Clusters of Survivors of COVID-19 Associated Acute Respiratory Failure According to Response to Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
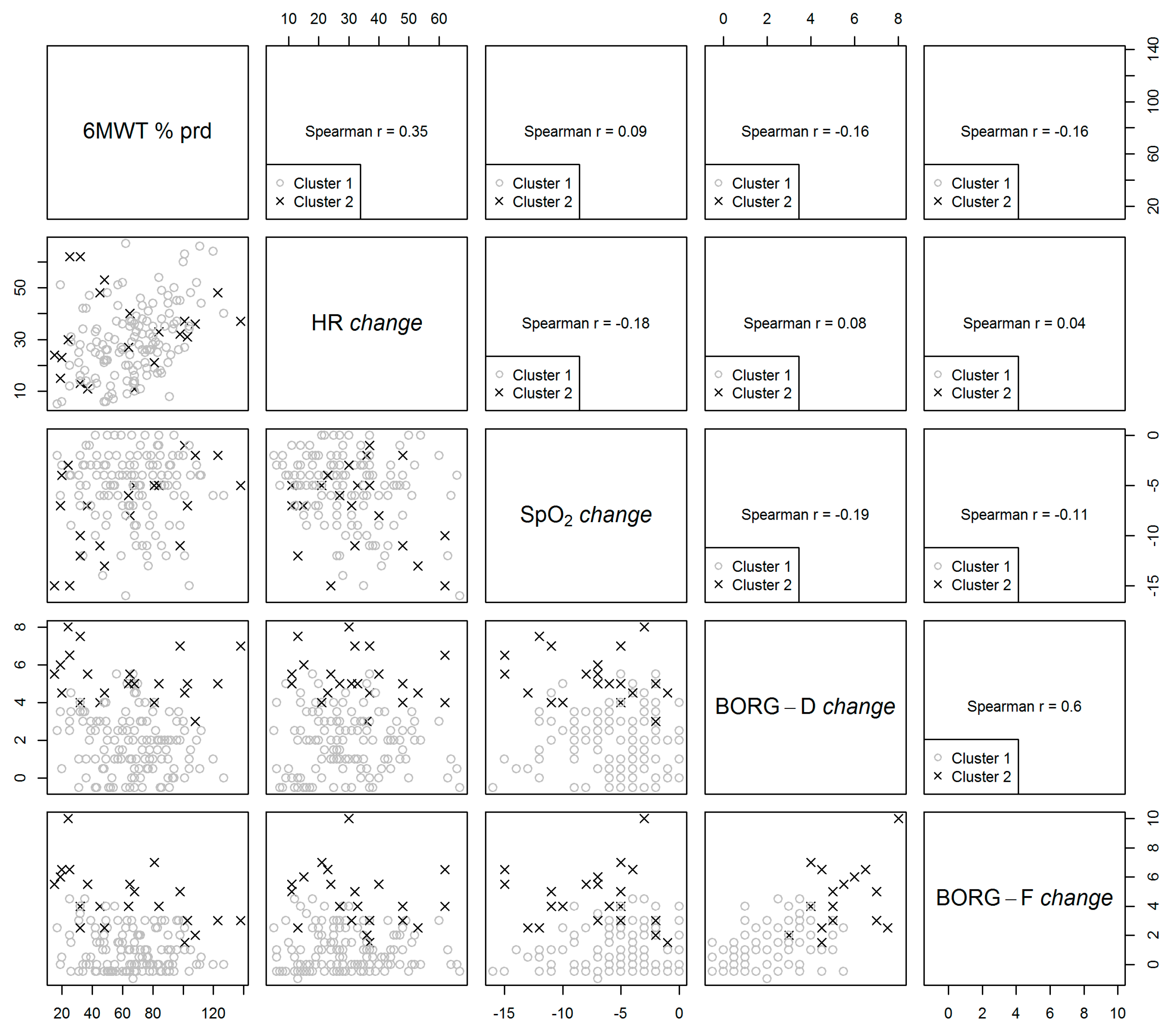
3.2. Correlation between Clustering Parameters
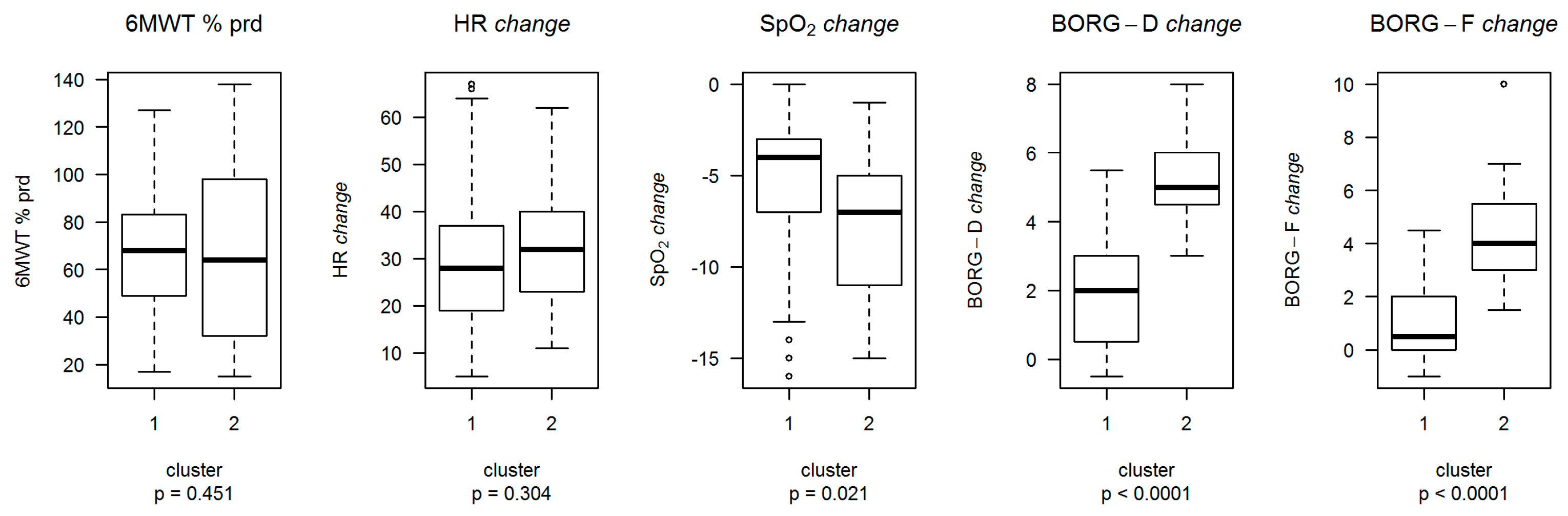
3.3. Clustering Analysis
3.4. Factors Associated to Variables Used for Clusters Definition
- (a)
- The need of walking aids was associated with lower 6MWT and % predicted. Participants from Pavia were more likely to be characterized by higher 6MWT and % predicted.
- (b)
- History of EI was associated with a positive shift in terms of HR change distribution, while SpO2 change, baseline HR, and time from admission to acute care hospitals were associated with a negative shift in terms of HR change distribution. The participants from Pavia were also characterized by a positive shift in terms of HR change distribution.
- (c)
- Body mass index was associated with a positive shift in terms of SpO2 change distribution, and the participants from Pavia centre were also likely to be characterized by a positive shift in terms of SpO2 change distribution.
- (d)
- Baseline Borg-D and the need of walking aids were associated with a positive shift in Borg-D change distribution. SpO2 change during the test was associated with a negative shift in Borg-D change distribution.
- (e)
- The need of walking aids and age were associated with a positive shift in terms of Borg-F change distribution.
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins Coronavirus Resource Center. Global Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 23 September 2021).
