Obesity Measures as Predictors of Type 2 Diabetes and Cardiovascular Diseases among the Jordanian Population: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
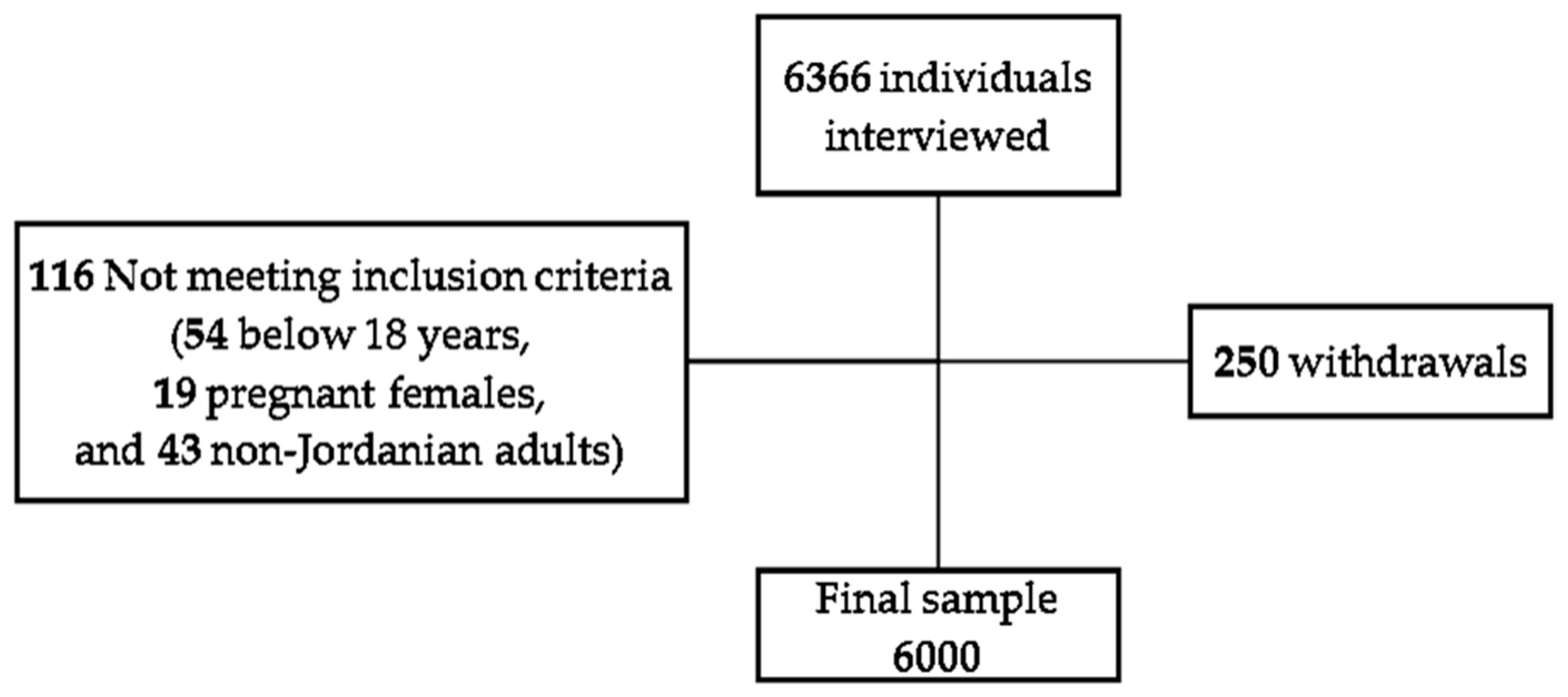
2.1. Study Design and Participants
2.2. Anthropometric Measurements
2.3. Statistical Analysis
2.4. Institutional Review Board Statement
3. Results
3.1. BMI Outcomes and Their Relation with Population Demographics
3.2. WC Outcomes and Their Relation with Population Demographics
3.3. The Relaltionship of T2D with BMI, WC, and Demographics
3.4. The Relationship between BMI, WC, and Demographics and CVD
3.5. BMI and WC and Their Association with the Risk of T2D
3.6. BMI and WC and Their Association with the Risk of CVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 April 2021).
- Hales, C.; Carroll, M.; Fryar, C.; Ogden, C. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; National Center for Health Statistics: Hyattsville, MD, USA, 2020. [Google Scholar]
- World Health Organization. European Health Information Gateway, Data, Indicators, Obesity. Available online: https://gateway.euro.who.int/en/indicators/h2020_9-obesity/ (accessed on 24 February 2021).
- Hoffman, D.J. Obesity in developing countries: Causes and implications. Food Nutr. Agric. 2001, 28, 35–44. [Google Scholar]
- Nikoloski, Z.; Williams, G. Obesity in the Middle East; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar] [CrossRef]
- World Health Organization. Jordan-WHO, Country Profiles. Available online: https://www.who.int/diabetes/country-profiles/jor_en.pdf (accessed on 24 February 2021).
- Centers for Disease Control and Prevention. Adult Obesity Causes and Cosequences. Available online: https://www.cdc.gov/obesity/adult/causes.html (accessed on 26 February 2021).
