Differences in Inter-Rectus Distance and Abdominopelvic Function between Nulliparous, Primiparous and Multiparous Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
2.4. Outcome Measures
2.4.1. IRD Measurement
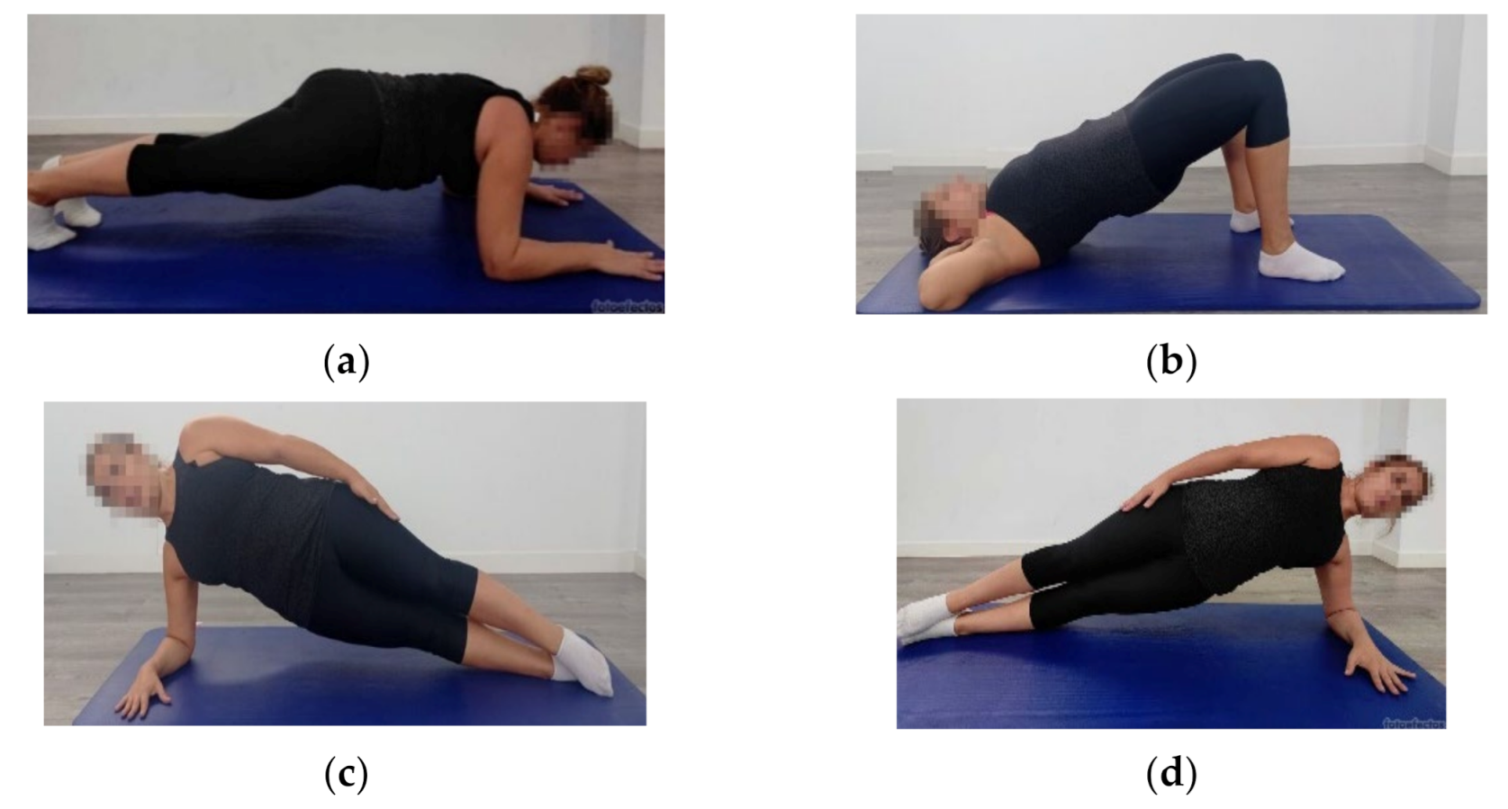
2.4.2. Abdominopelvic Muscle Function
2.5. Data Analyses
3. Results
4. Discussion
4.1. IRD Measurement
4.2. Abdominopelvic Muscle Function
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boissonnault, J.S.; Blaschak, M.J. Incidence of Diastasis Recti Abdominis During the Childbearing Year. Phys. Ther. 1988, 68, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Fernandes da Mota, P.G.; Pascoal, A.G.B.A.; Carita, A.I.A.D.; Bø, K. Prevalence and Risk Factors of Diastasis Recti Abdominis from Late Pregnancy to 6 Months Postpartum, and Relationship with Lumbo-Pelvic Pain. Man. Ther. 2015, 20, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Gitta, S.; Magyar, Z.; Tardi, P.; Füge, I.; Járomi, M.; Acs, P.; Garai, J.; Bódis, J.; Hock, M. A Rectus Diastasis Prevalenciája, Lehetséges Rizikófaktorai És Szövődményei. Orv. Hetil. 2017, 158, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Sperstad, J.B.; Tennfjord, M.K.; Hilde, G.; Ellström-Engh, M.; Bø, K. Diastasis Recti Abdominis during Pregnancy and 12 Months after Childbirth: Prevalence, Risk Factors and Report of Lumbopelvic Pain. Br. J. Sports Med. 2016, 50, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Lee, L.J.; McLaughlin, L. Stability, Continence and Breathing: The Role of Fascia Following Pregnancy and Delivery. J. Bodyw. Mov. Ther. 2008, 12, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Wade: Diastasis Recti and Low Back Pain - Google Scholar. Available online: https://scholar.google.com/scholar_lookup?hl=en&volume=17&publication_year=2005&pages=20-22&journal=Orthopaedic+Practice&author=Wade+M.&title=Diastasis+recti+and+low+back+pain (accessed on 20 October 2021).
- Hodges, P.W. Is There a Role for Transversus Abdominis in Lumbo-Pelvic Stability? Man. Ther. 1999, 4, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Coldron, Y.; Stokes, M.J.; Newham, D.J.; Cook, K. Postpartum Characteristics of Rectus Abdominis on Ultrasound Imaging. Man. Ther. 2008, 13, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hodges, P.W. Behavior of the Linea Alba During a Curl-up Task in Diastasis Rectus Abdominis: An Observational Study. J. Orthop. Sports Phys. Ther. 2016, 46, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Beamish, N.; Green, N.; Nieuwold, E.; McLean, L. Differences in Linea Alba Stiffness and Linea Alba Distortion Between Women With and Without Diastasis Recti Abdominis: The Impact of Measurement Site and Task. J. Orthop. Sports Phys. Ther. 2019, 49, 656–665. [Google Scholar] [CrossRef]
