Discrete-Event Simulation Modeling in Healthcare: A Comprehensive Review
Abstract
1. Introduction
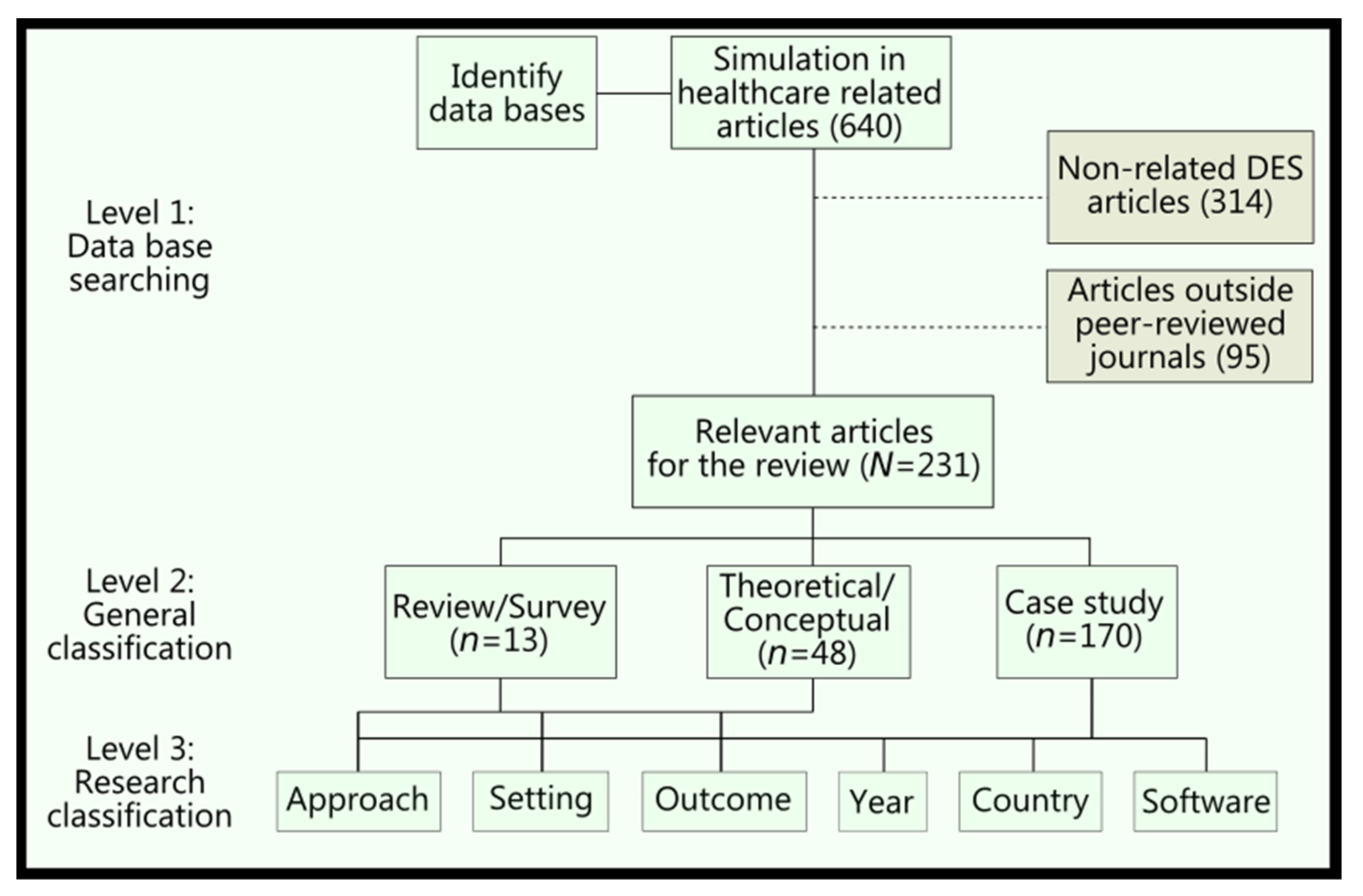
2. Methods
2.1. Search Strategy
2.2. Paper Inclusion Criteria
2.3. Review Methodology
- Time and efficiency;
- Financial and cost savings;
- Allocation of resources/schedule;
- Quality and defects;
- Patient health/safety.
3. Results
3.1. Review and Survey Papers
3.2. Theoretical/Conceptual Papers
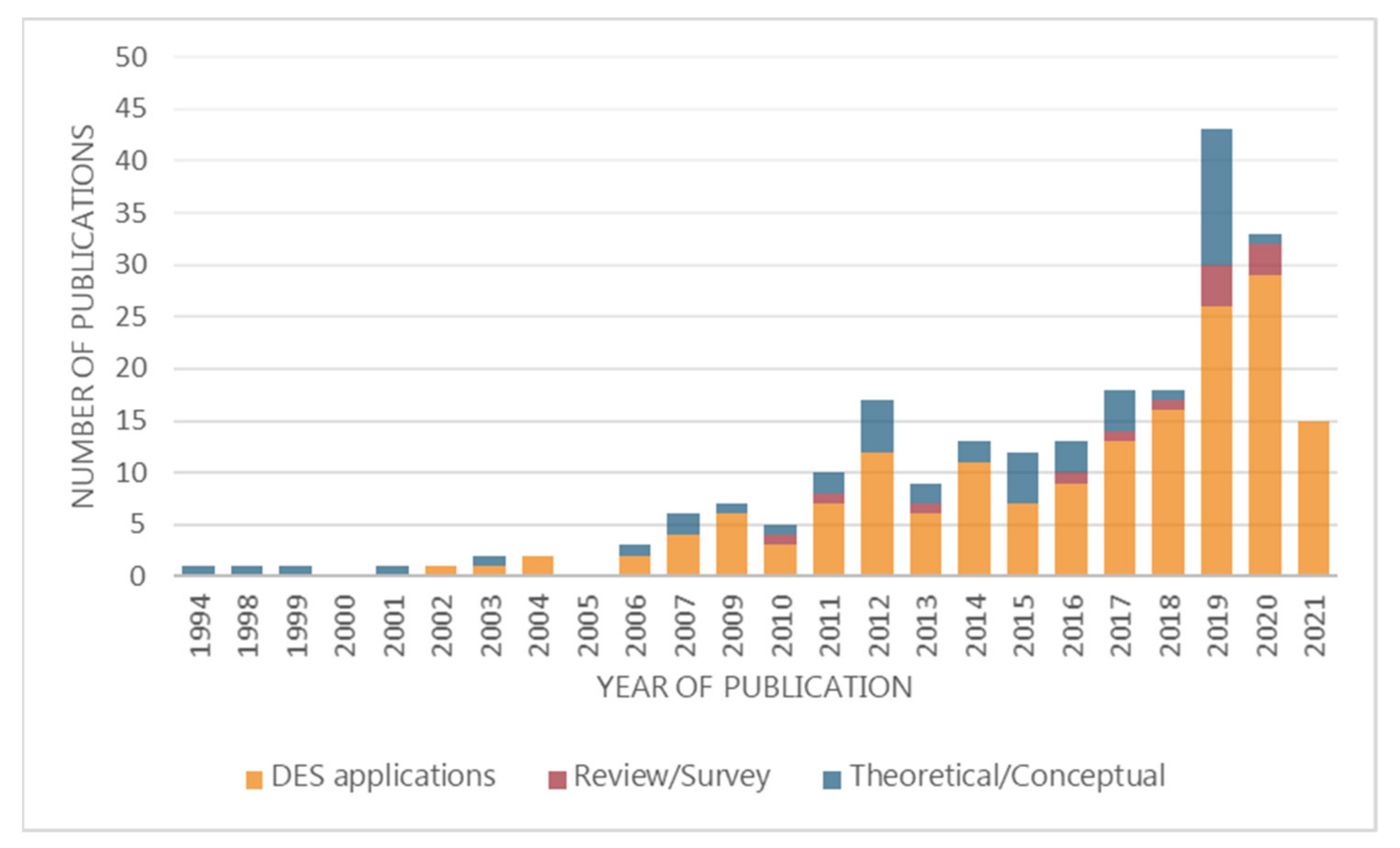
3.3. DES Applications Papers
3.3.1. Approach
3.3.2. Setting and Outcomes
3.3.3. Journals, Publishers, and Countries
3.3.4. Software Use
4. Discussion
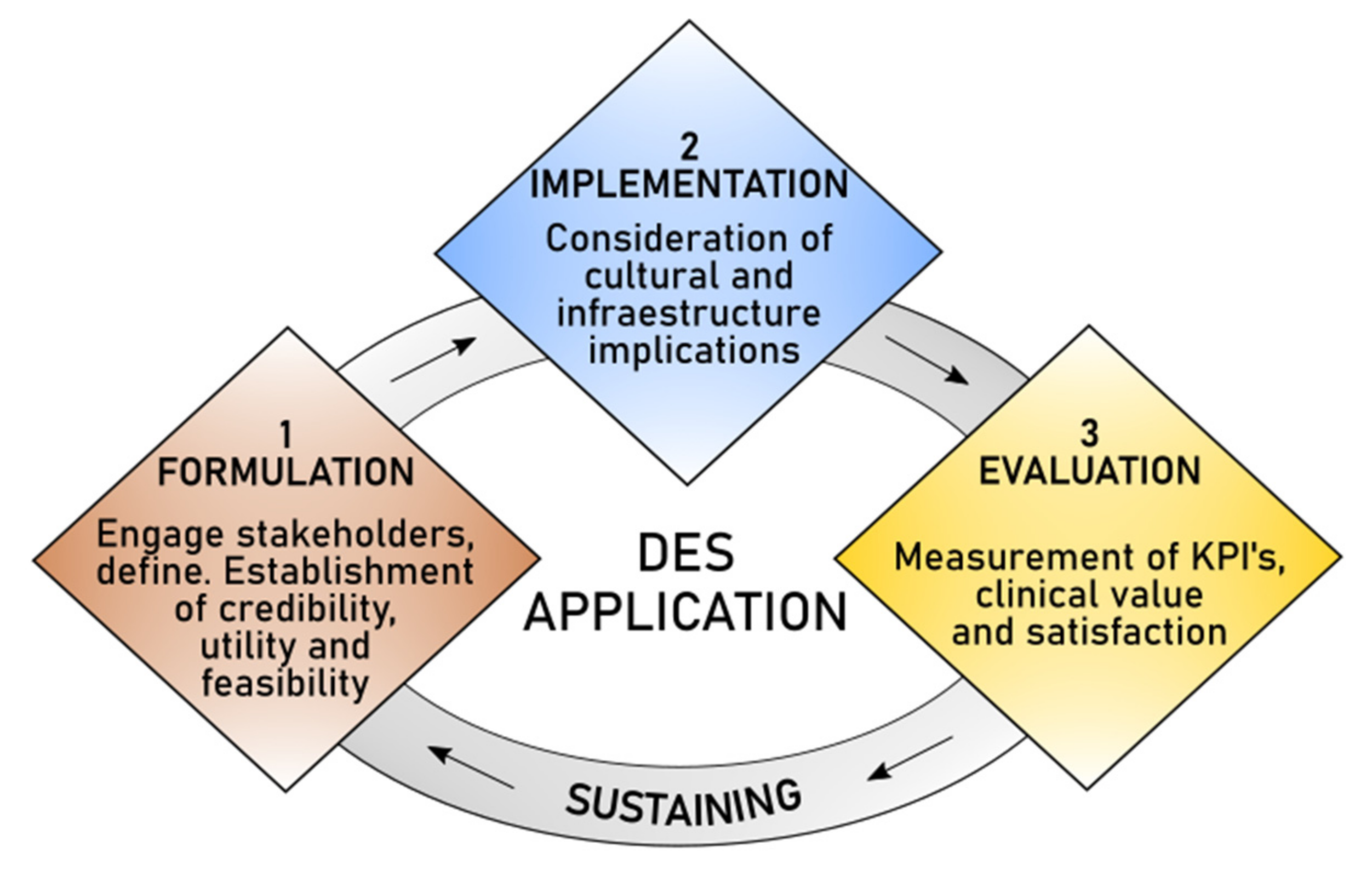
4.1. Key Elements to Formulate Models
4.2. Frameworks for Hybrid Models
4.3. Barriers for Implementation
4.4. Measuring Satisfaction and Clinical Value
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorwarth, M.; Rashwan, W.; Arisha, A. An analytical representation of flexible resource allocation in hospitals. Flex. Serv. Manuf. J. 2016, 28, 148–165. [Google Scholar] [CrossRef]
- Ben-Tovim, D.; Filar, J.; Hakendorf, P.; Qin, S.; Thompson, C.; Ward, D. Hospital Event Simulation Model: Arrivals to Discharge–Design, development and application. Simul. Model. Pract. Theory 2016, 68, 80–94. [Google Scholar] [CrossRef]
- Chahal, K.; Eldabi, T. Hybrid simulation and modes of governance in UK healthcare. Transform. Gov. People Process Policy 2011, 5, 143–154. [Google Scholar] [CrossRef]
- Koelling, P.; Schwandt, M.J. Health systems: A dynamic system—Benefits from system dynamics. In Proceedings of the Winter Simulation Conference, Orlando, FL, USA, 4 December 2005; Volume 2005, pp. 1321–1326. [Google Scholar]
- Arisha, A.; Rashwan, W. Modeling of healthcare systems: Past, current and future trends. Proc. Winter Simul. Conf. 2016, 1, 1523–1534. [Google Scholar] [CrossRef]
- Gillespie, J.; McClean, S.; FitzGibbons, F.; Scotney, B.; Dobbs, F.; Meenan, B.J. Do we need stochastic models for healthcare? The case of ICATS? J. Simul. 2014, 8, 293–303. [Google Scholar] [CrossRef]
- Marshall, D.A.; Burgos-Liz, L.; Ijzerman, M.J.; Osgood, N.D.; Padula, W.V.; Higashi, M.K.; Wong, P.K.; Pasupathy, K.S.; Crown, W. Applying dynamic simulation modeling methods in health care delivery research—The SIMULATE checklist: Report of the ISPOR simulation modeling emerging good practices task force. Value Health 2015, 18, 5–16. [Google Scholar] [CrossRef]
- Qureshi, S.M.; Purdy, N.; Mohani, A.; Neumann, W.P. Predicting the effect of nurse–patient ratio on nurse workload and care quality using discrete event simulation. J. Nurs. Manag. 2019, 27, 971–980. [Google Scholar] [CrossRef]
- Chahal, K.; Eldabi, T.; Young, T. A conceptual framework for hybrid system dynamics and discrete event simulation for healthcare. J. Enterp. Inf. Manag. 2013, 26, 50–74. [Google Scholar] [CrossRef]
- Landa, P.; Sonnessa, M.; Tànfani, E.; Testi, A. Multiobjective bed management considering emergency and elective patient flows. Int. Trans. Oper. Res. 2018, 25, 91–110. [Google Scholar] [CrossRef]
- Hajjarsaraei, H.; Shirazi, B.; Rezaeian, J. Scenario-based analysis of fast track strategy optimization on emergency department using integrated safety simulation. Saf. Sci. 2018, 107, 9–21. [Google Scholar] [CrossRef]
- Stahl, J.E.; Roberts, M.S.; Gazelle, S. Optimizing management and financial performance of the teaching ambulatory care clinic. J. Gen. Intern. Med. 2003, 18, 266–274. [Google Scholar] [CrossRef]
- Ramwadhdoebe, S.; Buskens, E.; Sakkers, R.J.B.; Stahl, J.E. A tutorial on discrete-event simulation for health policy design and decision making: Optimizing pediatric ultrasound screening for hip dysplasia as an illustration. Health Policy 2009, 93, 143–150. [Google Scholar] [CrossRef]
- Abubakar, A.M.; Adamu, A.; Abdulkadir, A.; Abdulkadir, H.S. Discrete Event Simulation of Clients Flow in Ante-natal Clinic. Asian J. Probab. Stat. 2020, 6, 63–78. [Google Scholar] [CrossRef]
- Weinstein, M.C. Recent developments in decision-analytic modelling for economic evaluation. Pharmacoeconomics 2006, 24, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Pongjetanapong, K.; O’Sullivan, M.; Walker, C.; Furian, N. Implementing complex task allocation in a cytology lab via HCCM using Flexsim HC. Simul. Model. Pract. Theory 2018, 86, 139–154. [Google Scholar] [CrossRef]
- Gunal, M.M. A guide for building hospital simulation models. Health Syst. 2012, 1, 17–25. [Google Scholar] [CrossRef]
- Brailsford, S.; Hilton, N. A comparison of discrete event simulation and system dynamics for modelling health care systems. Proc. ORAHS 2000 2001, 1, 18–39. [Google Scholar] [CrossRef]
- Raphael, J.; Helou, J.; Pritchard, K.I.; Naimark, D.M. Palbociclib in hormone receptor positive advanced breast cancer: A cost-utility analysis. Eur. J. Cancer 2017, 85, 146–154. [Google Scholar] [CrossRef]
- Dehghanimohammadabadi, M.; Keyser, T.K. Intelligent simulation: Integration of SIMIO and MATLAB to deploy decision support systems to simulation environment. Simul. Model. Pract. Theory 2017, 71, 45–60. [Google Scholar] [CrossRef]
- Zhang, X. Application of discrete event simulation in health care: A systematic review. BMC Health Serv. Res. 2018, 18, 687. [Google Scholar] [CrossRef]
- Lenin, R.B.; Lowery, C.L.; Hitt, W.C.; Manning, N.A.; Lowery, P.; Eswaran, H. Optimizing appointment template and number of staff of an OB/GYN clinic—Micro and macro simulation analyses. BMC Health Serv. Res. 2015, 15, 387. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Radnor, Z.J.; Burgess, N.; Worthington, C. SimLean: Utilising simulation in the implementation of lean in healthcare. Eur. J. Oper. Res. 2012, 219, 188–197. [Google Scholar] [CrossRef]
- Yip, K.C.M.; Huang, K.W.H.; Ho, E.W.Y.; Chan, W.K.; Lee, I.L.Y. Lessons from mixing OR methods in practice: Using DES and SD to explore a radiotherapy treatment planning process. Health Syst. 2017, 6, 102–111. [Google Scholar] [CrossRef]
- Viana, J.; Brailsford, S.C.; Harindra, V.; Harper, P.R. Combining discrete-event simulation and system dynamics in a healthcare setting: A composite model for Chlamydia infection. Eur. J. Oper. Res. 2014, 237, 196–206. [Google Scholar] [CrossRef]
- Cooper, K.; Brailsford, S.C.; Davies, R. Choice of modelling technique for evaluating health care interventions. J. Oper. Res. Soc. 2007, 58, 168–176. [Google Scholar] [CrossRef]
- Fialho, A.S.; Oliveira, M.D.; Sá, A.B. Using discrete event simulation to compare the performance of family health unit and primary health care centre organizational models in Portugal. BMC Health Serv. Res. 2011, 11, 274. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, B.G.; Choi, S.H.; Kim, T.G. Data modeling versus simulation modeling in the big data era: Case study of a greenhouse control system. Simulation 2017, 93, 579–594. [Google Scholar] [CrossRef]
- Marshall, D.A.; Burgos-Liz, L.; Ijzerman, M.J.; Crown, W.; Padula, W.V.; Wong, P.K.; Pasupathy, K.S.; Higashi, M.K.; Osgood, N.D. Selecting a dynamic simulation modeling method for health care delivery research—Part 2: Report of the ISPOR dynamic simulation modeling emerging good practices task force. Value Health 2015, 18, 147–160. [Google Scholar] [CrossRef]
- Günal, M.M.; Pidd, M. Discrete event simulation for performance modelling in health care: A review of the literature. J. Simul. 2010, 4, 42–51. [Google Scholar] [CrossRef]
- Karnon, J.; Haji Ali Afzali, H. When to use Discrete Event Simulation (DES) for the economic evaluation of health technologies? A review and critique of the costs and benefits of DES. Pharmacoeconomics 2014, 32, 547–558. [Google Scholar] [CrossRef]