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587. [Google Scholar] [CrossRef] [Green Version]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008. 2011. Available online: https://www.who.int/publications/i/item/9789241501491 (accessed on 1 April 2021).
- Collins, G.S.; Mallett, S.; Omar, O.; Yu, L.-M. Developing risk prediction models for type 2 diabetes: A systematic review of methodology and reporting. BMC Med. 2011, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Q.; Nyamdorj, R. Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur. J. Clin. Nutr. 2010, 64, 30–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hippisley-Cox, J.; Coupland, C.; Vinogradova, Y.; Robson, J.; May, M.; Brindle, P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: Prospective open cohort study. BMJ 2007, 335, 136. [Google Scholar] [CrossRef] [Green Version]
- Cooney, M.T.; Dudina, A.L.; Graham, I.M. Value and limitations of existing scores for the assessment of cardiovascular risk: A review for clinicians. J. Am. Coll. Cardiol. 2009, 54, 1209–1227. [Google Scholar] [CrossRef] [Green Version]
- Emerging Risk Factors Collaboration. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 2011, 377, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. World Health Organization/International Society of Hypertension (WH0/ISH) Risk Prediction Charts for 14 WHO Epidemiological Sub-Regions (Charts in Colour). Available online: https://www.who.int/ncds/management/WHO_ISH_Risk_Prediction_Charts.pdf?ua=1 (accessed on 7 May 2021).
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity paradox in cardiovascular disease: Where do we stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Heart Lung and Blood Institute. Assessing Your Weight and Health Risk. Available online: https://www.nhlbi.nih.gov/health/educational/lose_wt/risk.htm (accessed on 2 November 2021).
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Darsini, D.; Hamidah, H.; Notobroto, H.B.; Cahyono, E.A. Health risks associated with high waist circumference: A systematic review. J. Public Health Res. 2020, 9, 1811. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Available online: https://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (accessed on 5 July 2021).
- Kashiwagi, R.; Iwahashi, H.; Yamada, Y.; Sakaue, T.; Okita, T.; Kawachi, Y.; Iwamoto, R.; Saisho, K.; Tamba, S.; Yamamoto, K.; et al. Effective waist circumference reduction rate necessary to avoid the development of type 2 diabetes in Japanese men with abdominal obesity. Endocr. J. 2017, 64, 881–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. 6. Obesity management for the treatment of type 2 diabetes. Diabetes Care 2016, 39 (Suppl. 1), S47–S51. [Google Scholar] [CrossRef] [Green Version]
- Haase, C.L.; Lopes, S.; Olsen, A.H.; Satylganova, A.; Schnecke, V.; McEwan, P. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: Evidence from a UK primary care database. Int. J. Obes. 2021, 45, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Fanghänel, G.; Sánchez-Reyes, L.; Félix-García, L.; Violante-Ortiz, R.; Campos-Franco, E.; Alcocer, L.A. Impact of waist circumference reduction on cardiovascular risk in treated obese subjects. Cir. Cir. 2011, 79, 175–181. [Google Scholar]
- Department of Statistics. Population. Available online: http://dosweb.dos.gov.jo/ar/population/population-2/ (accessed on 27 February 2021).
- World Health Organization. Risk of Premature Death Due to Ncds, Jordan. Available online: https://www.who.int/nmh/countries/jor_en.pdf (accessed on 28 February 2021).
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 28 February 2021).
- Lee, R.D.; Nieman, D.C. Nutritional Assessment, 5th ed.; International ed.; McGraw-Hill Higher Education: New York, NY, USA, 2010. [Google Scholar]
- Deurenberg, P.; Weststrate, J.A.; Seidell, J.C. Body mass index as a measure of body fatness: Age-and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R. Biochemical assessment of nutritional status. In Nutritional Assessment: Ney York; Lee, R.D., Nieman, D.C., Eds.; McGraw-Hill: Boone, NC, USA; Appalachian State University: Boone, NC, USA, 2013. [Google Scholar]