- Fan, C.; Guidolin, D.; Ragazzo, S.; Fede, C.; Pirri, C.; Gaudreault, N.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Effects of Cesarean Section and Vaginal Delivery on Abdominal Muscles and Fasciae. Med. Kaunas Lith. 2020, 56, 260. [Google Scholar] [CrossRef]
- Kaufmann, R.L.; Reiner, C.S.; Dietz, U.A.; Clavien, P.A.; Vonlanthen, R.; Käser, S.A. Normal Width of the Linea Alba, Prevalence, and Risk Factors for Diastasis Recti Abdominis in Adults, a Cross-Sectional Study. Hernia J. Hernias Abdom. Wall Surg. 2021, 1–10. [Google Scholar] [CrossRef]
- Spitznagle, T.M.; Leong, F.C.; Van Dillen, L.R. Prevalence of Diastasis Recti Abdominis in a Urogynecological Patient Population. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2007, 18, 321–328. [Google Scholar] [CrossRef]
- Harada, B.S.; De Bortolli, T.T.; Carnaz, L.; De Conti, M.H.S.; Hijaz, A.; Driusso, P.; Marini, G. Diastasis Recti Abdominis and Pelvic Floor Dysfunction in Peri- and Postmenopausal Women: A Cross-Sectional Study. Physiother. Theory Pract. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Fuentes Aparicio, L.; Rejano-Campo, M.; Donnelly, G.M.; Vicente-Campos, V. Self-Reported Symptoms in Women with Diastasis Rectus Abdominis: A Systematic Review. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101995. [Google Scholar] [CrossRef]
- Toranto, I.R. The Relief of Low Back Pain with the WARP Abdominoplasty: A Preliminary Report. Plast. Reconstr. Surg. 1990, 85, 545–555. [Google Scholar] [CrossRef]
- Dalal, K.; Kaur, A.; Mitra, M. Correlation between Diastasis Rectus Abdominis and Lumbopelvic Pain and Dysfunction. Indian J. Physiother. Occup. Ther. 2014, 8, 210–214. [Google Scholar] [CrossRef]
- Benjamin, D.R.; van de Water, A.T.M.; Peiris, C.L. Effects of Exercise on Diastasis of the Rectus Abdominis Muscle in the Antenatal and Postnatal Periods: A Systematic Review. Physiotherapy 2014, 100, 1–8. [Google Scholar] [CrossRef]
- Bø, K.; Hilde, G.; Tennfjord, M.K.; Sperstad, J.B.; Engh, M.E. Pelvic Floor Muscle Function, Pelvic Floor Dysfunction and Diastasis Recti Abdominis: Prospective Cohort Study. Neurourol. Urodyn. 2017, 36, 716–721. [Google Scholar] [CrossRef]
- Braga, A.; Caccia, G.; Nasi, I.; Ruggeri, G.; Di Dedda, M.C.; Lamberti, G.; Salvatore, S.; Papadia, A.; Serati, M. Diastasis Recti Abdominis after Childbirth: Is It a Predictor of Stress Urinary Incontinence? J. Gynecol. Obstet. Hum. Reprod. 2019, 49, 101657. [Google Scholar] [CrossRef]
