Short-Term Cumulative Exposure to Ambient Traffic-Related Black Carbon and Blood Pressure: MMDA Traffic Enforcers’ Health Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Site
2.2. Data Collection
2.2.1. Health Assessment and Self-Administered Questionnaire
2.2.2. Blood Pressure Measurement
2.2.3. Black Carbon and Meteorological Measurement
2.3. Data Analysis
3. Results
3.1. Demographic Profile and Health Assessment
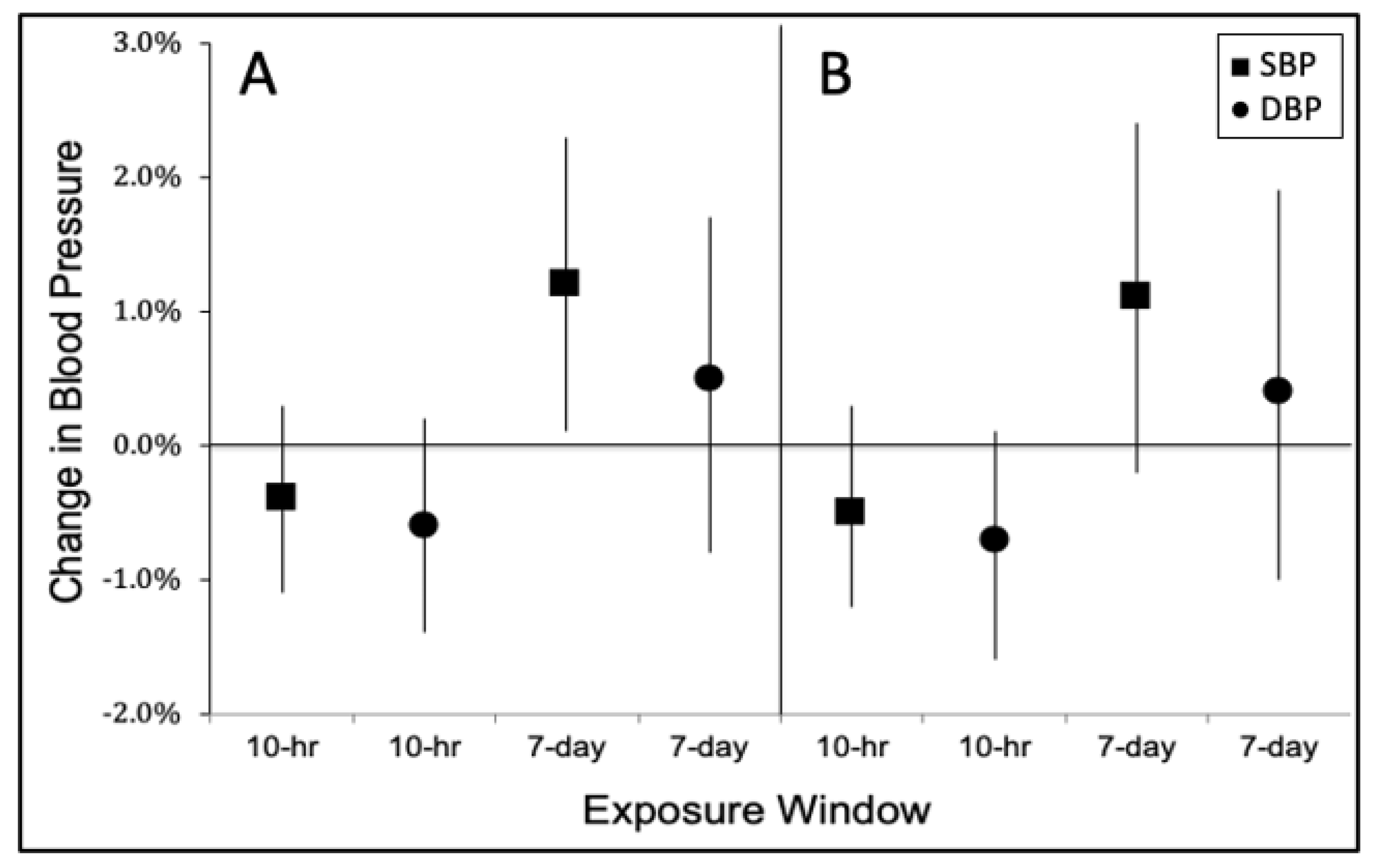
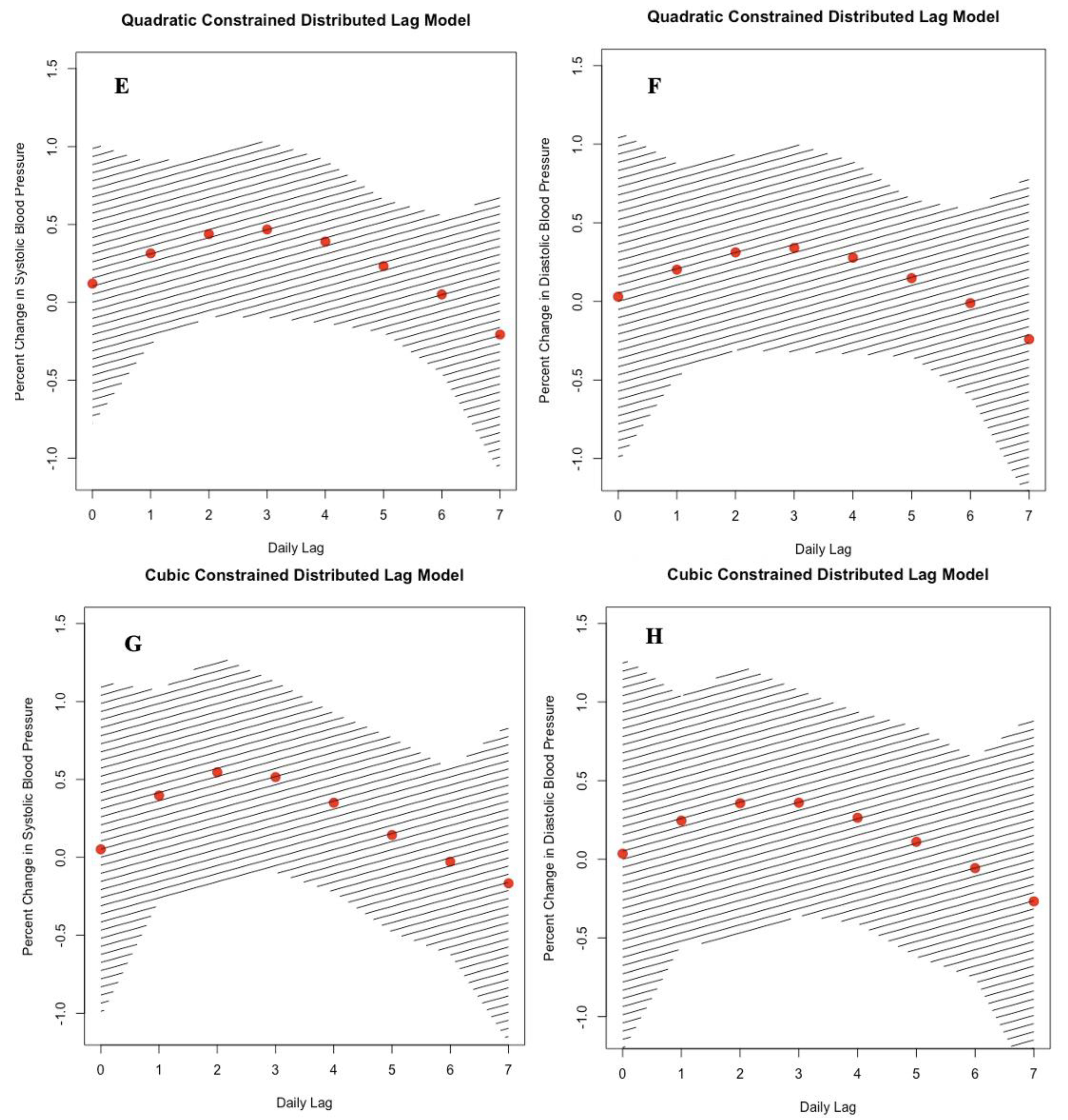
3.2. Effect of Black Carbon on Blood Pressure
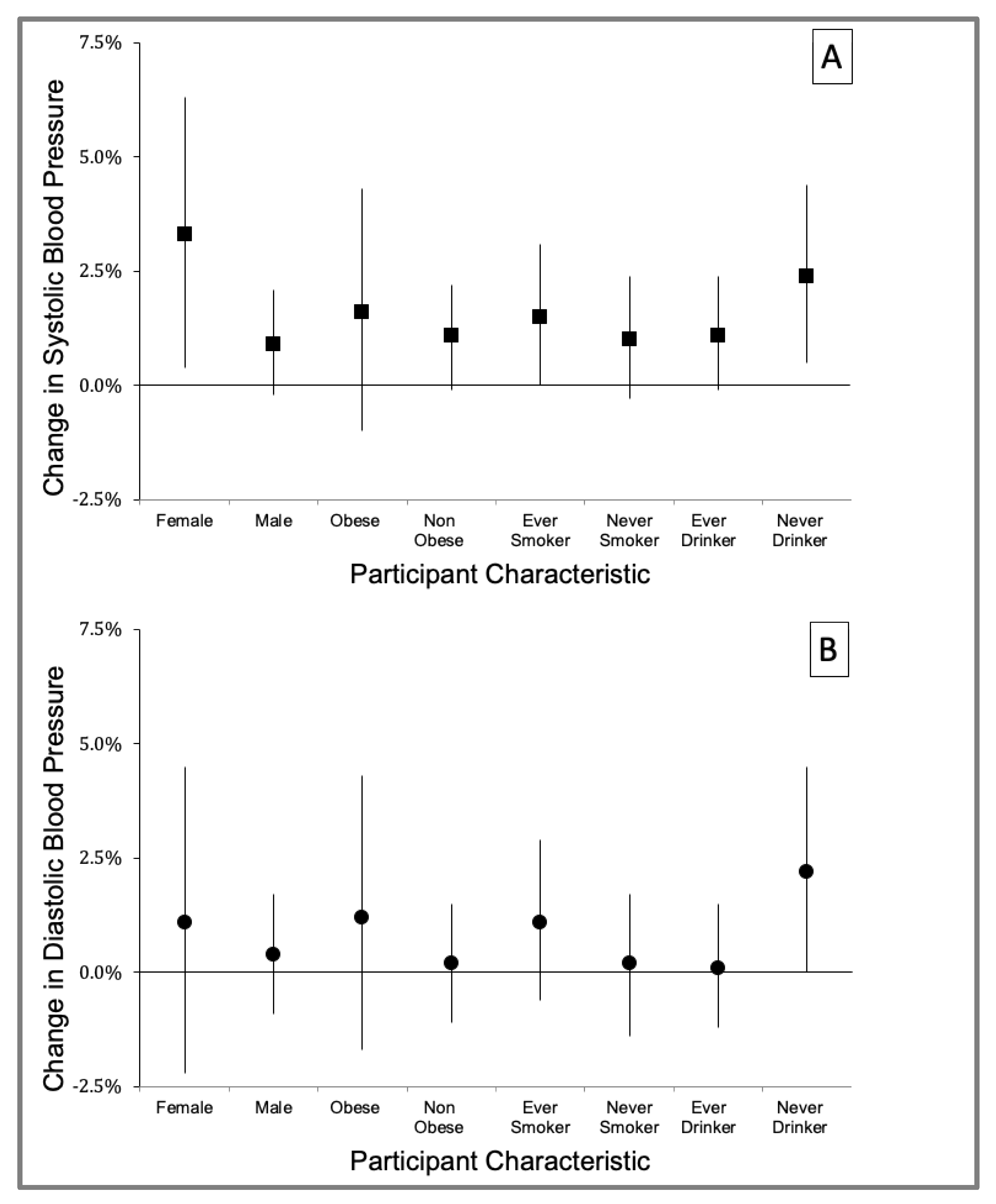
3.3. Effect Modification by Participant Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F. Global, regional, and national comparative risk assessment of behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. IARC evaluation of the carcinogenicity of outdoor air pollution. Environ. Risques St. 2014, 13, 347–352. [Google Scholar]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Borduas, N.; Donahue, N.M. The natural atmosphere. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 131–150. [Google Scholar]
- U.S. EPA. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2009); U.S. Environmental Protection Agenc: Washington, DC, USA, 2009.