- North American Association for the Study of Obesity; National Heart, Lung, Blood Institute; NHLBI Obesity Education Initiative. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults; National Institutes of Health: Long Beach, CA, USA; National Heart, Lung, and Blood Institute: Long Beach, CA, USA; NHLBI Obesity Education Initiative: Long Beach, CA, USA; North American Association for the Study of Obesity: Long Beach, CA, USA, 2000. [Google Scholar]
- Lacy, M.E.; Wellenius, G.A.; Carnethon, M.R.; Loucks, E.B.; Carson, A.P.; Luo, X.; Kiefe, C.I.; Gjelsvik, A.; Gunderson, E.P.; Eaton, C.B.; et al. Racial differences in the performance of existing risk prediction models for incident type 2 diabetes: The CARDIA study. Diabetes Care 2016, 39, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Ajlouni, K.; Khader, Y.; Batieha, A.; Jaddou, H.; El-Khateeb, M. An alarmingly high and increasing prevalence of obesity in Jordan. Epidemiol. Health 2020, 42, e2020040. [Google Scholar] [CrossRef] [PubMed]
- Khabazkhoob, M.; Emamian, M.H.; Hashemi, H.; Shariati, M.; Fotouhi, A. Prevalence of overweight and obesity in the middle-age population: A priority for the health system. Iran. J. Public Health 2017, 46, 827. [Google Scholar]
- Løvsletten, O.; Jacobsen, B.K.; Grimsgaard, S.; Njølstad, I.; Wilsgaard, T. Prevalence of general and abdominal obesity in 2015-2016 and 8-year longitudinal weight and waist circumference changes in adults and elderly: The Tromsø Study. BMJ Open 2020, 10, e038465. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Araújo, J.; Severo, M.; Correia, D.; Ramos, E.; Torres, D.; Lopes, C. Prevalence of general and abdominal obesity in Portugal: Comprehensive results from the National Food, nutrition and physical activity survey 2015–2016. BMC Public Health 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lee, Y.; Wu, Y.; Zhang, X.; Jin, C.; Huang, Z.; Wang, Y.; Wang, Z.; Kris-Etherton, P.; Wu, S.; et al. A prospective study of waist circumference trajectories and incident cardiovascular disease in China: The Kailuan Cohort Study. Am. J. Clin. Nutr. 2020, 113, 338–347. [Google Scholar] [CrossRef]
- Gurung, M.S.; Guwatudde, D.; Msaidié, M.; Houehanou, C.; Houinato, D.; Jorgensen, J.M.A.; Kagaruki, G.B.; Karki, K.B.; Labadarios, D.; Martins, J.S.; et al. Diabetes Prevalence and Its Relationship with Education, Wealth, and BMI in Twenty-Nine Low-and Middle-Income Countries. Diabetes Care 2020, 43, 1. [Google Scholar]
- Jeon, J.; Jung, K.J.; Jee, S.H. Waist circumference trajectories and risk of type 2 diabetes mellitus in Korean population: The Korean genome and epidemiology study (KoGES). BMC Public Health 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Laukkanen, J.A.; Li, Q.; Li, G. Body mass index is associated with type 2 diabetes mellitus in Chinese elderly. Clin. Interv. Aging 2017, 12, 745. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018, 3, 280–287. [Google Scholar] [CrossRef]
- Ganz, M.L.; Wintfeld, N.; Li, Q.; Alas, V.; Langer, J.; Hammer, M. The association of body mass index with the risk of type 2 diabetes: A case–control study nested in an electronic health records system in the United States. Diabetol. Metab. Syndr. 2014, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.C.; Cho, N.H.; Kim, J.H.; Hur, K.Y.; Jin, S.-M.; Lee, M.-K. Association of Body Mass Index with the Risk of Incident Type 2 Diabetes, Cardiovascular Disease, and All-Cause Mortality: A Community-Based Prospective Study. Endocrinol. Metab. 2020, 35, 416–424. [Google Scholar] [CrossRef]
- de Koning, L.; Merchant, A.T.; Pogue, J.; Anand, S.S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur. Heart J. 2007, 28, 850–856. [Google Scholar] [CrossRef]
- Feller, S.; Boeing, H.; Pischon, T. Body mass index, waist circumference, and the risk of type 2 diabetes mellitus: Implications for routine clinical practice. Dtsch. Ärzteblatt Int. 2010, 107, 470. [Google Scholar]
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 13 April 2021).