- Winck, J.C.; Ambrosino, N. COVID-19 pandemic and non invasive respiratory management: Every Goliath needs a David. An evidence based evaluation of problems. Pulmonology 2020, 26, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 2020, 27, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle Strength and Physical Performance in Patients without Previous Disabilities Recovering from COVID-19 Pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, C.; Paneroni, M.; Vitacca, M.; Ambrosino, N. Measures of physical performance in COVID-19 patients: A mapping review. Pulmonology 2021, 27, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Carlucci, A.; Paneroni, M.; Carotenuto, M.; Bertella, E.; Cirio, S.; Gandolfo, A.; Simonelli, C.; Vigna, M.; Lastoria, C.; Malovini, A.; et al. Prevalence of exercise-induced oxygen desaturation after recovery from SARS-CoV-2 pneumonia and use of lung ultrasound to predict need for pulmonary rehabilitation. Pulmonology 2021. [Google Scholar] [CrossRef] [PubMed]
- Vitacca, M.; Paneroni, M.; Brunetti, G.; Carlucci, A.; Balbi, B.; Spanevello, A.; Ambrosino, N. Characteristics of COVID-19 Pneumonia Survivors with Resting Normoxemia and Exercise-Induced Desaturation. Respir. Care 2021, 66, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.S.; Landau, S.; Leese, M.; Daniel Stahl, D. Cluster Analysis, 5th ed.; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2011; p. 352. ISBN 978-0-470-74991-3. [Google Scholar]
- Ceriana, P.; Nava, S.; Vitacca, M.; Carlucci, A.; Paneroni, M.; Schreiber, A.; Pisani, L.; Ambrosino, N. Noninvasive ventilation during weaning from prolonged mechanical ventilation. Pulmonology 2019, 25, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Maestri, R.; Bruschi, C.; Fracchia, C.; Pinna, G.D.; Fanfulla, F.; Ambrosino, N. Physiological and clinical characteristics of patients with COPD admitted to an inpatient pulmonary rehabilitation program: A real-life study. Pulmonology 2019, 25, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Vitacca, M.; Migliori, G.B.; Spanevello, A.; Melazzini, M.G.; Ambrosino, N.; COVID-19 ICS Maugeri IRCCS Network; Ceriana, P.; Fanfulla, F.; Braghiroli, A.; Fracchia, C.; et al. Management and outcomes of post-acute COVID-19 patients in Northern Italy. Eur. J. Intern. Med. 2020, 78, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Ippolito, M.; Vitale, F.; Accurso, G.; Iozzo, P.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Medical masks and Respirators for the Protection of Healthcare Workers from SARS-CoV-2 and other viruses. Pulmonology 2020, 26, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L.; Sherrill, D.L. Reference Equations for the Six-Minute Walk in Healthy Adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, G. Psychophysical basis of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Poulain, M.; Durand, F.; Palomba, B.; Ceugniet, F.; Desplan, J.; Varray, A.; Préfaut, C. 6-Minute Walk Testing Is More Sensitive than Maximal Incremental Cycle Testing for Detecting Oxygen Desaturation in Patients with COPD. Chest 2003, 123, 