- Zhang, X.; Lhachimi, S.K.; Rogowski, W.H. Reporting Quality of Discrete Event Simulations in Healthcare—Results from a Generic Reporting Checklist. Value Health 2020, 23, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Triantis, K.P.; Xue, H.; Wang, Y. The Diffusion of Discrete Event Simulation Approaches in Health Care Management in the Past Four Decades: A Comprehensive Review. MDM Policy Pract. 2020, 5, 238146832091524. [Google Scholar] [CrossRef] [PubMed]
- Hvitfeldt-Forsberg, H.; Mazzocato, P.; Glaser, D.; Keller, C.; Unbeck, M. Staffs’ and managers’ perceptions of how and when discrete event simulation modelling can be used as a decision support in quality improvement: A focus group discussion study at two hospital settings in Sweden. BMJ Open 2017, 7, e013869. [Google Scholar] [CrossRef] [PubMed]
- Alrabghi, A. Simulation in saudi healthcare: An empirical study revealing the current status and future prospects. South Afr. J. Ind. Eng. 2020, 31, 28–39. [Google Scholar] [CrossRef]
- Standfield, L.; Comans, T.; Scuffham, P. Markov modeling and discrete event simulation in health care: A systematic comparison. Int. J. Technol. Assess. Health Care 2014, 30, 165–172. [Google Scholar] [CrossRef]
- Zhang, C.; Grandits, T.; Härenstam, K.P.; Hauge, J.B.; Meijer, S. A systematic literature review of simulation models for non-technical skill training in healthcare logistics. Adv. Simul. 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Salleh, S.; Thokala, P.; Brennan, A.; Hughes, R.; Booth, A. Simulation Modelling in Healthcare: An Umbrella Review of Systematic Literature Reviews. Pharmacoeconomics 2017, 35, 937–949. [Google Scholar] [CrossRef]
- Hughes, V.S.; Ferreira De Azeredo-Da-Silva, A.L.; Hincapie, A.L. Health Economics and Outcomes Research (HEOR) Knowledge Needs Assessment for Latin America. Value Health Reg. Issues 2019, 20, 2–6. [Google Scholar] [CrossRef]
- Paul, S.A.; Reddy, M.C.; Deflitch, C.J. A systematic review of simulation studies investigating emergency department overcrowding. Simulation 2010, 86, 559–571. [Google Scholar] [CrossRef]
- Katsaliaki, K.; Mustafee, N. Applications of simulation within the healthcare context. J. Oper. Res. Soc. 2011, 62, 1598–1600. [Google Scholar] [CrossRef]
- Mustafee, N.; Taylor, S.; Katsaliaki, K.; Dwivedi, Y.; Williams, M. Motivations and barriers in using distributed supply chain simulation. Int. Trans. Oper. Res. 2012, 19, 733–751. [Google Scholar] [CrossRef]
- Jun, J.B.; Jacobson, S.H.; Swisher, J.R. Application of discrete-event simulation in health care clinics: A survey. J. Oper. Res. Soc. 1999, 50, 109–123. [Google Scholar] [CrossRef]
- Komashie, A.; Mousavi, A.; Gore, J. Quality management in healthcare and industry: A comparative review and emerging themes. J. Manag. Hist. 2007, 13, 359–370. [Google Scholar] [CrossRef]
- Fletcher, A.; Worthington, D. What is a “generic” hospital model?—A comparison of “generic” and “specific” hospital models of emergency patient flows. Health Care Manag. Sci. 2009, 12, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Eldabi, T.; Paul, R.J.; Young, T. Simulation modelling in healthcare: Reviewing legacies and investigating futures. J. Oper. Res. Soc. 2007, 58, 262–270. [Google Scholar] [CrossRef]
- Tako, A.A.; Kotiadis, K. PartiSim: A multi-methodology framework to support facilitated simulation modelling in healthcare. Eur. J. Oper. Res. 2015, 244, 555–564. [Google Scholar] [CrossRef]
- Maidstone, R. Discrete Event Simulation, System Dynamics and Agent Based Simulation: Discussion and Comparison. System 2012, 1, 1–6. [Google Scholar]
- Bayer, S. Simulation modelling and resource allocation in complex services. BMJ Qual. Saf. 2014, 23, 353–355. [Google Scholar] [CrossRef]
- Caro, J.J.; Möller, J.; Getsios, D. Discrete event simulation: The preferred technique for health economic evaluations? Value Health 2010, 13, 1056–1060. [Google Scholar] [CrossRef]
- Jun, G.T.; Morris, Z.; Eldabi, T.; Harper, P.; Naseer, A.; Patel, B.; Clarkson, J.P. Development of modelling method selection tool for health services management: From problem structuring methods to modelling and simulation methods. BMC Health Serv. Res. 2011, 11, 108. [Google Scholar] [CrossRef]
- Abe, T.K.; Beamon, B.M.; Storch, R.L.; Agus, J. Operations research applications in hospital operations: Part III. IIE Trans. Healthc. Syst. Eng. 2016, 6, 175–191. [Google Scholar] [CrossRef]
- Bean, D.M.; Taylor, P.; Dobson, R.J.B. A patient flow simulator for healthcare management education. BMJ Simul. Technol. Enhanc. Learn. 2019, 5, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Ahalt, V.; Argon, N.T.; Ziya, S.; Strickler, J.; Mehrotra, A. Comparison of emergency department crowding scores: A discrete-event simulation approach. Health Care Manag. Sci. 2018, 21, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Hamrock, E.; Paige, K.; Parks, J.; Scheulen, J.; Levin, S. Discrete event simulation for healthcare organizations: A tool for decision making. J. Healthc. Manag. 2013, 58, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.; Ghattas, M.; Mould, G. Exploring alternative routes to realising the benefits of simulation in healthcare. J. Oper. Res. Soc. 2012, 63, 1457–1466. [Google Scholar] [CrossRef]
- Enayati, S.; Mayorga, M.E.; Rajagopalan, H.K.; Saydam, C. Real-time ambulance redeployment approach to improve service coverage with fair and restricted workload for EMS providers. Omega 2018, 79, 67–80. [Google Scholar] [CrossRef]
- Dong, Y.; Chbat, N.W.; Gupta, A.; Hadzikadic, M.; Gajic, O. Systems modeling and simulation applications for critical care medicine. Ann. Intensive Care 2012, 2, 18. [Google Scholar] [CrossRef]
- Brown, J.; Karnon, J. Selecting a decision model for economic evaluation: A case study and review. Health Care Manag. Sci. 1998, 1, 133–140. [Google Scholar]
- Langellier, B.A.; Yang, Y.; Purtle, J.; Nelson, K.L.; Stankov, I.; Diez Roux, A.V. Complex Systems Approaches to Understand Drivers of Mental Health and Inform Mental Health Policy: A Systematic Review. Adm. Policy Ment. Health Ment. Health Serv. Res. 2019, 46, 128–144. [Google Scholar] [CrossRef]
- Caro, J.J.; Huybrechts, K.F.; Xenakis, J.G.; O’Brien, J.A.; Rajagopalan, K.; Lee, K. Budgetary impact of treating acute bipolar mania in hospitalized patients with quetiapine: An economic analysis of clinical trials. Curr. Med. Res. Opin. 2006, 22, 2233–2242. [Google Scholar] [CrossRef]
- Degeling, K.; Koffijberg, H.; Franken, M.D.; Koopman, M.; IJzerman, M.J. Comparing Strategies for Modeling Competing Risks in Discrete-Event Simulations: A Simulation Study and Illustration in Colorectal Cancer. Med. Decis. Mak. 2019, 39, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Kotiadis, K.; Tako, A.A. Facilitated post-model coding in discrete event simulation (DES): A case study in healthcare. Eur. J. Oper. Res. 2018, 266, 1120–1133. [Google Scholar] [CrossRef]
- Jahn, B.; Theurl, E.; Siebert, U.; Pfeiffer, K.P. Tutorial in medical decision modeling incorporating waiting lines and queues using discrete event simulation. Value Health 2010, 13, 501–506. [Google Scholar] [CrossRef]
- Kotiadis, K.; Tako, A.A.; Vasilakis, C. A participative and facilitative conceptual modelling framework for discrete event simulation studies in healthcare. J. Oper. Res. Soc. 2014, 65, 197–213. [Google Scholar] [CrossRef]
- Pinha, D.C.; Ahluwalia, R.S. Flexible resource management and its effect on project cost and duration. J. Ind. Eng. Int. 2019, 15, 119–133. [Google Scholar] [CrossRef]