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes# (accessed on 10 November 2021).
- OECD. Is Cardiovascular Disease Slowing Improvements in Life Expectancy?: OECD and the King’s Fund... Workshop Proceedings; OECD: Paris, France, 2020. [Google Scholar]
- Bhagavathula, A.; Shehab, A.; Ullah, A.; Rahmani, J. The Burden of Cardiovascular Disease Risk Factors in the Middle East: A Systematic Review and Meta-Analysis Focusing on Primary Prevention. Curr. Vasc. Pharmacol. 2020, 19, 379–389. [Google Scholar] [CrossRef]
- Al-Sahouri, A.; Merrell, J.; Snelgrove, S. Barriers to good glycemic control levels and adherence to diabetes management plan in adults with Type-2 diabetes in Jordan: A literature review. Patient Prefer. Adherence 2019, 13, 675. [Google Scholar] [CrossRef] [Green Version]
- Jelwan, Y.A.; Asbeutah, A.A.A.; Welty, F.K. Comprehensive Review of Cardiovascular Diseases, Diabetes, and Hypercholesterolemia in Lebanon. Cardiol. Rev. 2020, 28, 73–83. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Nayak, B.S.; Sobrian, A.; Latiff, K.; Pope, D.; Rampersad, A.; Lourenço, K.; Samuel, N. The association of age, gender, ethnicity, family history, obesity and hypertension with type 2 diabetes mellitus in Trinidad. Diabetes Metab. Syndr. Clin. Res. Rev. 2014, 8, 91–95. [Google Scholar] [CrossRef]
- Silveira, E.A.; de Souza Rosa, L.P.; de Souza Cardoso, C.K.; Noll, M. Type 2 diabetes mellitus in class II and III obesity: Prevalence, associated factors, and correlation between glycemic parameters and body mass index. Int. J. Environ. Res. Public Health 2020, 17, 3930. [Google Scholar] [CrossRef]
- Martinell, M.; Pingel, R.; Hallqvist, J.; Dorkhan, M.; Groop, L.; Rosengren, A.; Storm, P.; Stålhammar, J. Education, immigration and income as risk factors for hemoglobin A1c > 70 mmol/mol when diagnosed with type 2 diabetes or latent autoimmune diabetes in adult: A population-based cohort study. BMJ Open Diabetes Res. Care 2017, 5, e000346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Shon, C. Relationship between socioeconomic status and type 2 diabetes: Results from Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2012. BMJ Open 2014, 4, e005710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toshihiro, á.; Saito, K.; Takikawa, S.; Takebe, N.; Onoda, T.; Satoh, J. Psychosocial factors are independent risk factors for the development of Type 2 diabetes in Japanese workers with impaired fasting glucose and/or impaired glucose tolerance 1. Diabet. Med. 2008, 25, 1211–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Y.; Guo, Y.; Zheng, Y.; Huang, Z.; Sun, S.; Kowal, P.; Shi, Y.; Wu, F. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: Results from SAGE Wave 1. BMC Public Health 2018, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rosengren, A.; Smyth, A.; Rangarajan, S.; Ramasundarahettige, C.; Bangdiwala, S.I.; AlHabib, K.F.; Avezum, A.; Boström, K.B.; Chifamba, J.; Gulec, S.; et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: The Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob. Health 2019, 7, e748–e760. [Google Scholar] [CrossRef] [Green Version]
- Geldsetzer, P.; Manne-Goehler, J.; Theilmann, M.; Davies, J.I.; Awasthi, A.; Danaei, G.; Gaziano, T.A.; Vollmer, S.; Jaacks, L.M.; Bärnighausen, T.; et al. Geographic and sociodemographic variation of cardiovascular disease risk in India: A cross-sectional study of 797,540 adults. PLoS Med. 2018, 15, e1002581. [Google Scholar] [CrossRef] [Green Version]
- İzmirli, M.; Göktekin, Ö.; Bacaksız, A.; Uysal, Ö.; Kılıç, Ü. The effect of the SIRT1 2827 A > G polymorphism, resveratrol, exercise, age and occupation in Turkish population with cardiovascular disease. Anatol. J. Cardiol. 2015, 15, 103. [Google Scholar] [CrossRef] [Green Version]