1401–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitacca, M.; Paneroni, M.; Braghiroli, A.; Balbi, B.; Aliani, M.; Guido, P.; Fanfulla, F.; Pertosa, M.; Ceriana, P.; Zampogna, E.; et al. Exercise capacity and comorbidities in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2020, 16, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, M.N.; Carvalho, T.A.; Marson, F.A.D.L. Retraction in the era of COVID-19 and its influence on evidence-based medicine: Is science in jeopardy? Pulmonology 2021, 27, 97–106. [Google Scholar] [CrossRef] [PubMed]
| Variable | N | Value | Overall | Lumezzane | Pavia | p |
|---|---|---|---|---|---|---|
| N = 154 | N = 58 | N = 96 | ||||
| Sex | 154 | F | 45 (29.22%) | 23 (39.66%) | 22 (22.92%) | 0.0298 |
| M | 109 (70.78%) | 35 (60.34%) | 74 (77.08%) | |||
| Age, years | 154 | 69.5 (60.25:76.00) | 70 (61.25:75.75) | 68 (59.75:76.25) | 0.4432 | |
| BMI, Kg/m2 | 154 | 26.23 (23.46:29.38) | 26.64 (23.5:32.24) | 26.23 (23.48:28.40) | 0.2562 | |
| Obesity | 154 | Yes | 37 (24.03%) | 22 (37.93%) | 15 (15.62%) | 0.0024 |
| Arterial hypertension | 154 | Yes | 93 (60.39%) | 35 (60.34%) | 58 (60.42%) | 1 |
| Arrhythmias | 154 | Yes | 25 (16.23%) | 10 (17.24%) | 15 (15.62%) | 0.8197 |
| Diabetes | 154 | Yes | 32 (20.78%) | 14 (24.14%) | 18 (18.75%) | 0.5326 |
| Renal failure | 154 | Yes | 9 (5.84%) | 3 (5.17%) | 6 (6.25%) | 1 |
| Congestive heart failure | 154 | Yes | 14 (9.09%) | 11 (18.97%) | 3 (3.12%) | 0.0024 |
| Asthma | 154 | Yes | 10 (6.49%) | 4 (6.90%) | 6 (6.25%) | 1 |
| COPD | 154 | Yes | 13 (8.44%) | 3 (5.17%) | 10 (10.42%) | 0.3682 |
| OSAS | 154 | Yes | 13 (8.44%) | 6 (10.34%) | 7 (7.29%) | 0.5644 |
| LoS in acute care hospitals, days | 154 | 20 (11:33.8) | 26.5 (20:38.5) | 16 (10:22) | <0.0001 | |
| EI in acute care hospitals | 154 | Yes | 41 (26.62%) | 20 (34.48%) | 21 (21.88%) | 0.0954 |
| Tracheostomy | 154 | Yes | 28 (18.18%) | 14 (24.14%) | 14 (14.58%) | 0.1898 |
| NIV in acute care hospitals | 154 | Yes | 91 (59.09%) | 34 (58.62%) | 57 (59.38%) | 1 |
| O2 supply in acute care hospitals | 154 | Yes | 131 (85.06%) | 53 (91.38%) | 78 (81.25%) | 0.0969 |
| LoS in study centres, days | 154 | 17 (13:22) | 20 (16.25:28) | 15 (12:20) | <0.0001 | |
| PaO2, mmHg | 125 | 72.6 (65.7:81.3) | 71.3 (63.45:79.3) | 73.2 (66.5:81.6) | 0.2031 | |
| PaCO2, mmHg | 125 | 35.1 (32.4:38.6) | 36.3 (34:39.68) | 34.6 (31.5:37.5) | 0.0161 | |
| pH | 125 | 7.44 (7.42:7.46) | 7.44 (7.43:7.45) | 7.44 (7.42:7.46) | 0.7353 | |
| Need of walking aids | 153 | Yes | 47 (30.72%) | 15 (26.32%) | 32 (33.33%) | 0.3757 |
| Motor Barthel index, points | 99 | 90 (80:100) | 100 (90:100) | 90 (60:100) | 0.0018 | |
| CRP, mg/dL | 144 | 1.37 (0.68:3.76) | 3.4 (1.8:10.2) | 1 (0.42:2.06) | <0.0001 | |
| D-dimer, μg/mL | 121 | 760 (420:1280) | 635 (382.5:1137.5) | 920 (465:1460) | 0.0378 | |
| Ferritin, ng/mL | 122 | 419 (227.75:776.5) | 354.5 (213:518) | 457 (251:869.5) | 0.0300 | |
| Albumin, g/dL | 105 | 30.5 (26.9:34.7) | 34 (31.1:35.9) | 28.3 (25.78:30.83) | <0.0001 | |
| Haemoglobin, g/dL | 148 | 11.4 (10.5:12.5) | 11.3 (10.7:12.2) | 11.5 (10.45:12.65) | 0.7520 | |