- Keeler, J.; Albrecht, M.; Eberhardt, L.; Horn, L.; Donnelly, C.; Lowe, D.L. Diastasis Recti Abdominis: A Survey of Women’s Health Specialists for Current Physical Therapy Clinical Practice for Postpartum Women. J. Women’s Health Phys. Ther. 2012, 36, 131–142. [Google Scholar] [CrossRef]
- Bursch, S.G. Interrater Reliability of Diastasis Recti Abdominis Measurement. Phys. Ther. 1987, 67, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Mota, P.; Pascoal, A.G.; Sancho, F.; Bø, K. Test-Retest and Intrarater Reliability of 2-Dimensional Ultrasound Measurements of Distance between Rectus Abdominis in Women. J. Orthop. Sports Phys. Ther. 2012, 42, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; de Sá, R.A.M.; Coca Velarde, L.G. Diastasis of Rectus Abdominis in the Immediate Puerperium: Correlation between Imaging Diagnosis and Clinical Examination. Arch. Gynecol. Obstet. 2013, 288, 299–303. [Google Scholar] [CrossRef] [PubMed]
- van de Water, A.T.M.; Benjamin, D.R. Measurement Methods to Assess Diastasis of the Rectus Abdominis Muscle (DRAM): A Systematic Review of Their Measurement Properties and Meta-Analytic Reliability Generalisation. Man. Ther. 2016, 21, 41–53. [Google Scholar] [CrossRef]
- Liaw, L.-J.; Hsu, M.-J.; Liao, C.-F.; Liu, M.-F.; Hsu, A.-T. The Relationships between Inter-Recti Distance Measured by Ultrasound Imaging and Abdominal Muscle Function in Postpartum Women: A 6-Month Follow-up Study. J. Orthop. Sports Phys. Ther. 2011, 41, 435–443. [Google Scholar] [CrossRef]
- Keshwani, N.; Mathur, S.; McLean, L. Relationship Between Interrectus Distance and Symptom Severity in Women With Diastasis Recti Abdominis in the Early Postpartum Period. Phys. Ther. 2018, 98, 182–190. [Google Scholar] [CrossRef]
- Mota, P.; Pascoal, A.G.; Carita, A.I.; Bø, K. Normal Width of the Inter-Recti Distance in Pregnant and Postpartum Primiparous Women. Musculoskelet. Sci. Pract. 2018, 35, 34–37. [Google Scholar] [CrossRef]
- Rett, M.; Braga, M.; Bernardes, N.; Andrade, S. Prevalência de Diástase Dos Músculos Retoabdominais No Puerpério Imediato: Comparação Entre Primíparas e Multíparas. Braz. J. Phys. Ther. 2009, 13, 275–280. [Google Scholar] [CrossRef]
- Rett, M.T.; de Almeida, T.V.; Mendonça, A.C.R.; DeSantana, J.M.; de Lima Ferreira, A.P.; de Araújo, K.C.G.M. Fatores Materno-Infantis Associados à Diástase Dos Músculos Retos Do Abdome No Puerpério Imediato. Rev. Bras. Saúde Materno Infant. 2014, 14, 73–80. [Google Scholar] [CrossRef][Green Version]