- Brazier, J.; Ara, R.; Azzabi, I.; Busschbach, J.; Chevrou-Séverac, H.; Crawford, B.; Cruz, L.; Karnon, J.; Lloyd, A.; Paisley, S.; et al. Identification, Review, and Use of Health State Utilities in Cost-Effectiveness Models: An ISPOR Good Practices for Outcomes Research Task Force Report. Value Health 2019, 22, 267–275. [Google Scholar] [CrossRef]
- Traoré, M.K.; Zacharewicz, G.; Duboz, R.; Zeigler, B. Modeling and simulation framework for value-based healthcare systems. Simulation 2019, 95, 481–497. [Google Scholar] [CrossRef]
- Gillespie, J.; McClean, S.; Garg, L.; Barton, M.; Scotney, B.; Fullerton, K. A multi-phase DES modelling framework for patient-centred care. J. Oper. Res. Soc. 2016, 67, 1239–1249. [Google Scholar] [CrossRef]
- Young, T.; Soorapanth, S.; Wilkerson, J.; Millburg, L.; Roberts, T.; Morgareidge, D. The costs and value of modelling-based design in healthcare delivery: Five case studies from the US. Health Syst. 2018, 9, 253–262. [Google Scholar] [CrossRef]
- Matta, M.E.; Patterson, S.S. Evaluating multiple performance measures across several dimensions at a multi-facility outpatient center. Health Care Manag. Sci. 2007, 10, 173–194. [Google Scholar] [CrossRef]
- Kovalchuk, S.V.; Funkner, A.A.; Metsker, O.G.; Yakovlev, A.N. Simulation of patient flow in multiple healthcare units using process and data mining techniques for model identification. J. Biomed. Inform. 2018, 82, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Celano, G.; Costa, A.; Fichera, S.; Tringali, G. Linking Six Sigma to simulation: A new roadmap to improve the quality of patient care. Int. J. Health Care Qual. Assur. 2012, 25, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Mykoniatis, K.; Angelopoulou, A. A modeling framework for the application of multi-paradigm simulation methods. Simulation 2020, 96, 55–73. [Google Scholar] [CrossRef]
- Chang Junior, J.; Lima, F.; da Silva Fernandes, A.M.; de Almeida Guardia, F.; Da Silva, V.D.; Maccheri, G.A. Computer Simulation Model for Outpatient Clinics in a Brazilian Large Public Hospital Specialized in Cardiology. Braz. J. Oper. Prod. Manag. 2019, 16, 14–32. [Google Scholar] [CrossRef]
- Kisliakovskii, I.; Balakhontceva, M.; Kovalchuk, S.; Zvartau, N.; Konradi, A. Towards a simulation-based framework for decision support in healthcare quality assessment. Procedia Comput. Sci. 2017, 119, 207–214. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Marra, C.A.; Galanis, E.; Patrick, D.M. Cost effectiveness of Herpes Zoster Vaccine in Canada. Pharmacoeconomics 2009, 27, 991–1004. [Google Scholar] [CrossRef]
- Pradelli, L.; Eandi, M.; Povero, M.; Mayer, K.; Muscaritoli, M.; Heller, A.R.; Fries-Schaffner, E. Cost-effectiveness of omega-3 fatty acid supplements in parenteral nutrition therapy in hospitals: A discrete event simulation model. Clin. Nutr. 2014, 33, 785–792. [Google Scholar] [CrossRef]
- Feng, W.H.; Lou, Z.; Kong, N.; Wan, H. A multiobjective stochastic genetic algorithm for the pareto-optimal prioritization scheme design of real-time healthcare resource allocation. Oper. Res. Health Care 2017, 15, 32–42. [Google Scholar] [CrossRef]
- Katsaliaki, K.; Mustafee, N.; Taylor, S.J.E.; Brailsford, S. Comparing conventional and distributed approaches to simulation in a complex supply-chain health system. J. Oper. Res. Soc. 2009, 60, 43–51. [Google Scholar] [CrossRef]
- Uriarte, A.G.; Zúñiga, E.R.; Moris, M.U.; Ng, A.H. How can decision makers be supported in the improvement of an emergency department? A simulation, optimization and data mining approach. Oper. Res. Heath Care 2017, 15, 102–122. [Google Scholar] [CrossRef]
- Xiong, W.; Bair, A.; Sandrock, C.; Wang, S.; Siddiqui, J.; Hupert, N. Implementing telemedicine in medical emergency response: Concept of operation for a regional telemedicine hub. J. Med. Syst. 2012, 36, 1651–1660. [Google Scholar] [CrossRef]
- Hussein, N.A.; Abdelmaguid, T.F.; Tawfik, B.S.; Ahmed, N.G.S. Mitigating overcrowding in emergency departments using Six Sigma and simulation: A case study in Egypt. Oper. Res. Health Care 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Vile, J.L.; Allkins, E.; Frankish, J.; Garland, S.; Mizen, P.; Williams, J.E. Modelling patient flow in an emergency department to better understand demand management strategies. J. Simul. 2017, 11, 115–127. [Google Scholar] [CrossRef]
- De Boeck, K.; Carmen, R.; Vandaele, N. Needy boarding patients in emergency departments: An exploratory case study using discrete-event simulation. Oper. Res. Health Care 2019, 21, 19–31. [Google Scholar] [CrossRef]
- Rachuba, S.; Salmon, A.; Zhelev, Z.; Pitt, M. Redesigning the diagnostic pathway for chest pain patients in emergency departments. Health Care Manag. Sci. 2018, 21, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, F.; Mahootchi, M.; Sepehri, M.M. Resource planning in the emergency departments: A simulation-based metamodeling approach. Simul. Model. Pract. Theory 2015, 53, 123–138. [Google Scholar] [CrossRef]
- Lim, M.E.; Worster, A.; Goeree, R.; Tarride, J.É. Simulating an emergency department: The importance of modeling the interactions between physicians and delegates in a discrete event simulation. BMC Med. Inform. Decis. Mak. 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Wu, I.C.; Chen, T.L. Stochastic resource allocation in emergency departments with a multi-objective simulation optimization algorithm. Health Care Manag. Sci. 2017, 20, 55–75. [Google Scholar] [CrossRef]
- Choon, O.H.; Dali, Z.; Beng, P.T.; Magdalene, C.P.Y. Uncovering effective process improvement strategies in an emergency department using discrete event simulation. Health Syst. 2014, 3, 93–104. [Google Scholar] [CrossRef]
- Gul, M.; Guneri, A.F. A computer simulation model to reduce patient length of stay and to improve resource utilization rate in an emergency department service system. Int. J. Ind. Eng. Theory Appl. Pract. 2012, 19, 221–231. [Google Scholar]
- Bal, A.; Ceylan, C.; Taçoğlu, C. Using value stream mapping and discrete event simulation to improve efficiency of emergency departments. Int. J. Healthc. Manag. 2017, 10, 196–206. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.; Strome, T.; Weldon, E.; Chochinov, A. Evaluation of physician in triage impact on overcrowding in emergency department using discrete-event simulation. J. Proj. Manag. 2020, 5, 211–226. [Google Scholar] [CrossRef]
- Jat, M.N.; Rafique, R.A. Mass-Casualty Distribution for Emergency Healthcare: A Simulation Analysis. Int. J. Disaster Risk Sci. 2020, 11, 364–377. [Google Scholar] [CrossRef]
- Atalan, A.; Dönmez, C.C. Optimizing experimental simulation design for the emergency departments. Brazilian J. Oper. Prod. Manag. 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Valipoor, S.; Hatami, M.; Hakimjavadi, H.; Akçalı, E.; Swan, W.A.; De Portu, G. Data-Driven Design Strategies to Address Crowding and Boarding in an Emergency Department: A Discrete-Event Simulation Study. Health Environ. Res. Des. J. 2021, 14, 161–177. [Google Scholar] [CrossRef]
- Strauss, C.; Bildstein, G.; Efe, J.; Flacher, T.; Hofmann, K.; Huggler, M.; Stämpfli, A.; Schmid, M.; Schmid, E.; Gehring, C.; et al. Optimizing emergency medical service structures using a rule-based discrete event simulation—A practitioner’s point of view. Int. J. Environ. Res. Public Health 2021, 18, 2649. [Google Scholar] [CrossRef]
- Aboueljinane, L.; Sahin, E.; Jemai, Z.; Marty, J. A simulation study to improve the performance of an emergency medical service: Application to the French Val-de-Marne department. Simul. Model. Pract. Theory 2014, 47, 46–59. [Google Scholar] [CrossRef]
- Marchesi, J.F.; Hamacher, S.; Fleck, J.L. A stochastic programming approach to the physician staffing and scheduling problem. Comput. Ind. Eng. 2020, 142, 106281. [Google Scholar] [CrossRef]