- Zaitsu, M.; Kato, S.; Kim, Y.; Takeuchi, T.; Sato, Y.; Kobayashi, Y.; Kawachi, I. Occupational class and risk of cardiovascular disease incidence in Japan: Nationwide, multicenter, hospital-based case-control study. J. Am. Heart Assoc. 2019, 8, e011350. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, W.; Yuan, Z.; Wang, S.-m.; Yi, X.; Jia, H.; Xue, F. Association between obesity indices and type 2 diabetes mellitus among middle-aged and elderly people in Jinan, China: A cross-sectional study. BMJ Open 2016, 6, e012742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, R.-N.; Zhao, C.; Wang, C.; Niu, Y.-C.; Li, K.; Guo, F.-C.; Li, S.-T.; Sun, C.-H.; Li, Y. BMI is strongly associated with hypertension, and waist circumference is strongly associated with type 2 diabetes and dyslipidemia, in northern Chinese adults. J. Epidemiol. 2012, 22, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Shi, L.; Jia, J.; Li, Y.; Yang, Q.; Ruan, Y.; Chen, R.; Kan, H. Differentiating the associations of waist circumference and body mass index with cardiovascular disease risk in a Chinese population. Asia Pac. J. Public Health 2015, 27, NP457–NP467. [Google Scholar] [CrossRef]
- Borel, A.-L.; Coumes, S.; Reche, F.; Ruckly, S.; Pépin, J.-L.; Tamisier, R.; Wion, N.; Arvieux, C. Waist, neck circumferences, waist-to-hip ratio: Which is the best cardiometabolic risk marker in women with severe obesity? The SOON cohort. PLoS ONE 2018, 13, e0206617. [Google Scholar] [CrossRef] [Green Version]
- Han, T.S.; Al-Gindan, Y.Y.; Govan, L.; Hankey, C.R.; Lean, M.E.J. Associations of BMI, waist circumference, body fat, and skeletal muscle with type 2 diabetes in adults. Acta Diabetol. 2019, 56, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Decoda Study Group; Nyamdorj, R. BMI compared with central obesity indicators in relation to diabetes and hypertension in Asians. Obesity 2008, 16, 1622–1635. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-C.; Arthur, R.; Iyengar, N.M.; Kamensky, V.; Xue, X.; Wassertheil-Smoller, S.; Allison, M.A.; Shadyab, A.H.; Wild, R.A.; Sun, Y.; et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur. Heart J. 2019, 40, 2849–2855. [Google Scholar] [CrossRef]
- Janssen, I.; Katzmarzyk, P.T.; Ross, R. Waist circumference and not body mass index explains obesity-related health risk. Am. J. Clin. Nutr. 2004, 79, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regi, M.; Sharma, N. Body Adiposity Index versus Body Mass Index and Other Anthropometric Traits as Correlates of Cardiovascular Disease. Int. J. Res. Sci. Innov. (IJRSI) 2016, 3, 2321–2705. [Google Scholar]
- Perea, V.; Jiménez, A.; Flores, L.; Ortega, E.; Coves, M.J.; Vidal, J. Anthropometric indexes outperform bioelectrical impedance analysis-derived estimates of body composition in identification of metabolic abnormalities in morbid obesity. Surg. Obes. Relat. Dis. 2013, 9, 648–652. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.-H.; Han, S. Underweight: Another risk factor for cardiovascular disease?: A cross-sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine 2017, 96, 48. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Chou, Y.-C.; Huang, N.; Chou, Y.-J.; Hu, H.-Y.; Li, C.-P. Association of body mass index with all-cause and cardiovascular disease mortality in the elderly. PLoS ONE 2014, 9, e102589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tennant, I.A.; Barnett, A.T.; Thompson, D.S.; Kips, J.; Boyne, M.S.; Chung, E.E.; Chung, A.P.; Osmond, C.; Hanson, M.A.; Gluckman, P.D.; et al. Impaired cardiovascular structure and function in adult survivors of severe acute malnutrition. Hypertension 2014, 6, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Grey, K.; Gonzales, G.B.; Abera, M.; Lelijveld, N.; Thompson, D.; Berhane, M.; Abdissa, A.; Girma, T.; Kerac, M. Severe malnutrition or famine exposure in childhood and cardiometabolic non-communicable disease later in life: A systematic review. BMJ Glob. Health 2021, 6, e003161. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total | Male | p-Value | Female | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Under wt | Normal wt | Over wt | Obese | Under wt | Normal wt | Over wt | Obese | ||||
| Age (mean ± SD) | 41.5 ± 14.7 | 30.0 ± 13.6 | 35.8 ± 16.2 | 43.0 ± 16.0 | 47.9 ± 14.6 | <0.001 | 28.0 ± 9.8 | 33.0 ± 12.6 | 40.6 ± 13.6 | 47.0 ± 12.4 | <0.001 |