- Luben, T.J.; Nichols, J.L.; Dutton, S.J.; Kirrane, E.; Owens, E.O.; Datko-Williams, L.; Madden, M.; Sacks, J. A systematic review of cardiovascular emergency department visits, hospital admissions and mortality associated with ambient black carbon. Environ. Int. 2017, 107, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Apte, K.; Salvi, S. Household air pollution and its effects on health. F1000Research 2016, 5, 2593. [Google Scholar] [CrossRef] [PubMed]
- Autrup, H. Ambient Air Pollution and Adverse Health Effects. Procedia Soc. Behav. Sci. 2010, 2, 7333–7338. [Google Scholar] [CrossRef][Green Version]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Franchini, M. Health effects of ambient air pollution in developing countries. Int. J. Environ. Res. Public Health 2017, 14, 1048. [Google Scholar] [CrossRef] [PubMed]
- Apergis, N.; Gupta, R.; Lau, C.K.M.; Mukherjee, Z. US state-level carbon dioxide emissions: Does it affect health care expendture? Renew. Sustain. Energy Rev. 2018, 91, 521–530. [Google Scholar] [CrossRef]
- Cao, C.; Cui, X.; Cai, W.; Wang, C.; Xing, L.; Zhang, N.; Shen, S.; Bai, Y.; Deng, Z. Incorporating health co-benefits into regional carbon emission reduction policy making: A case study of China’s power sector. Appl. Energy 2019, 253. [Google Scholar] [CrossRef]
- Kwan, S.C.; Tainio, M.; Woodcock, J.; Sutan, R.; Hashim, J.H. The carbon savings and health co-benefits from the introduction of mass rapid transit system in Greater Kuala Lumpur, Malaysia. J. Transp. Health 2017, 6, 187–200. [Google Scholar] [CrossRef]
- Bayat, R.; Ashrafi, K.; Motlagh, M.S.; Hassanvand, M.S.; Daroudi, R.; Fink, G.; Künzli, N. Health impact and related cost of ambient air pollution in Tehran. Environ. Res. 2019, 176, 108547. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A., III; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef]
- Almetwally, A.A.; Bin-Jumah, M.; Allam, A. Ambient air pollution and its influence on human health and welfare: An overview. Environ. Sci. Pollut. Res. 2020, 27, 24815–24830. [Google Scholar] [CrossRef]
- Karl, T.R.; Melillo, J.M.; Peterson, T.C.; Hassol, S.J. Global Climate Change Impacts in the United States; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Eze, I.C.; Schaffner, E.; Fischer, E.; Schikowski, T.; Adam, M.; Imboden, M.; Tsai, M.; Carballo, D.; von Eckardstein, A.; Künzli, N. Long-term air pollution exposure and diabetes in a population-based Swiss cohort. Environ. Int. 2014, 70, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Rabito, F.A.; Yang, Q.; Zhang, H.; Werthmann, D.; Shankar, A.; Chillrud, S. The association between short-term residential black carbon concentration on blood pressure in a general population sample. Indoor Air 2020, 30, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, S.; Baumgartner, J.; Weichenthal, S. Impacts of exposure to black carbon, elemental carbon, and ultrafine par-ticles from indoor and outdoor sources on blood pressure in adults: A review of epidemiological evidence. Environ. Res. 2018, 161, 345–353. [Google Scholar] [CrossRef]
- Segersson, D.; Eneroth, K.; Gidhagen, L.; Johansson, C.; Omstedt, G.; Nylén, A.E.; Forsberg, B. Health Impact of PM10, PM2.5 and Black Carbon Exposure Due to Different Source Sectors in Stockholm, Gothenburg and Umea, Sweden. Int. J. Environ. Res. Public Health 2017, 14, 742. [Google Scholar] [CrossRef]
- Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and Temporal Variation in PM2.5 Chemical Composition in the United States for Health Effects Studies. Environ. Health Perspect. 2007, 115, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.; Hoek, G.; Simic-Lawson, M.; Fischer, P.; Van Bree, L.; Brink, H.T.; Keuken, M.; Atkinson, R.; Anderson, H.R.; Brunekreef, B.; et al. Black Carbon as an Additional Indicator of the Adverse Health Effects of Airborne Particles Compared with PM10and PM2.5. Environ. Health Perspect. 2011, 119, 1691–1699. [Google Scholar] [CrossRef]
- Louwies, T.; Nawrot, T.; Cox, B.; Dons, E.; Penders, J.; Provost, E.; Panis, L.I.; De Boever, P. Blood pressure changes in association with black carbon exposure in a panel of healthy adults are independent of retinal microcirculation. Environ. Int. 2015, 75, 81–86. [Google Scholar] [CrossRef]