- Maulla, R.S.; Smarta, P.A.; Harrisb, A.; Karasnehc, A.A.F. An evaluation of “fast track” in A&E: A discrete event simulation approach. Serv. Ind. J. 2009, 29, 923–941. [Google Scholar] [CrossRef]
- Hu, X.; Barnes, S.; Golden, B. Applying queueing theory to the study of emergency department operations: A survey and a discussion of comparable simulation studies. Int. Trans. Oper. Res. 2018, 25, 7–49. [Google Scholar] [CrossRef]
- Gearhart, R.S. Demand and capacity modelling for acute services using discrete event simulation. Health Syst. 2017, 6, 15–32. [Google Scholar] [CrossRef]
- Baril, C.; Gascon, V.; Vadeboncoeur, D. Discrete-event simulation and design of experiments to study ambulatory patient waiting time in an emergency department. J. Oper. Res. Soc. 2019, 70, 2019–2038. [Google Scholar] [CrossRef]
- Atalan, A.; Donmez, C.C. Employment of emergency advanced nurses of Turkey: A discrete-event simulation application. Processes 2019, 7, 48. [Google Scholar] [CrossRef]
- Ordu, M.; Demir, E.; Tofallis, C.; Gunal, M.M. A novel healthcare resource allocation decision support tool: A forecasting-simulation- optimization approach. J. Oper. Res. Soc. 2020, 72, 485–500. [Google Scholar] [CrossRef]
- Griffiths, J.D.; Jones, M.; Read, M.S.; Williams, J.E. A simulation model of bed-occupancy in a critical care unit. J. Simul. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Mould, G.; Bowers, J.; Dewar, C.; McGugan, E. Assessing the impact of systems modeling in the redesign of an Emergency Department. Health Syst. 2013, 2, 3–10. [Google Scholar] [CrossRef]
- Fu, X.; Presbitero, A.; Kovalchuk, S.V.; Krzhizhanovskaya, V.V. Coupling Game Theory and Discrete-Event Simulation for Model-Based Ambulance Dispatching. Procedia Comput. Sci. 2018, 136, 398–407. [Google Scholar] [CrossRef]
- Easter, B.; Houshiarian, N.; Pati, D.; Wiler, J.L. Designing efficient emergency departments: Discrete event simulation of internal-waiting areas and split flow sorting. Am. J. Emerg. Med. 2019, 37, 2186–2193. [Google Scholar] [CrossRef]
- Pawar, S.; Stanam, A. Developing a DEVS-JAVA Model to Simulate and Pre-test Changes to Emergency Care Delivery in a Safe and Efficient Manner. Lect. Notes Comput. Sci. (Incl. Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinform.) 2019, 11466 LNBI, 3–14. [Google Scholar] [CrossRef]
- Baia Medeiros, D.T.; Hahn-Goldberg, S.; Aleman, D.M.; O’Connor, E. Planning Capacity for Mental Health and Addiction Services in the Emergency Department: A Discrete-Event Simulation Approach. J. Healthc. Eng. 2019, 2019, 8973515. [Google Scholar] [CrossRef]
- Tsai, J.C.-H.; Weng, S.-J.; Liu, S.-C.; Tsai, Y.-T.; Gotcher, D.F.; Chen, C.-H.; Chou, C.-A.; Kim, S.-H. Adjusting Daily Inpatient Bed Allocation to Smooth Emergency Department Occupancy Variation. Healthcare 2020, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.T.; Campos, A.T.; de Lima Magacho, A.; Segismondi, L.C.; Vilela, F.F.; de Queiroz, J.A.; Montevechi, J.A.B. Lean thinking by integrating with discrete event simulation and design of experiments: An emergency department expansion. PeerJ Comput. Sci. 2020, 6, e284. [Google Scholar] [CrossRef] [PubMed]
- Onggo, B.S.S.; Proudlove, N.C.; D’Ambrogio, S.A.; Calabrese, A.; Bisogno, S.; Levialdi Ghiron, N. A BPMN extension to support discrete-event simulation for healthcare applications: An explicit representation of queues, attributes and data-driven decision points. J. Oper. Res. Soc. 2018, 69, 788–802. [Google Scholar] [CrossRef]
- Masterson, B.J.; Mihara, T.G.; Miller, G.; Randolph, S.C.; Forkner, M.E.; Crouter, A.L. Using models and data to support optimization of the military health system: A case study in an intensive care unit. Health Care Manag. Sci. 2004, 7, 217–224. [Google Scholar] [CrossRef]
- Hasan, I.; Bahalkeh, E.; Yih, Y. Evaluating intensive care unit admission and discharge policies using a discrete event simulation model. Simulation 2020, 96, 501–518. [Google Scholar] [CrossRef]
- Davodabadi, A.; Daneshian, B.; Saati, S.; Razavyan, S. Prioritization of patients in ICU: Composite approach of multiple-criteria decision-making and discrete event simulation. Brazilian J. Oper. Prod. Manag. 2021, 18, 1–21. [Google Scholar] [CrossRef]
- Zhu, Z.; Hen, B.H.; Teow, K.L. Estimating ICU bed capacity using discrete event simulation. Int. J. Health Care Qual. Assur. 2012, 25, 134–144. [Google Scholar] [CrossRef]
- Wilson, A.M.; Reynolds, K.A.; Verhougstraete, M.P.; Canales, R.A. Validation of a Stochastic Discrete Event Model Predicting Virus Concentration on Nurse Hands. Risk Anal. 2019, 39, 1812–1824. [Google Scholar] [CrossRef]
- Sun, N.Z.; Anand, P.A.; Snell, L. The importance of considering resource’s tasks when modeling healthcare services with discrete-event simulation: An approach using work sampling method. J. Simul. 2017, 11, 151–158. [Google Scholar] [CrossRef]
- Baril, C.; Gascon, V.; Miller, J.; Côté, N. Use of a discrete-event simulation in a Kaizen event: A case study in healthcare. Eur. J. Oper. Res. 2016, 249, 327–339. [Google Scholar] [CrossRef]
- D’Andrea, E.; Choudhry, N.K.; Raby, B.; Weinhouse, G.L.; Najafzadeh, M. A bronchial-airway gene-expression classifier to improve the diagnosis of lung cancer: Clinical outcomes and cost-effectiveness analysis. Int. J. Cancer 2020, 146, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Sewell, B.; Jones, M.; Gray, H.; Wilkes, H.; Lloyd-Bennett, C.; Beddow, K.; Bevan, M.; Fitzsimmons, D. Rapid cancer diagnosis for patients with vague symptoms: A cost-effectiveness study. Br. J. Gen. Pract. 2020, 70, e186–e192. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Hvitfeldt-Forsberg, H.; Unbeck, M.; Sköldenberg, O.G.; Stark, A.; Kelly-Pettersson, P.; Mazzocato, P. Operational strategies to manage non-elective orthopaedic surgical flows: A simulation modelling study. BMJ Open 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.J.; Persson, J.A. Analysing management policies for operating room planning using simulation. Health Care Manag. Sci. 2010, 13, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.B.; Dahl, F.A.; Barra, M. Towards a multimethodology in health care – synergies between Soft Systems Methodology and Discrete Event Simulation. Health Syst. 2013, 2, 11–23. [Google Scholar] [CrossRef]
- Reese, K.; Avansino, J.; Brumm, M.; Martin, L.; Day, T.E. Determining future capacity for an Ambulatory Surgical Center with discrete event simulation. Int. J. Healthc. Manag. 2020, 14, 920–925. [Google Scholar] [CrossRef]
- Burns, P.; Konda, S.; Alvarado, M. Discrete-event simulation and scheduling for Mohs micrographic surgery. J. Simul. 2020, 1–15. [Google Scholar] [CrossRef]
- Baril, C.; Gascon, V.; Cartier, S. Design and analysis of an outpatient orthopaedic clinic performance with discrete event simulation and design of experiments. Comput. Ind. Eng. 2014, 78, 285–298. [Google Scholar] [CrossRef]
- Standfield, L.; Comans, T.; Raymer, M.; O’Leary, S.; Moretto, N.; Scuffham, P. The Efficiency of Increasing the Capacity of Physiotherapy Screening Clinics or Traditional Medical Services to Address Unmet Demand in Orthopaedic Outpatients: A Practical Application of Discrete Event Simulation with Dynamic Queuing. Appl. Health Econ. Health Policy 2016, 14, 479–491. [Google Scholar] [CrossRef]
- Rohleder, T.R.; Lewkonia, P.; Bischak, D.P.; Duffy, P.; Hendijani, R. Using simulation modeling to improve patient flow at an outpatient orthopedic clinic. Health Care Manag. Sci. 2011, 14, 135–145. [Google Scholar] [CrossRef]