| Time from acute care hospital admission, days | 154 | 38 (29:53) | 51.5 (37:71) | 35 (26:42.25) | <0.0001 | |
| 6MWT, m | 154 | 336 (215:446.5) | 267.5 (180:372.5) | 393 (259.5:464) | <0.0001 | |
| 6MWT, % predicted | 154 | 67.5 (48:83.75) | 53 (37.5:70.5) | 73 (56.5:90) | <0.0001 | |
| Baseline HR, b/min | 154 | 84 (74:94) | 84.5 (75:92) | 84 (73.75:94.25) | 0.4761 | |
| Baseline SpO2, % | 154 | 96 (94:98) | 94.5 (93:96) | 97 (95:98) | <0.0001 | |
| Baseline Borg-D | 154 | 0.5 (0:0.5) | 0.5 (0.5:0.5) | 0 (0:1) | 0.0004 | |
| Baseline Borg-F | 154 | 0.5 (0:0.5) | 0.5 (0.5:0.5) | 0 (0:1) | 0.0404 |
| Variable | Value | C1 (n = 133) | C2 (n = 21) | p |
|---|---|---|---|---|
| Hospital | Lumezzane | 50 (37.59%) | 8 (38.1%) | 1 |
| Pavia | 83 (62.41%) | 13 (61.9%) | ||
| LoS in acute care hospitals, days | 20 (11:30) | 20 (10:39) | 0.5737 | |
| LoS in study centrrs, days | 18 (13:22) | 15 (13:23) | 0.6221 | |
| Time from acute care hospital admission, days | 38 (29:52) | 39 (25:57) | 0.8025 | |
| Gender | Females | 35 (26.32%) | 10 (47.62%) | 0.0688 |
| Males | 98 (73.68%) | 11 (52.38%) | ||
| Age, years | 66 (59:75) | 75 (71:77) | 0.0081 | |
| BMI, Kg/m2 | 26.22 (23.66:29.32) | 26.29 (22.49:32.05) | 0.7903 | |
| Obesity | Yes | 31 (23.31%) | 6 (28.57%) | 0.7879 |
| Hypertension | Yes | 81 (60.9%) | 12 (57.14%) | 0.8099 |
| Arrhytmias | Yes | 23 (17.29%) | 2 (9.52%) | 0.5329 |
| Diabetes | Yes | 28 (21.05%) | 4 (19.05%) | 1 |
| Renal failure | Yes | 5 (3.76%) | 4 (19.05%) | 0.0191 |
| Congestive heart failure | Yes | 10 (7.52%) | 4 (19.05%) | 0.1079 |
| Asthma | Yes | 9 (6.77%) | 1 (4.76%) | 1 |
| COPD | Yes | 8 (6.02%) | 5 (23.81%) | 0.0171 |
| OSAS | Yes | 9 (6.77%) | 4 (19.05%) | 0.0774 |
| PaO2, mmHg | 73.1 (65.85:81.57) | 70.5 (65.25:75.6) | 0.2329 | |
| PaCO2, mmHg | 34.95 (32.32:38.55) | 36.2 (33.6:39.5) | 0.3233 | |
| pH | 7.44 (7.42:7.46) | 7.43 (7.42:7.46) | 0.8998 | |
| Endotracheal Intubation | Yes | 39 (29.32%) | 2 (9.52%) | 0.0640 |
| Tracheostomy | Yes | 26 (19.55%) | 2 (9.52%) | 0.3638 |
| NIV | Yes | 81 (60.9%) | 10 (47.62%) | 0.3429 |
| O2 supply | Yes | 115 (86.47%) | 16 (76.19%) | 0.3147 |
| Need of walking aids | Yes | 38 (28.79%) | 9 (42.86%) | 0.2132 |
| Motor Barthel index, points | 95 (83.75:100) | 90 (65:100) | 0.2150 | |
| CRP, mg/dL | 1.39 (0.71:3.54) | 1 (0.51:6.67) | 0.8274 | |
| D-dimer, μg/mL | 750 (420:1270) | 1125 (522.5:1440) | 0.2634 | |
| Ferritin, ng/mL | 434 (231.5:794.5) | 356 (198.75:440.25) | 0.2000 | |
| Albumin, g/dL | 30.6 (26.9:34.6) | 29.35 (26.85:35.38) | 0.9431 | |
| Haemoglobin, g/dL | 11.5 (10.55:12.55) | 11.2 (10.5:11.7) | 0.2728 | |
| 6MWT, m | 355 (225:450) | 267 (150:372) | 0.0501 | |
| 6MWT, % predicted # | 68 (49:83) | 64 (32:98) | 0.4514 | |
| Baseline HR, b/mim | 84 (74:94) | 84 (69:92) | 0.4754 | |
| HRpeak, b/min | 154 (145:161) | 145 (143:149) | 0.0084 | |
| HR, % of maximal predicted | 74 (66:82) | 76 (72:81) | 0.2380 | |
| HR change, % # | 28 (19:37) | 32 (23:40) | 0.3044 | |
| Baseline SpO2, % | 96 (94:98) | 96 (95:97) | 0.7778 | |
| End exercise SpO2, % | 91 (88:94) | 89 (87:91) | 0.0806 | |
| SpO2 change, % # | −4 (−7:−3) | −7 (−11:−5) | 0.0206 | |