- Alas, H.D.; Müller, T.; Birmili, W.; Kecorius, S.; Cambaliza, M.O.; Simpas, J.B.B.; Cayetano, M.; Weinhold, K.; Vallar, E.; Galvez, M.C.; et al. Spatial Characterization of Black Carbon Mass Concentration in the Atmosphere of a Southeast Asian Megacity: An Air Quality Case Study for Metro Manila, Philippines. Aerosol Air Qual. Res. 2018, 18, 2301–2317. [Google Scholar] [CrossRef]
- Kecorius, S.; Madueño, L.; Vallar, E.; Alas, H.; Betito, G.; Birmili, W.; Cambaliza, M.O.; Catipay, G.; Gonzaga-Cayetano, M.; Galvez, M.C.; et al. Aerosol particle mixing state, refractory particle number size distributions and emission factors in a polluted urban environment: Case study of Metro Manila, Philippines. Atmos. Environ. 2017, 170, 169–183. [Google Scholar] [CrossRef]
- Kirrane, E.F.; Luben, T.; Benson, A.; Owens, E.; Sacks, J.; Dutton, S.; Madden, M.; Nichols, J. A systematic review of cardiovascular responses associated with ambient black carbon and fine particulate matter. Environ. Int. 2019, 127, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Biel, R.; Danieli, C.; Shekarrizfard, M.; Minet, L.; Abrahamowicz, M.; Baumgartner, J.; Liu, R.; Hatzopoulou, M.; Weichenthal, S. Acute cardiovascular health effects in a panel study of personal exposure to traffic-related air pollutants and noise in Toronto, Canada. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Zhang, Y.; Schauer, J.J.; Huang, W.; Wang, Y.; Ezzati, M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc. Natl. Acad. Sci. USA 2014, 111, 13229–13234. [Google Scholar] [CrossRef] [PubMed]
- Witters, K.; Dockx, Y.; Roodt, J.O.; Lefebvre, W.; Vanpoucke, C.; Plusquin, M.; Vangronsveld, J.; Janssen, B.G.; Nawrot, T.S. Dynamics of skin microvascular blood flow in 4–6-year-old children in association with pre- and postnatal black carbon and particulate air pollution exposure. Environ. Int. 2021, 157, 106799. [Google Scholar] [CrossRef]
- Lin, C.; Hu, D.; Jia, X.; Chen, J.; Deng, F.; Guo, X.; Heal, M.; Cowie, H.; Wilkinson, P.; Miller, M.R.; et al. The relationship between personal exposure and ambient PM2.5 and black carbon in Beijing. Sci. Total Environ. 2020, 737, 139801. [Google Scholar] [CrossRef]
- Yang, J.; Sakhvidi, M.J.Z.; de Hoogh, K.; Vienneau, D.; Siemiatyck, J.; Zins, M.; Goldberg, M.; Chen, J.; Lequy, E.; Jacquemin, B. Long-term exposure to black carbon and mortality: A 28-year follow-up of the GAZEL cohort. Environ. Int. 2021, 157, 106805. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Xu, Y.; Chen, M.; Zhang, H.; Huang, X.; Li, Z.; Pan, B.; Peng, R.; Zhu, Y.; Kan, H.; et al. Exposure to different fractions of diesel exhaust PM2.5 induces different levels of pulmonary inflammation and acute phase response. Ecotoxicol. Environ. Saf. 2021, 210, 111871. [Google Scholar] [CrossRef] [PubMed]
- Mordukhovich, I.; Wilker, E.; Suh, H.; Wright, R.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Black Carbon Exposure, Oxidative Stress Genes, and Blood Pressure in a Repeated-Measures Study. Environ. Health Perspect. 2009, 117, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Dubowsky, S.D.; Suh, H.; Schwartz, J.; Coull, B.A.; Gold, D.R. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ. Health Perspect. 2006, 114, 992–998. [Google Scholar] [CrossRef]
- Rückerl, R.; Ibald-Mulli, A.; Koenig, W.; Schneider, A. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am. J. Respir. Crit. Care Med. 2006, 173, 432. [Google Scholar] [CrossRef]
- Park, S.K.; O’Neill, M.S.; Vokonas, P.S.; Sparrow, D.; Schwartz, J. Effects of Air Pollution on Heart Rate Variability: The VA Normative Aging Study. Environ. Health Perspect. 2005, 113, 304–309. [Google Scholar] [CrossRef]
- Brook, R.D.; Sun, Z.; Brook, J.R.; Zhao, X.; Ruan, Y.; Yan, J.; Mukherjee, B.; Rao, X.; Duan, F.; Sun, L. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: The air pollution and cardiometabolic disease study. Hypertension 2016, 67, 77–85. [Google Scholar] [CrossRef]
- Environmental Management Bureau, E. Air Quality; Department of Environment and Natural Resources: Manila, Philippines, 2015.