- Anderson, G.H.; Jenkins, P.J.; McDonald, D.A.; Van Der Meer, R.; Morton, A.; Nugent, M.; Rymaszewski, L.A. Cost comparison of orthopaedic fracture pathways using discrete event simulation in a Glasgow hospital. BMJ Open 2017, 7, e014509. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.B.; Linville, B.A.; Slonim, A.D. Desktop microsimulation: A tool to improve efficiency in the medical office practice. J. Healthc. Qual. 2013, 35, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Pongjetanapong, K.; Walker, C.; O’Sullivan, M.; Lovell-Smith, M.; Furian, N. Exploring trade-offs between staffing levels and turnaround time in a pathology laboratory using discrete event simulation. Int. J. Health Plan. Manag. 2019, 34, e1119–e1134. [Google Scholar] [CrossRef]
- Fairley, M.; Scheinker, D.; Brandeau, M.L. Improving the efficiency of the operating room environment with an optimization and machine learning model. Health Care Manag. Sci. 2018, 22, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Slocum, R.F.; Jones, H.L.; Fletcher, M.T.; McConnell, B.M.; Hodgson, T.J.; Taheri, J.; Wilson, J.R. Improving chemotherapy infusion operations through the simulation of scheduling heuristics: A case study. Health Syst. 2020, 10. [Google Scholar] [CrossRef]
- Bowers, J.; Mould, G.; Marshall, C. Location of services and the impact on healthcare quality: Insights from a simulation of a musculoskeletal physiotherapy service. J. Oper. Res. Soc. 2015, 66, 1212–1221. [Google Scholar] [CrossRef]
- Sandelands, E. Discrete event simulation and pharmacy process re-engineering. Int. J. Health Care Qual. Assur. 1994, 7, 1–40. [Google Scholar] [CrossRef]
- Reynolds, M.; Vasilakis, C.; McLeod, M.; Barber, N.; Mounsey, A.; Newton, S.; Jacklin, A.; Franklin, B.D. Using discrete event simulation to design a more efficient hospital pharmacy for outpatients. Health Care Manag. Sci. 2011, 14, 223–236. [Google Scholar] [CrossRef]
- Borgman, N.J.; Vliegen, I.M.H.; Boucherie, R.J.; Hans, E.W. Appointment scheduling with unscheduled arrivals and reprioritization. Flex. Serv. Manuf. J. 2018, 30, 30–53. [Google Scholar] [CrossRef]
- Shakoor, M.; Al-Nasra, M.; Abu Jadayil, W.; Jaber, N.; Abu Jadayil, S. Evaluation of provided services at MRI department in a public hospital using discrete event simulation technique: A case study. Cogent Eng. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Badilla-Murillo, F.; Vargas-Vargas, B.; Víquez-Acuña, O.; García-Sanz-Calcedo, J. Analysis of the installed productive capacity in a medical angiography room through discrete event simulation. Processes 2020, 8, 660. [Google Scholar] [CrossRef]
- Shakoor, M.; Qureshi, M.R.; Jadayil, W.A.; Jaber, N.; Al-Nasra, M. Application of discrete event simulation for performance evaluation in private healthcare: The case of a radiology department. Int. J. Healthc. Manag. 2020, 14, 1303–1310. [Google Scholar] [CrossRef]
- Singla, S. Demand and Capacity Modelling in Healthcare Using Discrete Event Simulation. Open J. Model. Simul. 2020, 8, 88–107. [Google Scholar] [CrossRef]
- Zhu, Z. Impact of different discharge patterns on bed occupancy rate and bed waiting time: A simulation approach. J. Med. Eng. Technol. 2011, 35, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Monks, T.; Worthington, D.; Allen, M.; Pitt, M.; Stein, K.; James, M.A. A modelling tool for capacity planning in acute and community stroke services. BMC Health Serv. Res. 2016, 16, 530. [Google Scholar] [CrossRef]
- Hulshof, P.J.H.; Vanberkel, P.T.; Boucherie, R.J.; Hans, E.W.; van Houdenhoven, M.; van Ommeren, J.K.C.W. Analytical models to determine room requirements in outpatient clinics. OR Spectr. 2012, 34, 391–405. [Google Scholar] [CrossRef]
- Sormaz, D.N.; Malik, M. Data-driven Simulation Modelling for Progressive Care Units in Hospitals. Procedia Manuf. 2018, 17, 819–826. [Google Scholar] [CrossRef]
- Rohleder, T.R.; Bischak, D.P.; Baskin, L.B. Modeling patient service centers with simulation and system dynamics. Health Care Manag. Sci. 2007, 10, 1–12. [Google Scholar] [CrossRef]
- Mans, R.; Reijers, H.; Wismeijer, D.; Van Genuchten, M. A process-oriented methodology for evaluating the impact of IT: A proposal and an application in healthcare. Inf. Syst. 2013, 38, 1097–1115. [Google Scholar] [CrossRef]
- Amir, T.; Lee, B.; Woods, R.W.; Mullen, L.A.; Harvey, S.C. A Pilot of Data-Driven Modeling to Assess Potential for Improved Efficiency in an Academic Breast-Imaging Center. J. Digit. Imaging 2019, 32, 221–227. [Google Scholar] [CrossRef]
- Almashrafi, A.; Vanderbloemen, L. Quantifying the effect of complications on patient flow, costs and surgical throughputs. BMC Med. Inform. Decis. Mak. 2016, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Duru, G.; Roblin, X.; Savarieau, B.; Brunel, P.; Lamure, M.; Peyrin-Biroulet, L. Cost savings using a test-based de-escalation strategy for patients with Crohn’s disease in remission on optimized infliximab: A discrete event model study. Dig. Liver Dis. 2019, 51, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kongpakwattana, K.; Chaiyakunapruk, N. Application of Discrete-Event Simulation in Health Technology Assessment: A Cost-Effectiveness Analysis of Alzheimer’s Disease Treatment Using Real-World Evidence in Thailand. Value Health 2020, 23, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.; Asseburg, C.; Slee, A.; Nilsson, A.; Neslusan, C. Development and Internal Validation of a Discrete Event Simulation Model of Diabetic Kidney Disease Using CREDENCE Trial Data. Diabetes Ther. 2020, 11, 2657–2676. [Google Scholar] [CrossRef]
- Bae, S.; Karnon, J.; Crane, G.; Bessen, T.; Desai, J.; Crowe, P.; Neuhaus, S. Cost-effectiveness analysis of imaging surveillance in stage II and III extremity soft tissue sarcoma: An Australian perspective. Cost Eff. Resour. Alloc. 2020, 18, 5. [Google Scholar] [CrossRef]
- Dieleman, J.M.; Myles, P.S.; Bulfone, L.; Younie, S.; van Zaane, B.; McGiffin, D.; Moodie, M.; Gao, L. Cost-effectiveness of routine transoesophageal echocardiography during cardiac surgery: A discrete-event simulation study. Br. J. Anaesth. 2020, 124, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Furzer, J.; Tessier, L.; Hodgson, D.; Cotton, C.; Nathan, P.C.; Gupta, S.; Pechlivanoglou, P. Cost-Utility of Early Breast Cancer Surveillance in Survivors of Thoracic Radiation-Treated Adolescent Hodgkin Lymphoma. J. Natl. Cancer Inst. 2020, 112, 63–70. [Google Scholar] [CrossRef]
- Huygens, S.A.; Ramos, I.C.; Bouten, C.V.C.; Kluin, J.; Chiu, S.T.; Grunkemeier, G.L.; Takkenberg, J.J.M.; Rutten-van Mölken, M.P.M.H. Early cost-utility analysis of tissue-engineered heart valves compared to bioprostheses in the aortic position in elderly patients. Eur. J. Health Econ. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kongnakorn, T.; Ward, A.; Roberts, C.S.; O’Brien, J.A.; Proskorovsky, I.; Caro, J.J. Economic evaluation of atorvastatin for prevention of recurrent stroke based on the SPARCL trial. Value Health 2009, 12, 880–887. [Google Scholar] [CrossRef][Green Version]
- Väätäinen, S.; Soini, E.; Peltola, J.; Charokopou, M.; Taiha, M.; Kälviäinen, R. Economic Value of Adjunctive Brivaracetam Treatment Strategy for Focal Onset Seizures in Finland. Adv. Ther. 2020, 37, 477–500. [Google Scholar] [CrossRef]
- Geitona, M.; Stamuli, E.; Giannakodimos, S.; Kimiskidis, V.K.; Kountouris, V.; Charokopou, M.; Christou, P. Lacosamide as a first-line treatment option in focal epilepsy: A cost-utility analysis for the Greek healthcare system. J. Med. Econ. 2019, 22, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Eldabi, T.; Paul, R.J.; Taylor, S.J.E. Simulating economic factors in adjuvant breast cancer treatment. J. Oper. Res. Soc. 2000, 51, 465–475. [Google Scholar] [CrossRef]