| Age | |||||||||||
| ≤20 | 389 (6.5) | 9 (25.7) | 71 (15.3) | 25 (4.5) | 14 (2.7) | <0.001 | 25 (21.4) | 141 (13.0) | 70 (5.7) | 34 (1.7) | <0.001 |
| 21–30 | 1309 (21.8) | 14 (40.0) | 171 (36.8) | 121 (21.6) | 63 (12.1) | 62 (53.0) | 435 (40.0) | 256 (20.9) | 187 (9.4) | ||
| 31–40 | 1183 (19.7) | 4 (11.4) | 71 (15.3) | 119 (21.3) | 77 (14.8) | 17 (14.5) | 237 (21.8) | 304 (24.8) | 354 (17.8) | ||
| 41–50 | 1394 (23.2) | 6 (17.1) | 55 (11.8) | 120 (21.4) | 150 (28.7) | 9 (7.7) | 168 (15.4) | 296 (24.2) | 590 (29.7) | ||
| 51–60 | 1094 (18.2) | 0 (0.0) | 47 (10.1) | 85 (15.2) | 110 (21.1) | 2 (1.7) | 69 (6.3) | 201 (16.4) | 580 (29.2) | ||
| >60 | 631 (10.5) | 2 (5.7) | 50 (10.8) | 90 (16.1) | 108 (20.7) | 2 (1.7) | 38 (3.5) | 98 (8.0) | 243 (12.2) | ||
| Income Level (JOD) 2 | |||||||||||
| <350 | 2332 (38.9) | 8 (22.9) | 134 (28.8) | 171 (30.5) | 175 (33.5) | 0.042 | 47 (40.2) | 401 (36.9) | 475 (38.8) | 921 (46.3) | <0.001 |
| 350–799 | 2926 (48.8) | 16 (45.7) | 247 (53.1) | 285 (50.9) | 278 (53.3) | 58 (49.6) | 527 (48.4) | 611 (49.9) | 904 (45.5) | ||
| ≥800 | 742 (12.4) | 11 (31.4) | 84 (18.1) | 104 (18.6) | 69 (13.2) | 12 (10.3) | 160 (14.7) | 139 (11.3) | 163 (8.2) | ||
| Job Nature | |||||||||||
| Administrative | 846 (14.1) | 6 (17.1) | 86 (18.5) | 122 (21.8) | 129 (24.7) | 0.11 | 9 (7.7) | 139 (12.8) | 171 (14.0) | 184 (9.3) | <0.001 |
| Non-administrative | 5154 (85.9) | 29 (82.9) | 379 (81.5) | 438 (78.2) | 393 (75.3) | 108 (92.3) | 949 (87.2) | 1054 (86.0) | 1804 (90.7) | ||
| Educational Level | |||||||||||
| <High School | 437 (7.3) | 1 (2.9) | 27 (5.8) | 31 (5.5) | 40 (7.7) | 0.082 | 5 (4.3) | 60 (5.5) | 96 (7.8) | 177 (8.9) | <0.001 |
| High School | 3396 (56.6) | 20 (57.1) | 242 (52.0) | 308 (55.0) | 307 (58.8) | 53 (45.3) | 520 (47.8) | 639 (52.2) | 1307 (65.7) | ||
| University | 2019 (33.7) | 14 (40.0) | 184 (39.6) | 196 (35.0) | 158 (30.3) | 56 (47.9) | 481 (44.2) | 459 (37.5) | 471 (23.7) | ||
| Postgraduate | 148 (2.5) | 0 (0.0) | 12 (2.6) | 25 (4.5) | 17 (3.3) | 3 (2.6) | 27 (2.5) | 31 (2.5) | 33 (1.7) | ||
| Variable | Total 2 | Male | p-Value | Female | p-Value | ||
|---|---|---|---|---|---|---|---|
| Enlarged WC 2 | Normal WC | Enlarged WC | Normal WC | ||||
| Age (mean ± SD) | 49.3 ± 14.3 | 39.5 ± 16.3 | <0.001 | 46.4 ± 12.5 | 36.2 ± 13.7 | <0.001 | |
| Age | |||||||
| ≤20 | 384 (6.5) | 6 (1.4) | 111 (9.8) | <0.001 | 38 (1.8) | 229 (10.6) | <0.001 |
| 21–30 | 1288 (21.8) | 44 (10.3) | 323 (28.4) | 227 (10.5) | 694 (32.0) | ||
| 31–40 | 1161 (19.7) | 61 (14.3) | 206 (18.1) | 407 (18.8) | 487 (22.4) | ||
| 41–50 | 1375 (23.3) | 126 (29.4) | 200 (17.6) | 648 (29.9) | 401 (18.5) | ||
| 51–60 | 1075 (18.2) | 92 (21.5) | 149 (13.1) | 596 (27.5) | 238 (11.0) | ||
| >60 | 621 (10.5) | 99 (23.1) | 147 (12.9) | 254 (11.7) | 121 (5.6) | ||
| Income Level (JOD) 3 | |||||||
| <350 | 2296 (38.9) | 153 (35.7) | 328 (28.9) | 0.026 | 997 (45.9) | 818 (37.7) | <0.001 |
| 350–799 | 2878 (48.7) | 204 (47.7) | 615 (54.1) | 990 (45.6) | 1069 (49.3) | ||
| ≥800 | 730 (12.4) | 71 (16.6) | 193 (17.0) | 183 (8.4) | 283 (13.0) | ||
| Job Nature | |||||||
| Administrative | 837 (14.2) | 105 (24.5) | 236 (20.8) | 0.114 | 233 (10.7) | 263 (12.1) | 0.166 |
| Non-administrative | 5067 (85.8) | 323 (75.5) | 900 (79.2) | 1937 (89.3) | 1907 (87.9) | ||
| Educational Level | |||||||
| <High School | 433 (7.3) | 23 (5.4) | 76 (6.7) | 0.017 | 195 (9.0) | 139 (6.4) | <0.001 |
| High School | 3338 (56.5) | 255 (59.6) | 610 (53.7) | 1392 (64.1) | 1081 (49.8) | ||
| University | 1985 (33.6) | 129 (30.1) | 417 (36.7) | 548 (25.3) | 891 (41.1) | ||
| Postgraduate | 148 (2.5) | 21 (4.9) | 33 (2.9) | 35 (1.6) | 59 (2.7) | ||
| Variable | Total | Male | p-Value | Female | p-Value | ||
|---|---|---|---|---|---|---|---|
| NFG 2 | T2D 2 | NFG | T2D | ||||
| BMI Classification | |||||||
| Underweight | 152 (2.53) | 35 (2.5) | 0 (0.0) | <0.001 | 116 (3.0) | 1 (0.2) | <0.001 |
| Normal weight | 1553 (25.9) | 436 (31.7) | 29 (14.1) | 1037 (26.9) | 51 (9.1) | ||
| Overweight | 1785 (29.8) | 477 (34.7) | 83 (40.3) | 1086 (28.1) | 139 (24.9) | ||