- Pearl, J. Causal diagrams for empirical research. Biometrika 1995, 82, 669–688. [Google Scholar] [CrossRef]
- Fitzmaurice, G.M.; Laird, N.M.; Ware, J.H. Applied Lngitudinal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 998. [Google Scholar]
- Baja, E.S.; Schwartz, J.D.; Wellenius, G.; Coull, B.A.; Zanobetti, A.; Vokonas, P.S.; Suh, H. Traffic-Related Air Pollution and QT Interval: Modification by Diabetes, Obesity, and Oxidative Stress Gene Polymorphisms in the Normative Aging Study. Environ. Health Perspect. 2010, 118, 840–846. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. 2009, 3, 332–350. [Google Scholar] [CrossRef]
- Ding, N.; Wang, X.; Weisskopf, M.G.; Sparrow, D.; Schwartz, J.; Hu, H.; Park, S.K. Lead-Related Genetic Loci, Cumulative Lead Exposure and Incident Coronary Heart Disease: The Normative Aging Study. PLoS ONE 2016, 11, e0161472. [Google Scholar] [CrossRef]
- Rapisarda, V.; Ledda, C.; Ferrante, M.; Fiore, M.; Cocuzza, S.; Bracci, M.; Fenga, C. Blood pressure and occupational ex-posure to noise and lead (Pb) A cross-sectional study. Toxicol. Ind. Health 2016, 32, 1729–1736. [Google Scholar] [CrossRef]
- Wilker, E.H.; Baccarelli, A.; Suh, H.; Vokonas, P.; Wright, R.O.; Schwartz, J. Black Carbon Exposures, Blood Pressure, and Interactions with Single Nucleotide Polymorphisms in MicroRNA Processing Genes. Environ. Health Perspect. 2010, 118, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Nwanaji-Enwerem, J.C.; Wang, W.; Nwanaji-Enwerem, O.; Vokonas, P.; Baccarelli, A.; Weisskopf, M.; Herndon, L.W.; Wiggs, J.L.; Park, S.K.; Schwartz, J. Association of Long-term Ambient Black Carbon Exposure and Oxidative Stress Allelic Variants With Intraocular Pressure in Older Men. JAMA Ophthalmol. 2019, 137, 129. [Google Scholar] [CrossRef]
- Hudda, N.; Eliasziw, M.; Hersey, S.O.; Reisner, E.; Brook, R.D.; Zamore, W.; Durant, J.L.; Brugge, D. Effect of Reducing Ambient Traffic-Related Air Pollution on Blood Pressure: A Randomized Crossover Trial. Hypertension 2021, 77, 823–832. [Google Scholar] [CrossRef]
- Mann, J.K.; Lutzker, L.; Holm, S.M.; Margolis, H.G.; Neophytou, A.M.; Eisen, E.A.; Costello, S.; Tyner, T.; Holland, N.; Tindula, G.; et al. Traffic-related air pollution is associated with glucose dysregulation, blood pressure, and oxidative stress in children. Environ. Res. 2021, 195, 110870. [Google Scholar] [CrossRef] [PubMed]
- Madueño, L.; Kecorius, S.; Birmili, W.; Müller, T.; Simpas, J.; Vallar, E.; Galvez, M.C.; Cayetano, M.; Wiedensohler, A. Aerosol Particle and Black Carbon Emission Factors of Vehicular Fleet in Manila, Philippines. Atmosphere 2019, 10, 603. [Google Scholar] [CrossRef]
- Boquet, Y. Battling congestion in Manila: The EDSA problem. Transp. Commun. Bull. Asia Pac. 2013, 82, 45–69. [Google Scholar]
- Chung, M.; Wang, D.D.; Rizzo, A.M.; Gachette, D.; Delnord, M.; Parambi, R.; Kang, C.-M.; Brugge, D. Association of PNC, BC, and PM2.5 Measured at a Central Monitoring Site with Blood Pressure in a Predominantly Near Highway Population. Int. J. Environ. Res. Public Health 2015, 12, 2765–2780. [Google Scholar] [CrossRef]
- Weichenthal, S.; Hatzopoulou, M.; Goldberg, M.S. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: A cross-over study. Part. Fibre Toxicol. 2014, 11, 1–16. [Google Scholar] [CrossRef]
