrasts, EC2 vs. CS1 in T1 (5.3 vs. 6.5 cm) and in T2 (3.7 vs. 5 cm). |
| Takahashi et al. 2000 [33] | W (n: 17) and M (n: 5), Adults with cervical omo-brachial Pain | EC1: CRET-CAP50/651 kHz (n: 22) | 1. Severity of symptoms 2. Skin temperature 3. Safety | T0 Baseline T1 After intervention T2 15 min after intervention | Symptom severity: a significant decrease in T1 vs. T0 (6.32 ± 3.36 vs. 9.5 ± 4.75 pts). Skin temperature: significant increase in T2 vs. T0 (30.6–31.3 vs. 29.3–29.8 °C) and T1 vs. T0 (29.7–28.8 vs. 29.3–29.8 °C). Safety: no adverse effect. |
| Ibánez-Vera et al. 2019 [34] | W (n: 13) and M (n: 6), Constipation in adults with Intellectual Developmental Disorders | EC1: CRET-850 kHz + Vacuum therapy (n: 19) | 1. Number of stools 2. Episodes of irritability 3. Scale fecal characteristics | T0 During intervention (14 days) T1 After intervention (14 days) | Number of stools: increase in T1 vs. T0 (10.23 ± 2.32 vs. 8.85 ± 2.34), irritability episodes: decrease in T1 vs. T0 (14 ± 4.26 vs. 15.95 ± 7.05), the scale of characteristics: better in T1 vs. T0 (2.47 ± 0.61 vs. 1.89 ± 0.65 points) significantly. |
| Ganzit et al. 2000 [35] | W (n: 3) and M (n: 27), Adult athletes with various muscle injuries | EC1: CRET-500 kHz (grade I, II, III muscle injury) (n: 30) | 1. Injury resolution by ultrasound image (Days/Sessions) | T0 Baseline T1 After intervention T2 2 weeks after intervention | Injury resolution: by group mean difference, Grade-I: 5.1 sessions in 8 days, Grade-II: 8.6 sessions in 14 days and Grade-III: 11.7 sessions in 19 days. |
| Sodré et al. 2019 [36] | M, Adults with urinary incontinence after radical prostatectomy (n: 10) | EC1: CRET-0.5/1 MHz (n: 10) | 1. Incontinence questionnaire (Short form/Overactive bladder) 2. Loss test 3. Global visual incontinence scale | T0 Baseline T1 After intervention | Incontinence questionnaire: improvement T1 vs. T0 in overactive bladder (6.0 pts. (4.7–8.7) vs. 5.0 pts. (0.7–6.7), mean and standard deviation). Loss test: improvement in T1 vs. T0 (2.0 g (0.0–9.0) vs. 6.5 g (1.7–50.0)), visual incontinence scale: decrease in T1 vs. T0 (4.0 cm (1.7–5.0) vs. 7.0 cm (5.0–8.5)). |
| Rafaetá et al. 2007 [37] | W (n: 15) and M (n: 5), adults with cervicalgia | EC1: CRET-N/A (n: 20) | 1. Pain 2. Cervicalgia questionnaire 3. Visual Scale of improvement | T0 Baseline T1 After intervention T2 2 months after intervention | Pain: Unspecified statistical contrasts, T1 vs. T0 (2.79 vs. 6.63 cm) and in T2 vs. T0 (2.55 vs. 6.63 cm). Questionnaire: without statistical contrast, decreased T1 vs. T0 (16.67 vs. 37.95%) and T2 vs. T0 (12.54 vs. 37.95%). |
| Takahashi et al. 1999 [38] | W (n: 27) and M (n: 10), Adults with low back pain | EC1: CRET-CAP50/650 kHz (n: 37) | 1. Severity of symptoms 2. Skin temperature 3. Safety | T0 Baseline T1 After intervention T2 15 min after intervention | Symptom severity: a significant decrease in T1 vs. T0 (6.2 ± 4.0 vs. 11.5 ± 4.9 pts). Skin temperature: significant increase in T2 vs. T0 (31.1–31.3 vs. 29.2–29.5 °C) and T1 vs. T0 (30.2–30.5 vs. 29.2–29.5 °C). Safety: no adverse effect. |
| Osti et al. 2014 [39] | W (n: 30) and M (n: 36), Adults with low back pain | EC1: Laser + CRET-450/600 kHz (n: 66) | 1. Pain 2. Physical disability test due to low back pain | T0 Baseline T1 8 weeks after intervention | Pain: decreases in T1 vs. T0 (2.63 ± 2.74 vs. 8.1 ± 1.58 cm), disability test: decrease in T1 vs. T0 (23.5 (± 19.8) vs. 53.0 (± 13.0) pts.), even when separating the group by extension of pain, both variables were still significant. |
| Fernández-Cuadros et al. 2020 [40] | W, Adults with chronic pelvic Pain and dyspareunia (n: 37) | EC1: CRET-448 kHz + Biofeedback + PFE (n: 37) | 1. Pain 2. Muscle strength | T0 Baseline T1 After intervention | Pain: decrease in T1 vs. T0 (3.75 ± 2.21 vs. 7.27 ± 1.34 cm), muscle strength: evidence of increased maximum strength in T1 vs. T0 (35.35 ± 20.4 vs. 25.56 ± 15.9 mmHg) as well as the mean force (7.18 ± 4.46 vs. 4.86 ± 3.53 mmHg). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sousa-De Sousa, L.; Tebar Sanchez, C.; Maté-Muñoz, J.L.; Hernández-Lougedo, J.; Barba, M.; Lozano-Estevan, M.d.C.; Garnacho-Castaño, M.V.; García-Fernández, P. Application of Capacitive-Resistive Electric Transfer in Physiotherapeutic Clinical Practice and Sports. Int. J. Environ. Res. Public Health 2021, 18, 12446. https://doi.org/10.3390/ijerph182312446
De Sousa-De Sousa L, Tebar Sanchez C, Maté-Muñoz JL, Hernández-Lougedo J, Barba M, Lozano-Estevan MdC, Garnacho-Castaño MV, García-Fernández P. Application of Capacitive-Resistive Electric Transfer in Physiotherapeutic Clinical Practice and Sports. International Journal of Environmental Research and Public Health. 2021; 18(23):12446. https://doi.org/10.3390/ijerph182312446
Chicago/Turabian StyleDe Sousa-De Sousa, Luis, Cristina Tebar Sanchez, José Luis Maté-Muñoz, Juan Hernández-Lougedo, Manuel Barba, Maria del Carmen Lozano-Estevan, Manuel Vicente Garnacho-Castaño, and Pablo García-Fernández. 2021. "Application of Capacitive-Resistive Electric Transfer in Physiotherapeutic Clinical Practice and Sports" International Journal of Environmental Research and Public Health 18, no. 23: 12446. https://doi.org/10.3390/ijerph182312446
APA StyleDe Sousa-De Sousa, L., Tebar Sanchez, C., Maté-Muñoz, J. L., Hernández-Lougedo, J., Barba, M., Lozano-Estevan, M. d. C., Garnacho-Castaño, M. V., & García-Fernández, P. (2021). Application of Capacitive-Resistive Electric Transfer in Physiotherapeutic Clinical Practice and Sports. International Journal of Environmental Research and Public Health, 18(23), 12446. https://doi.org/10.3390/ijerph182312446