| Obese | 2510 (41.8) | 428 (31.1) | 94 (45.6) | 1620 (42.0) | 368 (65.8) | ||
| WC 3 | |||||||
| Normal | 3306 (56.0) | 1022 (75.1) | 114 (56.2) | <0.001 | 2008 (52.9) | 162 (29.6) | <0.001 |
| Enlarged | 2598 (44.0) | 339 (24.9) | 89 (43.8) | 1785 (47.1) | 385 (70.4) | ||
| Age | |||||||
| ≤20 | 389 (6.5) | 119 (8.6) | 0 (0.0) | <0.001 | 270 (7.0) | 0 (0.0) | <0.001 |
| 21–30 | 1309 (21.8) | 364 (26.5) | 5 (2.4) | 927 (24.0) | 13 (2.3) | ||
| 31–40 | 1183 (19.7) | 261 (19.0) | 10 (4.9) | 875 (22.7) | 37 (6.6) | ||
| 41–50 | 1394 (23.2) | 288 (20.9) | 43 (20.9) | 972 (25.2) | 91 (16.3) | ||
| 51–60 | 1094 (18.2) | 186 (13.5) | 56 (27.2) | 595 (15.4) | 257 (46.0) | ||
| >60 | 631 (10.5) | 158 (11.5) | 92 (44.7) | 220 (5.7) | 161 (28.8) | ||
| Income Level (JOD) 4 | |||||||
| <350 | 2332 (38.9) | 407 (29.6) | 81 (39.3) | 0.001 | 1579 (40.9) | 265 (47.4) | <0.001 |
| 350–799 | 2926 (48.8) | 743 (54.0) | 83 (40.3) | 1835 (47.6) | 265 (47.4) | ||
| ≥800 | 742 (12.4) | 226 (16.4) | 42 (20.4) | 445 (11.5) | 29 (5.2) | ||
| Job Nature | |||||||
| Administrative | 846 (14.1) | 315 (22.9) | 28 (13.6) | 0.002 | 481 (12.5) | 22 (3.9) | <0.001 |
| Non-administrative | 5154 (85.9) | 1061 (77.1) | 178 (86.4) | 3378 (87.5) | 537 (96.1) | ||
| Educational Level | |||||||
| <High School | 437 (7.3) | 76 (5.5) | 23 (11.2) | 0.001 | 259 (6.7) | 79 (14.1) | <0.001 |
| High School | 3396 (56.6) | 751 (54.6) | 126 (61.2) | 2130 (55.2) | 389 (69.6) | ||
| University | 2019 (33.7) | 501 (36.4) | 51 (24.8) | 1378 (35.7) | 89 (15.9) | ||
| Postgraduate | 148 (2.5) | 48 (3.5) | 6 (2.9) | 92 (2.4) | 2 (0.4) | ||
| Variable | Total | Male | p-Value | Female | p-Value | ||
|---|---|---|---|---|---|---|---|
| Presence 2 | Absence 2 | Presence | Absence | ||||
| BMI Classification | |||||||
| Underweight | 152 (2.53) | 0 (0.0) | 35 (2.4) | <0.001 | 1 (0.4) | 116 (2.8) | <0.001 |
| Normal weight | 1553 (25.9) | 24 (20.0) | 441 (30.2) | 43 (16.8) | 1045 (25.1) | ||
| Overweight | 1785 (29.8) | 35 (29.2) | 525 (35.9) | 40 (15.6) | 1185 (28.5) | ||
| Obese | 2510 (41.8) | 61 (50.8) | 461 (31.5) | 172 (67.2) | 1816 (43.6) | ||
| WC 3 | |||||||
| Normal | 3306 (56.0) | 57 (47.9) | 1079 (74.7) | <0.001 | 68 (27.3) | 2102 (51.4) | <0.001 |
| Enlarged | 2598 (44.0) | 62 (52.1) | 366 (25.3) | 181 (72.7) | 1989 (48.6) | ||
| Age | |||||||
| ≤20 | 389 (6.5) | 4 (3.3) | 115 (7.9) | <0.001 | 3 (1.20) | 267 (6.40) | <0.001 |
| 21–30 | 1309 (21.8) | 5 (4.2) | 364 (24.9) | 21 (8.20) | 919 (22.1) | ||
| 31–40 | 1183 (19.7) | 8 (6.7) | 263 (18.0) | 34 (13.3) | 878 (21.1) | ||
| 41–50 | 1394 (23.2) | 15 (12.5) | 316 (21.6) | 52 (20.3) | 1011 (24.3) | ||
| 51–60 | 1094 (18.2) | 33 (27.5) | 209 (14.3) | 82 (32.0) | 770 (18.5) | ||
| >60 | 631 (10.5) | 55 (45.8) | 195 (13.3) | 64 (25.0) | 317 (7.6) | ||
| Income Level (JOD) 4 | |||||||
| <350 | 2332 (38.9) | 47 (39.2) | 441 (30.2) | 0.036 | 147 (57.4) | 1697 (40.8) | <0.001 |
| 350–799 | 2926 (48.8) | 61 (50.8) | 765 (52.3) | 96 (37.5) | 2004 (48.1) | ||
| ≥800 | 742 (12.4) | 12 (10.0) | 256 (17.5) | 13 (5.10) | 461 (11.1) | ||
| Job Nature | |||||||
| Administrative | 846 (14.1) | 15 (12.5) | 328 (22.4) | 0.011 | 12 (4.70) | 491 (11.8) | <0.001 |
| Non-administrative | 5154 (85.9) | 105 (87.5) | 1134 (77.6) | 244 (95.3) | 3671 (88.2) | ||
| Educational Level | |||||||
| <High School | 437 (7.3) | 6 (5.0) | 93 (6.4) | 0.116 | 26 (10.2) | 312 (7.5) | <0.001 |
| High School | 3396 (56.6) | 79 (65.8) | 798 (54.6) | 177 (69.1) | 2342 (56.3) | ||
| University | 2019 (33.7) | 31 (25.8) | 521 (35.6) | 53 (20.7) | 1414 (34.0) | ||
| Postgraduate | 148 (2.5) | 4 (3.3) | 50 (3.4) | 0 (0.0) | 94 (2.3) | ||
| Model | Variable | All Population | p-Value | Male | p-Value | Female | p-Value |
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||
| Model # 1 | |||||||
| BMI classification | |||||||
| <25 kg/m2 | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 | |
| ≥25 kg/m2 | 2.9 (2.30–3.80) | 2.5 (1.60–3.81) | 3.1 (2.26–4.35) | ||||
| WC 2 | |||||||
| Normal | Reference | <0.001 | Reference | 0.001 | Reference | <0.001 | |