| Baseline Borg-D | 0.5 (0:0.5) | 0.5 (0.5:2) | 0.0291 | |
| End exercise Borg-D | 2 (1:3) | 6 (5:7) | <0.0001 | |
| Borg-D change # | 2 (0.5:3) | 5 (4.5:6) | <0.0001 | |
| Baseline Borg-F | 0.5 (0:0.5) | 0.5 (0:0.5) | 0.7279 | |
| End exercise Borg-F | 1 (0:3) | 5 (4:7) | <0.0001 | |
| Borg-F change # | 0.5 (0:2) | 4 (3:5.5) | <0.0001 |
| 6MWT (% Predicted) | ||
| Variable | Coefficient (95% CI) | p value |
| Intercept | 61.92 (56.24:67.60) | |
| Hospital = Pavia # | 20.03 (13.21:26.85) | <0.0001 |
| IWalking Aids = Yes | −27.28 (−34.45:−20.10) | <0.0001 |
| HR change | ||
| Variable | Coefficient (95% CI) | p value |
| Intercept | 54.97 (40.04:69.9) | |
| Hospital = Pavia # | 5.37 (0.71:10.04) | 0.0243 |
| Baseline HR, b/m # | −0.33 (−0.48:−0.18) | <0.0001 |
| SpO2 change | −1.12 (−1.67:−0.57) | <0.0001 |
| Time from hospital admission, days | −0.19 (−0.29:−0.08) | 0.0009 |
| EI = Yes | 5.84 (0.98:10.71) | 0.0189 |
| SpO2 change | ||
| Variable | Coefficient (95% CI) | p value |
| Intercept | −35.21 (−59.74:−10.69) | |
| Hospital = Pavia # | 1.42 (0.2:2.65) | 0.0228 |
| Baseline SpO2, % # | 0.25 (−0.01:0.5) | 0.0603 |
| BMI | 0.2 (0.1:0.3) | <0.0001 |
| Borg-D change | ||
| Variable | Coefficient (95% CI) | p value |
| Intercept | 0.67 (−0.09:1.42) | |
| Hospital = Pavia # | 0.59 (−0.02:1.2) | 0.0591 |
| Baseline Borg-D # | 0.48 (0.04:0.92) | 0.0317 |
| Walking Aids = Yes | 1.01 (0.38:1.64) | 0.0018 |
| SpO2 change | −0.13 (−0.21:−0.04) | 0.0027 |
| Borg-F change | ||
| Variable | Coefficient (95% CI) | p value |
| Intercept | −1.39 (−3.30:0.53) | |
| Hospital = Pavia # | 0.17 (−0.44:0.78) | 0.5844 |
| Baseline Borg-F # | 0 (−0.35:0.35) | 0.9971 |
| Walking Aids = Yes | 1.10 (0.45:1.75) | 0.0010 |
| Age | 0.04 (0.01:0.06) | 0.0112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitacca, M.; Paneroni, M.; Malovini, A.; Carlucci, A.; Binda, C.; Sanci, V.; Ambrosino, N. Clusters of Survivors of COVID-19 Associated Acute Respiratory Failure According to Response to Exercise. Int. J. Environ. Res. Public Health 2021, 18, 11868. https://doi.org/10.3390/ijerph182211868
Vitacca M, Paneroni M, Malovini A, Carlucci A, Binda C, Sanci V, Ambrosino N. Clusters of Survivors of COVID-19 Associated Acute Respiratory Failure According to Response to Exercise. International Journal of Environmental Research and Public Health. 2021; 18(22):11868. https://doi.org/10.3390/ijerph182211868
Chicago/Turabian StyleVitacca, Michele, Mara Paneroni, Alberto Malovini, Annalisa Carlucci, Chiara Binda, Vincenzo Sanci, and Nicolino Ambrosino. 2021. "Clusters of Survivors of COVID-19 Associated Acute Respiratory Failure According to Response to Exercise" International Journal of Environmental Research and Public Health 18, no. 22: 11868. https://doi.org/10.3390/ijerph182211868
APA StyleVitacca, M., Paneroni, M., Malovini, A., Carlucci, A., Binda, C., Sanci, V., & Ambrosino, N. (2021). Clusters of Survivors of COVID-19 Associated Acute Respiratory Failure According to Response to Exercise. International Journal of Environmental Research and Public Health, 18(22), 11868. https://doi.org/10.3390/ijerph182211868