- Association, W.M. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. Lond. Engl. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Edad Media a la Maternidad por Orden del Nacimiento Según Nacionalidad (Española/Extranjera) de la Madre. 1579. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=1579 (accessed on 17 November 2021).
- Steeves, J.A.; Tudor-Locke, C.; Murphy, R.A.; King, G.A.; Fitzhugh, E.C.; Bassett, D.R.; Van Domelen, D.; Schuna, J.M.; Harris, T.B. Daily Physical Activity by Occupational Classification in US Adults: NHANES 2005-2006. J. Phys. Act. Health 2018, 15, 900–911. [Google Scholar] [CrossRef]
- Norton, K.; Norton, L.; Sadgrove, D. Position Statement on Physical Activity and Exercise Intensity Terminology. J. Sci. Med. Sport 2010, 13, 496–502. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An Update of Activity Codes and MET Intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and Validity of the Visual Analogue Scale for Disability in Patients with Chronic Musculoskeletal Pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef]
- Gould, D.; Kelly, D.; Goldstone, L.; Gammon, J. Examining the Validity of Pressure Ulcer Risk Assessment Scales: Developing and Using Illustrated Patient Simulations to Collect the Data. J. Clin. Nurs. 2001, 10, 697–706. [Google Scholar] [CrossRef]
- Schellenberg, K.; Lang, J.M.; Chan, K.M.; Burnham, R. A Clinical Tool for Office Assessment of Lumbar Spine Stabilization Endurance: Prone and Supine Bridge Maneuvers. Am. J. Phys. Med. Rehabil. 2007, 86.5, 380–386. [Google Scholar] [CrossRef]
- McGill, S.M.; Childs, A.; Liebenson, C. Endurance Times for Low Back Stabilization Exercises: Clinical Targets for Testing and Training from a Normal Database. Arch. Phys. Med. Rehabil. 1999, 80, 941–944. [Google Scholar] [CrossRef]
- Pascoal, A.G.; Dionisio, S.; Cordeiro, F.; Mota, P. Inter-Rectus Distance in Postpartum Women Can Be Reduced by Isometric Contraction of the Abdominal Muscles: A Preliminary Case-Control Study. Physiotherapy 2014, 100, 344–348. [Google Scholar] [CrossRef]
- Gluppe, S.B.; Engh, M.E.; Bø, K. Immediate Effect of Abdominal and Pelvic Floor Muscle Exercises on Interrecti Distance in Women With Diastasis Recti Abdominis Who Were Parous. Phys. Ther. 2020, 100, 1372–1383. [Google Scholar] [CrossRef]