- Hoffmann, B.; Luttmann-Gibson, H.; Cohen, A.; Zanobetti, A.; De Souza, C.; Foley, C.; Suh, H.; Coull, B.A.; Schwartz, J.; Mittleman, M.; et al. Opposing Effects of Particle Pollution, Ozone, and Ambient Temperature on Arterial Blood Pressure. Environ. Health Perspect. 2012, 120, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Kubesch, N.; de Nazelle, A.; Guerra, S.; Westerdahl, D.; Martinez, D.; Bouso, L.; Carrasco-Turigas, G.; Hoffmann, B.; Nieuwenhuijsen, M.J. Arterial blood pressure responses to short-term exposure to low and high traffic-related air pollution with and without moderate physical activity. Eur. J. Prev. Cardiol. 2014, 22, 548–557. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Z.; Ruan, Y.; Yan, J.; Mukherjee, B.; Yang, F.; Duan, F.; Sun, L.; Liang, R.; Lian, H. Personal black carbon exposure influences ambulatory blood pressure: Air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension 2014, 63, 871–877. [Google Scholar] [CrossRef]
- Liu, L.; Ruddy, T.; Dalipaj, M.; Poon, R.; Szyszkowicz, M.; You, H.; Dales, R.E.; Wheeler, A.J. Effects of Indoor, Outdoor, and Personal Exposure to Particulate Air Pollution on Cardiovascular Physiology and Systemic Mediators in Seniors. J. Occup. Environ. Med. 2009, 51, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, X.; Liu, F.; Yang, X.; Liu, Q.; Xing, X.; Cao, J.; Li, J.; Huang, K.; Yan, W.; et al. Impacts of Short-Term Fine Particulate Matter Exposure on Blood Pressure Were Modified by Control Status and Treatment in Hypertensive Patients. Hypertension 2021, 78, 174–183. [Google Scholar] [CrossRef]
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; A Chirinos, J.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.-P.; Hering, D.; et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on hypertension. Lancet 2016, 388, 2665–2712. [Google Scholar] [CrossRef]
- Brook, R.D.; Urch, B.; Dvonch, J.T.; Bard, R.L.; Speck, M.; Keeler, G.; Morishita, M.; Marsik, F.J.; Kamal, A.S.; Kaciroti, N.; et al. Insights into the Mechanisms and Mediators of the Effects of Air Pollution Exposure on Blood Pressure and Vascular Function in Healthy Humans. Hypertension 2009, 54, 659–667. [Google Scholar] [CrossRef]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef]
- Rafee, L.; Abedini, M.; Javanmard, S.H.; Sarrafzadegan, N.; Mansourian, M. Association of GSTT1 and GSTM1 poly-morphisms with blood pressure: A Bayesian modeling of continuous data. J. Res. Med Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 200. [Google Scholar]
- Elliott, J.L.; Lal, S. Blood Pressure, Sleep Quality and Fatigue in Shift Working Police Officers: Effects of a Twelve Hour Roster System on Cardiovascular and Sleep Health. Int. J. Environ. Res. Public Health 2016, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, F.; Nawi, N.G.; Van, M.H.; Byass, P.; Berhane, Y.; Bonita, R.; Wall, S. Association between body mass index and blood pressure across three populations in Africa and Asia. J. Hum. Hypertens. 2006, 21, 28–37. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Puett, R.C.; Hart, J.E.; Yanosky, J.D.; Paciorek, C.; Schwartz, J.; Suh, H.; Speizer, F.E.; Laden, F. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ. Health Perspect. 2009, 117, 1697–1701. [Google Scholar] [CrossRef]