- Wang, H.I.; Roman, E.; Crouch, S.; Aas, E.; Burton, C.; Patmore, R.; Smith, A. A Generic Model for Follicular Lymphoma: Predicting Cost, Life Expectancy, and Quality-Adjusted-Life-Year Using UK Population–Based Observational Data. Value Health 2018, 21, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Brailsford, S.C.; Gutjahr, W.J.; Rauner, M.S.; Zeppelzauer, W. Combined discrete-event simulation and ant colony optimisation approach for selecting optimal screening policies for diabetic retinopathy. Comput. Manag. Sci. 2007, 4, 59–83. [Google Scholar] [CrossRef]
- Ekman, M.; Lindgren, P.; Miltenburger, C.; Meier, G.; Locklear, J.C.; Chatterton, M. Lou Cost effectiveness of quetiapine in patients with acute bipolar depression and in maintenance treatment after an acute depressive episode. Pharmacoeconomics 2012, 30, 513–530. [Google Scholar] [CrossRef]
- Hartz, S.; Getsios, D.; Tao, S.; Blume, S.; Maclaine, G. Evaluating the cost effectiveness of donepezil in the treatment of Alzheimer’s disease in Germany using discrete event simulation. BMC Neurol. 2012, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Van Karnebeek, C.D.M.; Mohammadi, T.; Tsao, N.; Sinclair, G.; Sirrs, S.; Stockler, S.; Marra, C. Health economic evaluation of plasma oxysterol screening in the diagnosis of Niemann-Pick Type C disease among intellectually disabled using discrete event simulation. Mol. Genet. Metab. 2015, 114, 226–232. [Google Scholar] [CrossRef]
- Brailsford, S.C.; Harper, P.R.; Sykes, J. Incorporating human behaviour in simulation models of screening for breast cancer. Eur. J. Oper. Res. 2012, 219, 491–507. [Google Scholar] [CrossRef]
- Qin, Y.; Freebairn, L.; Atkinson, J.A.; Qian, W.; Safarishahrbijari, A.; Osgood, N.D. Multi-scale simulation modeling for prevention and public health management of diabetes in pregnancy and sequelae. Lect. Notes Comput. Sci. (Incl. Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinform.) 2019, 11549 LNCS, 256–265. [Google Scholar] [CrossRef]
- McKinley, K.W.; Babineau, J.; Roskind, C.G.; Sonnett, M.; Doan, Q. Discrete event simulation modelling to evaluate the impact of a quality improvement initiative on patient flow in a paediatric emergency department. Emerg. Med. J. 2020, 37, 193–199. [Google Scholar] [CrossRef]
- Ren, Y.; Phan, M.; Luong, P.; Wu, J.; Shell, D.; Barras, C.D.; Kok, H.K.; Burney, M.; Tahayori, B.; Seah, H.M.; et al. Geographic Service Delivery for Endovascular Clot Retrieval: Using Discrete Event Simulation to Optimize Resources. World Neurosurg. 2020, 141, e400–e413. [Google Scholar] [CrossRef] [PubMed]
- Di Mascolo, M.; Gouin, A. A generic simulation model to assess the performance of sterilization services in health establishments. Health Care Manag. Sci. 2013, 16, 45–61. [Google Scholar] [CrossRef]
- White, D.L.; Torabi, E.; Froehle, C.M. Ice-Breaker vs. Standalone: Comparing Alternative Workflow Modes of Mid-level Care Providers. Prod. Oper. Manag. 2017, 26, 2089–2106. [Google Scholar] [CrossRef]
- Ignone, G.; Mossa, G.; Mummolo, G.; Pilolli, R.; Ranieri, L. Increasing public healthcare network performance by de hospitalization: A patient pathway perspective. Strateg. Outsourc. Int. J. 2013, 6, 85–107. [Google Scholar] [CrossRef]
- Swisher, J.R.; Jacobson, S.H.; Jun, J.B.; Balci, O. Modeling and analyzing a physician clinic environment using discrete-event (visual) simulation. Comput. Oper. Res. 2001, 28, 105–125. [Google Scholar] [CrossRef]
- Knight, V.A.; Williams, J.E.; Reynolds, I. Modelling patient choice in healthcare systems: Development and application of a discrete event simulation with agent-based decision making. J. Simul. 2012, 6, 92–102. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Kon, A.W.M.; Wai, C.S.L.; Ang, W.B. Patient flow improvement for an ophthalmic specialist outpatient clinic with aid of discrete event simulation and design of experiment. Health Care Manag. Sci. 2015, 18, 137–155. [Google Scholar] [CrossRef]
- Lahr, M.M.H.; Maas, W.J.; Van Der Zee, D.J.; Uyttenboogaart, M.; Buskens, E. Rationale and design for studying organisation of care for intra-arterial thrombectomy in the Netherlands: Simulation modelling study. BMJ Open 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Chalk, D.; Trent, N.; Vennam, S.; McGrane, J.; Mantle, M. Reducing delays in the diagnosis and treatment of muscle-invasive bladder cancer using simulation modelling. J. Clin. Urol. 2019, 12, 129–133. [Google Scholar] [CrossRef]
- Hashim, S.; Tahar, R.M.; Abu bakar, E.M. Simulation study for improving patient treatment services. J. ICT 2003, 2, 87–104. [Google Scholar]
- Cochran, J.K.; Bharti, A. Stochastic bed balancing of an obstetrics hospital. Health Care Manag. Sci. 2006, 9, 31–45. [Google Scholar] [CrossRef]
- Devapriya, P.; Strömblad, C.T.B.; Bailey, M.D.; Frazier, S.; Bulger, J.; Kemberling, S.T.; Wood, K.E. StratBAM: A Discrete-Event Simulation Model to Support Strategic Hospital Bed Capacity Decisions. J. Med. Syst. 2015, 39, 130. [Google Scholar] [CrossRef]
- White, D.L.; Froehle, C.M.; Klassen, K.J. The effect of integrated scheduling and capacity policies on clinical efficiency. Prod. Oper. Manag. 2011, 20, 442–455. [Google Scholar] [CrossRef]
- Burns, A.S.; Santos, A.; Cheng, C.L.; Chan, E.; Fallah, N.; Atkins, D.; Dvorak, M.F.; Ho, C.; Ahn, H.; Paquet, J.; et al. Understanding Length of Stay after Spinal Cord Injury: Insights and Limitations from the Access to Care and Timing Project. J. Neurotrauma 2017, 34, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Pendharkar, S.R.; Bischak, D.P.; Rogers, P.; Flemons, W.; Noseworthy, T.W. Using patient flow simulation to improve access at a multidisciplinary sleep centre. J. Sleep Res. 2015, 24, 320–327. [Google Scholar] [CrossRef]
- Zhong, X.; Lee, H.K.; Williams, M.; Kraft, S.; Sleeth, J.; Welnick, R.; Hauschild, L.; Li, J. Workload balancing: Staffing ratio analysis for primary care redesign. Flex. Serv. Manuf. J. 2018, 30, 6–29. [Google Scholar] [CrossRef]
- Perez, E.; Anandhan, V.; Novoa, C. A Simulation-Based Planning Methodology for Decreasing Patient Waiting Times in Pure Walk-In Clinics. Int. J. Inf. Syst. Serv. Sect. 2020, 12, 34–54. [Google Scholar] [CrossRef]
- Gulhane, K.; Khan, A.; Joshi, R. Enhancing operational efficiency of hospitals using discrete event simulation. Int. J. Manag. 2020, 11, 32–43. [Google Scholar] [CrossRef]
- Das, A. Impact of the COVID-19 pandemic on the workflow of an ambulatory endoscopy center: An assessment by discrete event simulation. Gastrointest. Endosc. 2020, 92, 914–924. [Google Scholar] [CrossRef]
- Doneda, M.; Yalçındağ, S.; Marques, I.; Lanzarone, E. A discrete-event simulation model for analysing and improving operations in a blood donation centre. Vox Sang. 2021, 1, 1–16. [Google Scholar] [CrossRef]
- McKinley, K.W.; Chamberlain, J.M.; Doan, Q.; Berkowitz, D. Reducing Pediatric ED Length of Stay by Reducing Diagnostic Testing: A Discrete Event Simulation Model. Pediatr. Qual. Saf. 2021, 6, e396. [Google Scholar] [CrossRef] [PubMed]
- Yemane, A.; Abrha, H.; Gidey, K. Performance Measurement and Improvement of Healthcare Service Using Discrete Event Simulation in Bahir Dar Clinic. J. Optim. Ind. Eng. 2021, 14, 57–67. [Google Scholar] [CrossRef]
- Fernandez, M.B.; Herrera, M.M.; Trejos, C.; Romero, O.R. Resources allocation in service planning using discrete event simulation. Ing. Univ. 2021, 25, 1–22. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Mohsin, S.K.; Mohammed, S.J. The Effectiveness of Using Discrete Event Simulation to Optimize the Quality of Service of Outpatient in Iraq: A Case Study. Iraqi J. Ind. Res. 2021, 8, 40–49. [Google Scholar] [CrossRef]