- Deering, R.E.; Cruz, M.; Senefeld, J.W.; Pashibin, T.; Eickmeyer, S.; Hunter, S.K. Impaired Trunk Flexor Strength, Fatigability, and Steadiness in Postpartum Women. Med. Sci. Sports Exerc. 2018, 50, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Fukano, M.; Tsukahara, Y.; Takei, S.; Nose-Ogura, S.; Fujii, T.; Torii, S. Recovery of Abdominal Muscle Thickness and Contractile Function in Women after Childbirth. Int. J. Environ. Res. Public. Health 2021, 18, 2130. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; Botelho, S.; Marques, J.; Amorim, C.F.; Lanza, A.H.; Palma, P.; Riccetto, C. Are Transversus Abdominis/Oblique Internal and Pelvic Floor Muscles Coactivated during Pregnancy and Postpartum? Neurourol. Urodyn. 2013, 32, 416–419. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Physical Activity and Exercise During Pregnancy and the Postpartum Period: ACOG Committee Opinion, Number 804. Obstet. Gynecol. 2020, 135, e178–e188. [Google Scholar] [CrossRef]


| Total (n = 75) | Nulliparous (n = 25) | Primiparous (n = 25) | Multiparous (n = 25) | Differences among Groups | |
|---|---|---|---|---|---|
| Sample characteristics | |||||
| Age (y) | 34.15 (3.84) | 33.24 (4.58) | 34.16 (2.56) | 35.04 (4.04) | F = 1.39; p = 0.26 |
| Weight (kg) | 62.73 (10.62) | 62.60 (9.17) | 65.08 (13.08) | 60.60 (9.28) | H = 1.57; p = 0.36 |
| Body mass index (kg/m2) | 22.67 (3.36) | 22.34 (2.93) | 23.45 (4.18) | 22.26 (2.98) | H = 0.71; p = 0.41 |
| Height (cm) | 166.16 (5.93) | 167.32 (6.72) | 166.08 (5.32) | 165.08 (5.69) | F = 1.05; p = 0.41 |
| Waist circumference (cm) | 83.15 (9.77) | 80.40 (9.91) | 86.00 (10.08) | 83.29 (8.89) | H = 1.80; p = 0.14 |
| Profession physical activity classification, n (%) | |||||
| High | 19 (28.4%) | 8 (36.4%) | 7 (28.0%) | 4 (20.0%) | χ2 = 3.61; p = 0.48 |
| Medium | 14 (20.9%) | 5 (22.7%) | 3 (12.0%) | 6 (30.0%) | |
| Low | 34 (50.7%) | 9 (40.9%) | 15 (60.0%) | 10 (50.0%) | |
| Current exercise (Yes/No), n (%) | 45 (60%)/30 (40%) | 22 (88%)/3 (12%) | 14 (56%)/11 (44%) | 9 (36%)/16 (64%) | χ2 = 13.36; p = 0.001 * |
| Exercise frequency (h/w) | 13.03 (17.17) | 18.10 (22.05) | 5.50 (3.62) | 10.66 (9.49) | H = 7.14; p = 0.028 ** |
| Exercise intensity, n (%) | |||||
| Vigorous | 12 (16.0%) | 7 (28.0%) | 4 (16.0%) | 1 (4.0%) | χ2 = 17.42; p = 0.007 *** |
| Moderate | 16 (21.3%) | 8 (32.0%) | 6 (24.0%) | 2 (8.0%) | |
| Light | 17 (22.7%) | 7 (28.0%) | 4 (16.0%) | 6 (24.0%) | |
| Sedentary | 30 (40.0%) | 3 (12.0%) | 11 (44.0%) | 16 (64.0%) | |
| Abdominal pain last 24 h (Yes/No), n (%) | 6 (8%)/69 (92%) | 4 (16%)/21 (84%) | 1 (4%)/24 (96%) | 1 (4%)/24 (96%) | χ2 = 2.84; p = 0.24 |
| VAS (0–100) | 41.17 (19.70) | 49.25 (19.21) | 30.00 (0.00) | 20.00 (0.00) | - |