- Linderman, G.C.; Lu, J.; Lu, Y.; Sun, X.; Xu, W.; Nasir, K.; Schulz, W.; Jiang, L.; Krumholz, H.M. Association of Body Mass Index with Blood Pressure among 1.7 Million Chinese Adults. JAMA Netw. Open 2018, 1, e181271. [Google Scholar] [CrossRef]
- Bagtasa, G.; Chung-Shin, Y. Influence of local meteorology on the chemical characteristics of fine particulates in Metropolitan Manila in the Philippines. Atmos. Pollut. Res. 2020, 11, 1359–1369. [Google Scholar] [CrossRef]
- Regencia, Z.J.G.; Dalmacion, G.V.; Quizon, D.B.; Quizon, K.B.; Duarte, N.E.P.; Baja, E.S. Airborne heavy metals and blood pressure: Modification by sex and obesity in the MMDA traffic enforcers’ health study. Atmos. Pollut. Res. 2020, 11, 2244–2250. [Google Scholar] [CrossRef]
- Sarnat, J.A.; Brown, K.W.; Schwartz, J.; Coull, B.A.; Koutrakis, P. Ambient gas concentrations and personal particulate matter exposures: Implications for studying the health effects of particles. Epidemiology 2005, 385–395. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Mean ± SD, % |
|---|---|
| Age, years | 37.2 ± 8.7 |
| Male | 90.1 |
| Cholesterol level, mg/dL | 203.5 ± 38.4 |
| Glucose fasting, mg/dL | 102.6 ± 47.5 |
| Body mass index, kg/m2 | 25.9 ± 4.2 |
| Obese (BMI ≥ 30 kg/m2) | 16.4 |
| Hypertensive | 15.8 |
| Ever cigarette smoker | 44.1 |
| Ever drinker | 76.3 |
| College graduate | 36.2 |
| Work experience, years | 9.7 ± 8.6 |
| Systolic blood pressure, mmHg | 128.2 ± 16.2 |
| Diastolic blood pressure, mmHg | 78.1 ± 11.1 |
| Variable | Lag | Mean ± SD | Median | IQR |
|---|---|---|---|---|
| Black carbon, μg/m3 | 0-h | 10.6 ± 10.4 | 6.1 | 10.8 |
| 10-h | 8.1 ± 5.1 | 7.3 | 7.1 | |
| 7-d | 9.3 ± 8.6 | 5.8 | 8.5 | |
| Temperature, °C a | 32.0 ± 2.6 | 32.1 | 3.4 | |
| Relative humidity, % b | 55.8 ± 11.7 | 54.4 | 15.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regencia, Z.J.G.; Dalmacion, G.V.; Ligsay, A.D.; Baja, E.S. Short-Term Cumulative Exposure to Ambient Traffic-Related Black Carbon and Blood Pressure: MMDA Traffic Enforcers’ Health Study. Int. J. Environ. Res. Public Health 2021, 18, 12122. https://doi.org/10.3390/ijerph182212122
Regencia ZJG, Dalmacion GV, Ligsay AD, Baja ES. Short-Term Cumulative Exposure to Ambient Traffic-Related Black Carbon and Blood Pressure: MMDA Traffic Enforcers’ Health Study. International Journal of Environmental Research and Public Health. 2021; 18(22):12122. https://doi.org/10.3390/ijerph182212122
Chicago/Turabian StyleRegencia, Zypher Jude G., Godofreda V. Dalmacion, Antonio D. Ligsay, and Emmanuel S. Baja. 2021. "Short-Term Cumulative Exposure to Ambient Traffic-Related Black Carbon and Blood Pressure: MMDA Traffic Enforcers’ Health Study" International Journal of Environmental Research and Public Health 18, no. 22: 12122. https://doi.org/10.3390/ijerph182212122
APA StyleRegencia, Z. J. G., Dalmacion, G. V., Ligsay, A. D., & Baja, E. S. (2021). Short-Term Cumulative Exposure to Ambient Traffic-Related Black Carbon and Blood Pressure: MMDA Traffic Enforcers’ Health Study. International Journal of Environmental Research and Public Health, 18(22), 12122. https://doi.org/10.3390/ijerph182212122






