Modification of Apremilast from Pills to Aerosol a Future Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
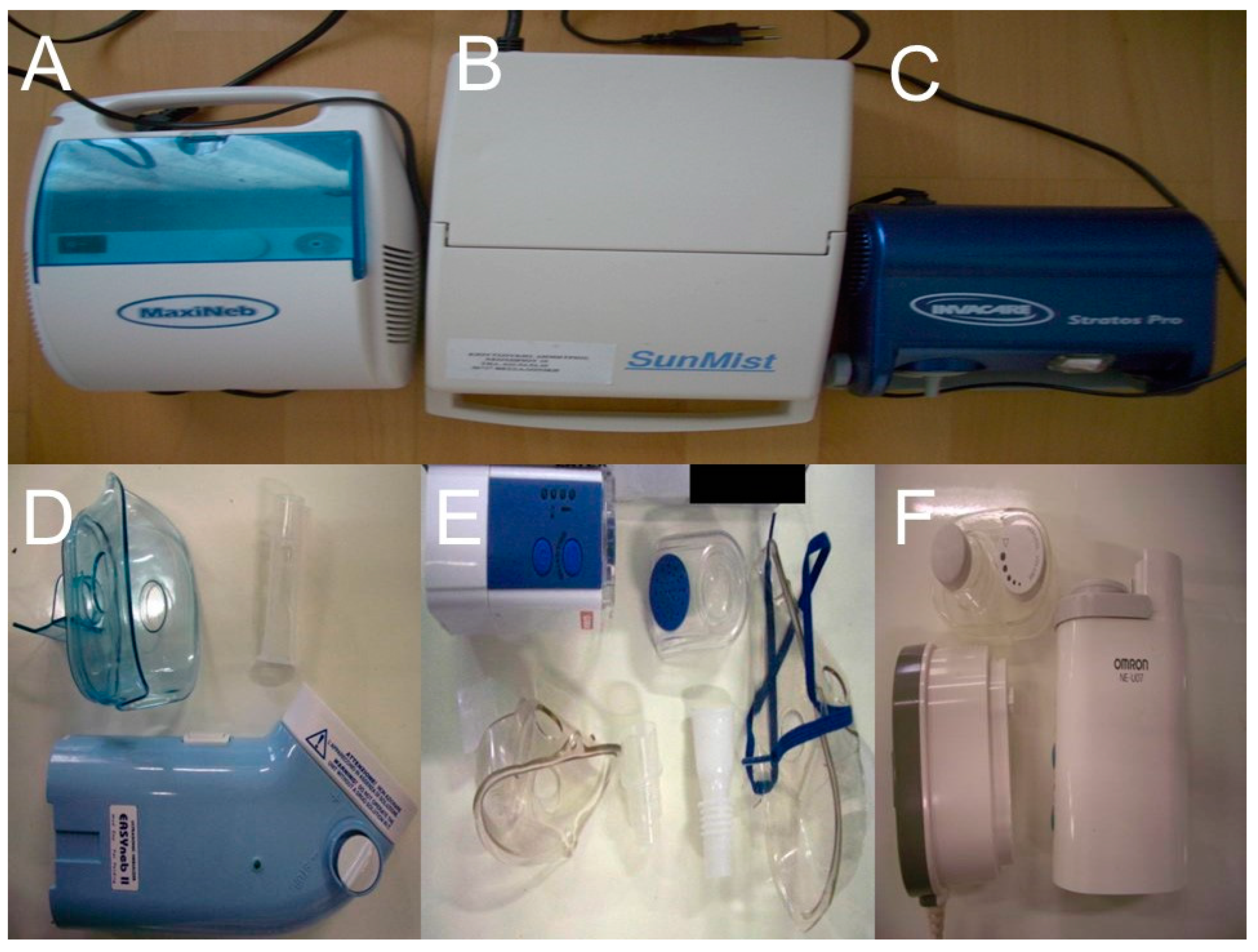
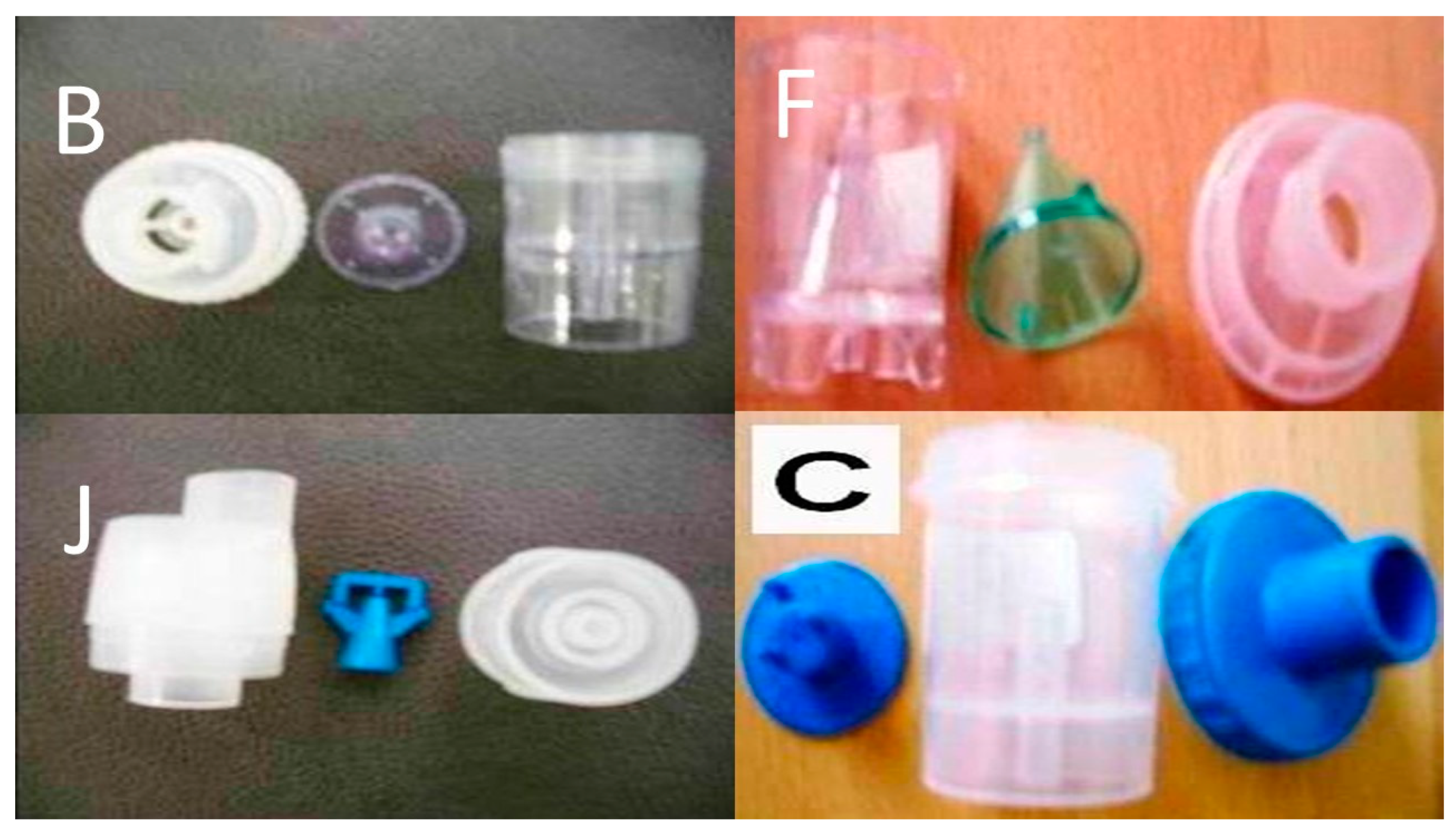