| Low back pain last 24 h (Yes/No), n (%) | 26 (34.7%)/49 (65.3%) | 11 (44%)/14 (56%) | 5 (20%)/20 (80%) | 10 (40%)/15 (60%) | χ2 = 3.25; p = 0.20 |
| VAS (0–100) | 41.42 (23.18) | 43.73 (22.92) | 51.20 (25.49) | 34.00 (22.36) | F = 1.01; p = 0.38 |
| Postpartum sample characteristics | |||||
| Birth weight of baby (kg) | - | - | 3.31 (0.33) | 3.27 (0.34) | t = 0.42; p = 0.68 |
| Weight gain during pregnancy (kg) | - | - | 12.30 (3.68) | 12.14 (4.10) | t = 0.15; p = 0.89 |
| Soft tissue damage, n (%) | |||||
| Not altered | - | - | 4 (16%) | 10 (40%) | χ2 = 4.62; p = 0.20 |
| Episiotomy | - | - | 10 (40%) | 6 (24%) | |
| Perineal tearing | - | - | 10 (40%) | 9 (36%) | |
| Both | - | - | 1 (4%) | 0 (0%) | |
| Exercise before pregnancy (Yes/No), n (%) | - | - | 18 (72%)/7 (28%) | 16 (64%)/9 (36%) | χ2 = 0.39; p = 0.54 |
| Frequency exercise (h/w) | - | - | 7.53 (5.88) | 11.89 (23.43) | t = −0.77; p = 0.45 |
| Exercise during pregnancy (Yes/No), n (%) | - | - | 19 (76%)/6 (24%) | 10 (40%)/15 (60%) | χ2 = 6.65; p = 0.010 |
| Frequency exercise (h/w) | - | - | 12.32 (15.84) | 19.3 (23.9) | t = −0.85; p = 0.41 |
| Nulliparous (n = 25) | Primiparous (n = 25) | Multiparous (n = 25) | Primiparous vs Nulliparous p [95% CI]; d | Multiparous vs Nulliparous p [95% CI]; d | Primiparous vs Multiparous p [95% CI]; d | ||
|---|---|---|---|---|---|---|---|
| IRD (cm) | |||||||
| REST | 2 cm above | 1.23 (0.46) | 1.99 (0.64) | 2.60 (0.79) | 0.001 [0.29 to 1.24]; 1.38 | <0.001 [0.91 to 1.83]; 3.38 | 0.012 [−1.1 to −0.11]; 1.27 |
| 2 cm below | 0.52 (0.33) | 1.23 (0.55) | 1.43 (0.63) | <0.001 [1.09 to 0.33]; 1.56 | <0.001[1.28 to 0.55]; 1.81 | 0.61 [−0.60 to 0.19]; 0.35 | |
| ADIM | 2 cm above | 1.31 (0.42) | 2.13 (0.59) | 2.78 (0.67) | <0.001 [0.41 to 1.24]; 1.39 | <0.001 [1.06 to 1.87]; 2.60 | 0.002 [−1.08 to −0.21]; 1.04 |
| 2 cm below | 0.59 (0.33) | 1.40 (0.53) | 1.83 (0.65) | <0.001 [0.42 to 1.19]; 1.50 | <0.001 [0.87 to 1.62]; 2.40 | 0.027 [−0.84 to −0.04]; 0.07 | |
| CURL-UP | 2 cm above | 1.09 (0.51) | 1.68 (0.49) | 1.91 (0.59) | 0.002 [0.19 to 0.99]; 1.18 | <0.001 [0.43 to 1.20]; 1.49 | 0.53 [−0.64 to 0.19]; 0.42 |
| 2 cm below | 0.50 (0.28) | 1.06 (0.51) | 1.23 (0.55) | <0.001 [0.23 to 0.90]; 1.38 | <0.001 [0.41 to 1.06]; 1.70 | 0.73 [−0.52 to 0.18]; 0.32 | |
| REST vs ADIM p [95% CI]; d | |||||||
| 2 cm above | 0.05 [−0.17 to 0.01]; 0.2 | 0.002 [−0.24 to −0.04]; 0.23 | <0.001 [−0.28 to −0.09]; 0.39 | ||||
| 2 cm below | 0.37 [−0.19 to 0.04]; 2.81 | 0.011 [−0.31 to −0.03]; 0.31 | <0.001 [−0.53 to −0.27]; 0.62 | ||||
| REST vs CURL-UP p [95% CI]; d | |||||||