- Abdoli, M.; Bahadori, M.; Ravangard, R.; Babaei, M.; Aminjarahi, M. Comparing 2 Appointment Scheduling Policies Using Discrete-Event Simulation. Qual. Manag. Health Care 2021, 30, 112–120. [Google Scholar] [CrossRef]
- Haddad, M.G.; Zouein, P.P.; Salem, J.; Otayek, R. Case Study of Lean in Hospital Admissions to Inspire Culture Change. EMJ 2016, 28, 209–223. [Google Scholar] [CrossRef]
- Study, S.C.; Restrepo-morales, J.A.; Andrés, E.; Betancur, G.; Gabriel, J.; López, V. Customer Service Multichannel Model in a Health Care Service Provider: A Discrete Competitividad y Gestión. Innovar 2019, 29, 89–102. [Google Scholar] [CrossRef]
- Heim, J.A.; Huang, H.; Zabinsky, Z.B.; Dickerson, J.; Wellner, M.; Astion, M.; Cruz, D.; Vincent, J.; Jack, R. Design and implementation of a combined influenza immunization and tuberculosis screening campaign with simulation modelling. J. Eval. Clin. Pract. 2015, 21, 727–734. [Google Scholar] [CrossRef]
- Parks, J.K.; Engblom, P.; Hamrock, E.; Satjapot, S.; Levin, S. Designed to fail: How computer simulation can detect fundamental flaws in clinic flow. J. Healthc. Manag. 2011, 56, 135–144. [Google Scholar] [CrossRef]
- Dehlendorff, C.; Kulahci, M.; Andersen, K.K. Designing simulation experiments with controllable and uncontrollable factors for applications in healthcare. J. R. Stat. Soc. Ser. C Appl. Stat. 2011, 60, 31–49. [Google Scholar] [CrossRef]
- Chemweno, P.; Thijs, V.; Pintelon, L.; Van Horenbeek, A. Discrete event simulation case study: Diagnostic path for stroke patients in a stroke unit. Simul. Model. Pract. Theory 2014, 48, 45–57. [Google Scholar] [CrossRef]
- Elliott, T.M.; Lee, X.J.; Foeglein, A.; Harris, P.N.; Gordon, L.G. A hybrid simulation model approach to examine bacterial genome sequencing during a hospital outbreak. BMC Infect. Dis. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Karnon, J. Alternative decision modelling techniques for the evaluation of health care technologies: Markov processes versus discrete event simulation. Health Econ. 2003, 12, 837–848. [Google Scholar] [CrossRef]
- Demir, E.; Southern, D. Enabling better management of patients: Discrete event simulation combined with the STAR approach. J. Oper. Res. Soc. 2017, 68, 577–590. [Google Scholar] [CrossRef][Green Version]
- Qiao, Y.; Ran, L.; Li, J. Optimization of Teleconsultation Using Discrete-Event Simulation from a Data-Driven Perspective. Telemed. e-Health 2019, 26, 112–123. [Google Scholar] [CrossRef]
- Cudney, E.A.; Baru, R.A.; Guardiola, I.; Materla, T.; Cahill, W.; Phillips, R.; Mutter, B.; Warner, D.; Masek, C. A decision support simulation model for bed management in healthcare. Int. J. Health Care Qual. Assur. 2019, 32, 499–515. [Google Scholar] [CrossRef]
- Demir, E.; Southern, D.; Verner, A.; Amoaku, W. A simulation tool for better management of retinal services. BMC Health Serv. Res. 2018, 18, 759. [Google Scholar] [CrossRef]
- Peres, I.T.; Hamacher, S.; Oliveira, F.L.C.; Barbosa, S.D.J.; Viegas, F. Simulation of Appointment Scheduling Policies: A Study in a Bariatric Clinic. Obes. Surg. 2019, 29, 2824–2830. [Google Scholar] [CrossRef]
- Rezaeiahari, M.; Khasawneh, M.T. Simulation optimization approach for patient scheduling at destination medical centers. Expert Syst. Appl. 2020, 140, 112881. [Google Scholar] [CrossRef]
- Kozlowski, D.; Worthington, D. Use of queue modelling in the analysis of elective patient treatment governed by a maximum waiting time policy. Eur. J. Oper. Res. 2015, 244, 331–338. [Google Scholar] [CrossRef]
- Qureshi, S.M.; Purdy, N.; Neumann, W.P. Development of a Methodology for Healthcare System Simulations to Quantify Nurse Workload and Quality of Care. IISE Trans. Occup. Ergon. Hum. Factors 2020, 8, 27–41. [Google Scholar] [CrossRef]
- Dutta, D.; Parry, F.; Obaid, M.; Ramadurai, G. Mechanical thrombectomy in stroke – planning for service expansion using discrete event simulation. Future Healthc. J. 2020, 7, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Melman, G.J.; Parlikad, A.K.; Cameron, E.A.B. Balancing scarce hospital resources during the COVID-19 pandemic using discrete-event simulation. Health Care Manag. Sci. 2021, 19, 356–374. [Google Scholar] [CrossRef] [PubMed]
- Bhosekar, A.; Ekşioğlu, S.; Işık, T.; Allen, R. A discrete event simulation model for coordinating inventory management and material handling in hospitals. Ann. Oper. Res. 2021, 1, 1–28. [Google Scholar] [CrossRef]
- Improta, G.; Guizzi, G.; Ricciardi, C.; Giordano, V.; Ponsiglione, A.M.; Converso, G.; Triassi, M. Agile six sigma in healthcare: Case study at santobono pediatric hospital. Int. J. Environ. Res. Public Health 2020, 17, 1052. [Google Scholar] [CrossRef]
- Gosavi, A.; Cudney, E.A.; Murray, S.L.; Masek, C.M. Analysis of Clinic Layouts and Patient-Centered Procedural Innovations Using Discrete-Event Simulation. EMJ 2016, 28, 134–144. [Google Scholar] [CrossRef]
- Bakker, M.; Tsui, K.L. Dynamic resource allocation for efficient patient scheduling: A data-driven approach. J. Syst. Sci. Syst. Eng. 2017, 26, 448–462. [Google Scholar] [CrossRef]
- Nikakhtar, A.; Hsiang, S.M. Incorporating the dynamics of epidemics in simulation models of healthcare systems. Simul. Model. Pract. Theory 2014, 43, 67–78. [Google Scholar] [CrossRef]
- Aslani, N.; Zhang, J. Integration of simulation and DEA to determine the most efficient patient appointment scheduling model for a specific healthcare setting. J. Ind. Eng. Manag. 2014, 7, 785–815. [Google Scholar] [CrossRef]
- Bilodeau, B.L.; Stanford, D.A.; Goldszmidt, M.; Appleton, A. Simulated co-location of patients admitted to an inpatient internal medicine teaching unit: Potential impacts on efficiency and physician-nurse collaboration. INFOR Inf. Syst. Oper. Res. 2019, 58, 109–123. [Google Scholar] [CrossRef]
- Kalwer, M.A.; Mari, S.I.; Memon, M.S.; Tanwari, A.; Siddiqui, A.A. Simulation Based Approach for Improving Outpatient Clinic Operations. Mehran Univ. Res. J. Eng. Technol. 2020, 39, 153–170. [Google Scholar] [CrossRef]
- Bae, K.-H.; Jones, M.; Evans, G.; Antimisiaris, D. Simulation modelling of patient flow and capacity planning for regional long-term care needs: A case study. Health Syst. 2019, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lamé, G.; Jouini, O.; Stal-Le Cardinal, J. Combining Soft Systems Methodology, ethnographic observation, and discrete-event simulation: A case study in cancer care. J. Oper. Res. Soc. 2019, 71, 1545–1562. [Google Scholar] [CrossRef]
- Demirli, K.; Al Kaf, A.; Simsekler, M.C.E.; Jayaraman, R.; Khan, M.J.; Tuzcu, E.M. Using lean techniques and discrete-event simulation for performance improvement in an outpatient clinic. Int. J. Lean Six Sigma 2021, 12, 1260–1288. [Google Scholar] [CrossRef]
- Lam, C.; Meinert, E.; Yang, A.; Cui, Z. Comparison between centralized and decentralized supply chains of autologous chimeric antigen receptor T-cell therapies: A UK case study based on discrete event simulation. Cytotherapy 2021, 23, 433–451. [Google Scholar] [CrossRef]
- England, T.J.; Harper, P.R.; Crosby, T.; Gartner, D.; Arruda, E.F.; Foley, K.G.; Williamson, I.J. Examining the diagnostic pathway for lung cancer patients in Wales using discrete event simulation. Transl. Lung Cancer Res. 2021, 10, 1368–1382. [Google Scholar] [CrossRef]
- Saidani, M.; Kim, H. A Discrete Event Simulation-Based Model to Optimally Design and Dimension Mobile COVID-19 Saliva-Based Testing Stations. Simul. Healthc. J. Soc. Simul. Healthc. 2021, 16, 151–152. [Google Scholar] [CrossRef]