| Enlarged | 1.7 (1.46–2.06) | 1.8 (1.28–2.44) | 1.8 (1.49–2.26) | ||||
| Model # 2 | |||||||
| BMI classification | |||||||
| <25 kg/m2 | Reference | <0.001 | Reference | 0.014 | Reference | 0.005 | |
| ≥25 kg/m2 | 1.8 (1.32–2.32) | 1.8 (1.12–2.85) | 1.7 (1.17–2.39) | ||||
| WC | |||||||
| Normal | Reference | 0.007 | Reference | 0.117 | Reference | 0.059 | |
| Enlarged | 1.3 (1.07–1.56) | 1.3 (0.90–1.81) | 1.3 (0.99–1.58) | ||||
| Model # 3 | |||||||
| BMI classification | |||||||
| <25 kg/m2 | Reference | <0.001 | Reference | 0.013 | Reference | 0.005 | |
| ≥25 kg/m2 | 1.8 (1.32–2.34) | 1.8 (1.14–2.88) | 1.7 (1.17–2.38) | ||||
| WC | |||||||
| Normal | Reference | 0.010 | Reference | 0.167 | Reference | 0.061 | |
| Enlarged | 1.3 (1.06–1.55) | 1.3 (0.90–1.82) | 1.3 (0.99–1.57) | ||||
| Model # 4 | |||||||
| BMI classification | |||||||
| <25 kg/m2 | Reference | <0.001 | Reference | 0.011 | Reference | 0.008 | |
| ≥25 kg/m2 | 1.7 (1.30–2.30) | 1.8 (1.15–2.94) | 1.6 (1.13–2.33) | ||||
| WC | |||||||
| Normal | Reference | 0.016 | Reference | 0.18 | Reference | 0.109 | |
| Enlarged | 1.3 (1.04–1.52) | 1.3 (0.89–1.82) | 1.2 (0.96–1.53) |
| Model | Variable | All Population | p-Value | Male | p-Value | Female | p-Value |
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||
| Model # 1 | |||||||
| BMI classification | |||||||
| <25 kg/m2 | Reference | 0.383 | Reference | 0.44 | Reference | 0.818 | |
| ≥25 kg/m2 | 1.2 (0.84–1.57) | 1.2 (0.73–2.07) | 1.1 (0.71–1.55) | ||||
| WC 2 | |||||||
| Normal | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 | |
| Enlarged | 2.5 (1.93–3.20) | 3.0 (1.95–4.53) | 2.8 (1.98–3.84) | ||||
| Model # 2 | |||||||
| BMI classification | |||||||
| <25 kg/m2 | Reference | 0.078 | Reference | 0.595 | Reference | 0.079 | |
| ≥25 kg/m2 | 0.8 (0.54–1.03) | 0.9 (0.50–1.48) | 0.7 (0.46–1.04) | ||||
| WC | |||||||
| Normal | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 | |
| Enlarged | 2.0 (1.55–2.59) | 2.3 (1.50–3.56) | 2.2 (1.54–3.01) | ||||
| Model # 3 | |||||||
| BMI classification | |||||||
| <25 kg/m2 | Reference | 0.087 | Reference | 0.642 | Reference | 0.079 | |
| ≥25 kg/m2 | 0.8 (0.54–1.04) | 0.9 (0.51–1.51) | 0.7 (0.46–1.04) | ||||
| WC | |||||||
| Normal | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 | |
| Enlarged | 2.0 (1.53–2.57) | 2.3 (1.51–3.61) | 2.2 (1.54–3.02) | ||||
| Model # 4 | |||||||
| BMI classification | |||||||
| <25 kg/m2 | Reference | 0.75 | Reference | 0.631 | Reference | 0.066 | |
| ≥25 kg/m2 | 0.7 (0.54–1.03) | 0.9 (0.51–1.51) | 0.7 (0.45–1.03) | ||||
| WC | |||||||
| Normal | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 | |
| Enlarged | 1.9 (1.47–2.47) | 2.2 (1.45–1.48) | 2.1 (1.48–2.92) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhalidy, H.; Orabi, A.; Alnaser, K.; Al-Shami, I.; Alzboun, T.; Obeidat, M.D.; Liu, D. Obesity Measures as Predictors of Type 2 Diabetes and Cardiovascular Diseases among the Jordanian Population: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 12187. https://doi.org/10.3390/ijerph182212187
Alkhalidy H, Orabi A, Alnaser K, Al-Shami I, Alzboun T, Obeidat MD, Liu D. Obesity Measures as Predictors of Type 2 Diabetes and Cardiovascular Diseases among the Jordanian Population: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2021; 18(22):12187. https://doi.org/10.3390/ijerph182212187
Chicago/Turabian StyleAlkhalidy, Hana, Aliaa Orabi, Khadeejah Alnaser, Islam Al-Shami, Tamara Alzboun, Mohammad D. Obeidat, and Dongmin Liu. 2021. "Obesity Measures as Predictors of Type 2 Diabetes and Cardiovascular Diseases among the Jordanian Population: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 18, no. 22: 12187. https://doi.org/10.3390/ijerph182212187
APA StyleAlkhalidy, H., Orabi, A., Alnaser, K., Al-Shami, I., Alzboun, T., Obeidat, M. D., & Liu, D. (2021). Obesity Measures as Predictors of Type 2 Diabetes and Cardiovascular Diseases among the Jordanian Population: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 18(22), 12187. https://doi.org/10.3390/ijerph182212187