| 2 cm above | 0.38 [−0.08 to 0.35]; 0.29 | 0.008 [0.07 to 0.56]; 0.56 | <0.001 [0.45 to 0.92]; 1.59 | ||||
| 2 cm below | 1.00 [−0.19 to 0.22]; 3.30 | 0.26 [−0.07 to 0.40]; 0.32 | 0.08 [−0.02 to 0.42]; 0.34 | ||||
| ADIM vs CURL-UP p [95% CI]; d | |||||||
| 2 cm above | 0.026 [0.02 to 0.42]; 0.52 | <0.001 [0.23 to 0.69]; 0.85 | <0.001 [0.66 to 1.09]; 1.39 | ||||
| 2 cm below | 0.75 [−0.10 to 0.29]; 0.29 | 0.002 [0.11 to 0.56]; 0.65 | <0.001 [0.39 to 0.82]; 1.00 | ||||
| Abdominopelvic muscle function (s) | |||||||
| PRONE | 21.05 (20.87) | 11.21 (4.17) | 12.60 (3.40) | 0.028[0.79 to 18.87]; 0.65 | 0.08 [−0.59 to 17.48]; 0.57 | 1.00 [−10.51 to 7.75]; 0.37 | |
| SUPINE | 43.95 (34.35) | 25.64 (12.34) | 27.97 (11.66) | 0.020[2.29 to 34.32]; 0.71 | 0.05 [−0.04 to 31.98]; 0.62 | 1.00 [−18.68 to 14.00]; 0.02 | |
| LEFT | 14.21 (15.78) | 8.61 (4.00) | 8.21 (3.03) | 0.16 [−1.35 to 12.55]; 0.49 | 0.11[−0.94 to 12.96]; 0.53 | 1.00 [−6.68 to 7.50]; 0.01 | |
| RIGHT | 14.07 (15.87) | 7.95 (2.67) | 9.63 (3.96) | 0.10 [−0.84 to 13.08]; 0.54 | 0.37 [−2.52 to 11.40]; 0.38 | 1.00 [−8.78 to 5.42]; 0.50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasch-Bernat, M.; Pérez-Alenda, S.; Carrasco, J.J.; Valls-Donderis, B.; Dueñas, L.; Fuentes-Aparicio, L. Differences in Inter-Rectus Distance and Abdominopelvic Function between Nulliparous, Primiparous and Multiparous Women. Int. J. Environ. Res. Public Health 2021, 18, 12396. https://doi.org/10.3390/ijerph182312396
Balasch-Bernat M, Pérez-Alenda S, Carrasco JJ, Valls-Donderis B, Dueñas L, Fuentes-Aparicio L. Differences in Inter-Rectus Distance and Abdominopelvic Function between Nulliparous, Primiparous and Multiparous Women. International Journal of Environmental Research and Public Health. 2021; 18(23):12396. https://doi.org/10.3390/ijerph182312396
Chicago/Turabian StyleBalasch-Bernat, Mercè, Sofía Pérez-Alenda, Juan J. Carrasco, Begoña Valls-Donderis, Lirios Dueñas, and Laura Fuentes-Aparicio. 2021. "Differences in Inter-Rectus Distance and Abdominopelvic Function between Nulliparous, Primiparous and Multiparous Women" International Journal of Environmental Research and Public Health 18, no. 23: 12396. https://doi.org/10.3390/ijerph182312396
APA StyleBalasch-Bernat, M., Pérez-Alenda, S., Carrasco, J. J., Valls-Donderis, B., Dueñas, L., & Fuentes-Aparicio, L. (2021). Differences in Inter-Rectus Distance and Abdominopelvic Function between Nulliparous, Primiparous and Multiparous Women. International Journal of Environmental Research and Public Health, 18(23), 12396. https://doi.org/10.3390/ijerph182312396