- Zhuhadar, L.P.; Thrasher, E. Data analytics and its advantages for addressing the complexity of healthcare: A simulated zika case study example. Appl. Sci. 2019, 9, 2208. [Google Scholar] [CrossRef]
- Gonsalves, T.; Itoh, K. Service optimization with patient satisfaction in healthcare systems. J. Simul. 2009, 3, 150–162. [Google Scholar] [CrossRef]
- Zhu, Z.; Heng, B.H.; Teow, K.L. Analysis of factors causing long patient waiting time and clinic overtime in outpatient clinics. J. Med. Syst. 2012, 36, 707–713. [Google Scholar] [CrossRef]
- Diamant, A.; Milner, J.; Quereshy, F. Dynamic Patient Scheduling for Multi-Appointment Health Care Programs. Prod. Oper. Manag. 2018, 27, 58–79. [Google Scholar] [CrossRef]
- Swisher, J.R.; Jacobson, S.H. Evaluating the design of a family practice healthcare clinic using discrete-event simulation. Health Care Manag. Sci. 2002, 5, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Bard, J.F.; Shu, Z.; Morrice, D.J.; Wang, D.E.; Poursani, R.; Leykum, L. Improving patient flow at a family health clinic. Health Care Manag. Sci. 2016, 19, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Almagooshi, S. Simulation Modelling in Healthcare: Challenges and Trends. Procedia Manuf. 2015, 3, 301–307. [Google Scholar] [CrossRef]
- Muravev, D.; Hu, H.; Rakhmangulov, A.; Mishkurov, P. Multi-agent optimization of the intermodal terminal main parameters by using AnyLogic simulation platform: Case study on the Ningbo-Zhoushan Port. Int. J. Inf. Manag. 2021, 57, 102133. [Google Scholar] [CrossRef]
- Postema, B.F.; Haverkort, B.R. An anylogic simulation model for power and performance analysis of data centres. Lect. Notes Comput. Sci. (Incl. Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinform.) 2015, 9272, 258–272. [Google Scholar] [CrossRef]
- Van Lent, W.A.; Vanberkel, P.; Van Harten, W.H. A review on the relation between simulation and improvement in hospitals. BMC Med. Inform. Decis. Mak. 2012, 12, 18. [Google Scholar] [CrossRef]

| Scope: | Operational, tactical |
| Purpose: | Decisions: Optimizations, predictions, and comparisons |
| Perspective: | Analytic, emphasis on detail complexity |
| Importance of variability: | High |
| Importance of tracking individuals: | High |
| Number of entities: | Large |
| Control: | Waiting (queues) |
| Relative timescale: | Short |
| Resolution of models: | Individual entities, attributes, decisions, and events |
| Data sources: | Numeric with some critical elements |
| Lower boundary of technical preparation: | Qualitative workflow |
| Model elements: | Physical, tangible, information |
| Model outputs: | Prediction points, performance measurements |
| Tools: | Arena, Simul8, FlexSim/FlexSim Healthcare, ProModel/MedModel, Simio, AnyLogic, TreeAge, ExtendSim |
| Setting/Approach | DES | DES + Markov | DES + SD or ABS | DES + Others | Total |
|---|---|---|---|---|---|
| General Healthcare | [30,31,32,33,34,35] | [36] | [37] | [38,39] | 10 |
| Emergency Unit | [40] | 1 | |||
| Medical Center | [21] | [41] | 2 | ||
| Total | 7 | 1 | 2 | 3 | 13 |
| Setting/Approach | DES | DES + Optimization or Math Model | DES + Lean or Six Sigma | DES + SD or ABS or Monte Carlo | DES + Others | Total | % |
|---|---|---|---|---|---|---|---|
| General Healthcare | [42,43,44,45] | [20] | [3,15,18,43,46,47,48,49] | [26,50,51,52] | 17 | 36 | |
| Emergency Unit | [53,54,55,56] | [57] | [9] | 6 | 13 | ||
| Intensive Unit | [58] | 1 | 2 | ||||
| Operating Room | [22] | 1 | 2 | ||||
| Pediatric | [13] | 1 | 2 | ||||
| Therapy | [24,59] | 2 | 4 | ||||
| Psychiatry | [60] | 1 | 2 | ||||
| Patient State | [61,62,63,64] | [65] | 5 | 10 | |||
| Medical Center | [66,67,68,69,70] | [2,71,72] | [73] | [7,17,74,75,76] | 14 | 29 | |
| Total | 18 | 5 | 2 | 18 | 5 | 48 | |
| % | 38 | 10 | 4 | 38 | 10 | 100 |
| Setting/Outcome | Time and Efficiency | Financial and Cost Savings | Allocation of Resources/Schedule | Public Health | Others | Total | % |
|---|---|---|---|---|---|---|---|
| Clinic | [77,78] | [79,80] | 4 | 2.3 | |||
| Emergency Unit | [10,11,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104] | [105,106,107,108,109,110,111,112,113] | [1,114] | 37 | 21.8 | ||
| Intensive Unit | [115,116,117] | [118] | 4 | 2.4 | |||
| Laboratory | [16] | 1 | 0.5 | ||||
| Nursing | [119] | [8] | 2 | 1.2 | |||
| Oncology | [120,121] | [122,123] | 4 | 2.4 | |||
| Operating Room | [124,125,126] | [127,128] | 5 | 3 | |||
| Orthopedic | [129,130,131] | [132] | [6,133] | 6 | 3.6 | ||
| Pathology | [134] | 1 | 0.5 | ||||
| Pediatric | [135] | 1 | 0.5 | ||||
| Therapy | [136,137] | 2 | 1.2 | ||||
| Pharmacy | [138,139] | 2 | 1.2 | ||||
| Radiology | [140,141,142,143,144] | 5 | 3 | ||||
| Support Areas | [145] | [146,147,148] | [149] | 5 | 3 | ||
| Dental Area | [150] | 1 | 0.5 | ||||
| Mammography | [151] | 1 | 0.5 | ||||
| Patient State | [152] | [19,153,154,155,156,157,158,159,160,161,162,163] | [164,165,166,167,168,169,170,171,172] | 22 | 13 | ||
| Medical Device | [173] | 1 | 0.5 | ||||
| Medical Center | [12,14,22,27,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202] | [203,204,205,206] | [207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223] | [25,224,225,226,227,228] | [229,230,231,232,233,234] | 66 | 38.9 |
| Total | 84 | 21 | 36 | 19 | 10 | 170 | |
| % | 49.4 | 12.3 | 21.2 | 11.2 | 5.9 | 100 |
| Article | Journal | Publisher | Number of Citations | Publication Year | Average Citations per Year (until 2021) |
|---|---|---|---|---|---|
| [182] | Health Care Management Science | Springer | 117 | 2006 | 7.8 |
| [131] | Health Care Management Science | Springer | 113 | 2011 | 11.3 |
| [204] | Health Economics | Wiley | 100 | 2003 | 5.6 |
| [233] | Health Care Management Science | Springer | 69 | 2002 | 3.6 |
| [25] | European Journal of Operational Research | Elsevier | 67 | 2014 | 9.6 |
| [149] | Health Care Management Science | Springer | 66 | 2007 | 4.7 |
| [184] | Production and Operations Management | Wiley | 57 | 2011 | 5.7 |
| [125] | Health Care Management Science | Springer | 55 | 2010 | 5.0 |
| [87] | Simulation Modelling Practice and Theory | Elsevier | 50 | 2015 | 8.3 |
| [121] | European Journal of Operational Research | Elsevier | 49 | 2016 | 9.8 |
| Country/Publisher | Springer | Elsevier | Taylor & Francis | Palgrave | Others | Total | % |
|---|---|---|---|---|---|---|---|
| US | 11 | 8 | 5 | 0 | 20 | 44 | 25.9 |
| UK | 5 | 4 | 4 | 9 | 11 | 33 | 19.4 |
| Canada | 4 | 4 | 3 | 1 | 9 | 21 | 12.4 |
| Others | 14 | 18 | 6 | 3 | 31 | 72 | 42.3 |
| Total | 34 | 34 | 18 | 13 | 71 | 170 | |
| % | 20 | 20 | 10.5 | 7.7 | 41.8 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Serrano, J.I.; Peimbert-García, R.E.; Cárdenas-Barrón, L.E. Discrete-Event Simulation Modeling in Healthcare: A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 12262. https://doi.org/10.3390/ijerph182212262
Vázquez-Serrano JI, Peimbert-García RE, Cárdenas-Barrón LE. Discrete-Event Simulation Modeling in Healthcare: A Comprehensive Review. International Journal of Environmental Research and Public Health. 2021; 18(22):12262. https://doi.org/10.3390/ijerph182212262
Chicago/Turabian StyleVázquez-Serrano, Jesús Isaac, Rodrigo E. Peimbert-García, and Leopoldo Eduardo Cárdenas-Barrón. 2021. "Discrete-Event Simulation Modeling in Healthcare: A Comprehensive Review" International Journal of Environmental Research and Public Health 18, no. 22: 12262. https://doi.org/10.3390/ijerph182212262
APA StyleVázquez-Serrano, J. I., Peimbert-García, R. E., & Cárdenas-Barrón, L. E. (2021). Discrete-Event Simulation Modeling in Healthcare: A Comprehensive Review. International Journal of Environmental Research and Public Health, 18(22), 12262. https://doi.org/10.3390/ijerph182212262