2.2. Nebulizers and Residual Cups
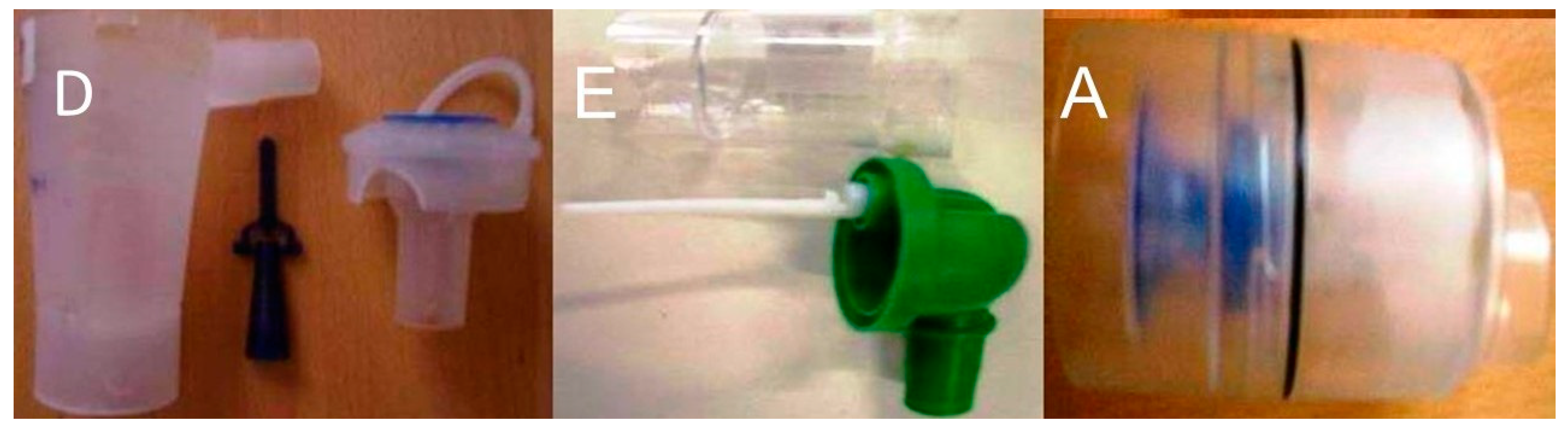
2.2.1. Jet-Nebulizers and Residual Cups
2.2.2. Ultrasound Nebulizers
2.3. Measurement of Droplet Size and Droplet Size Distribution
2.4. Milling
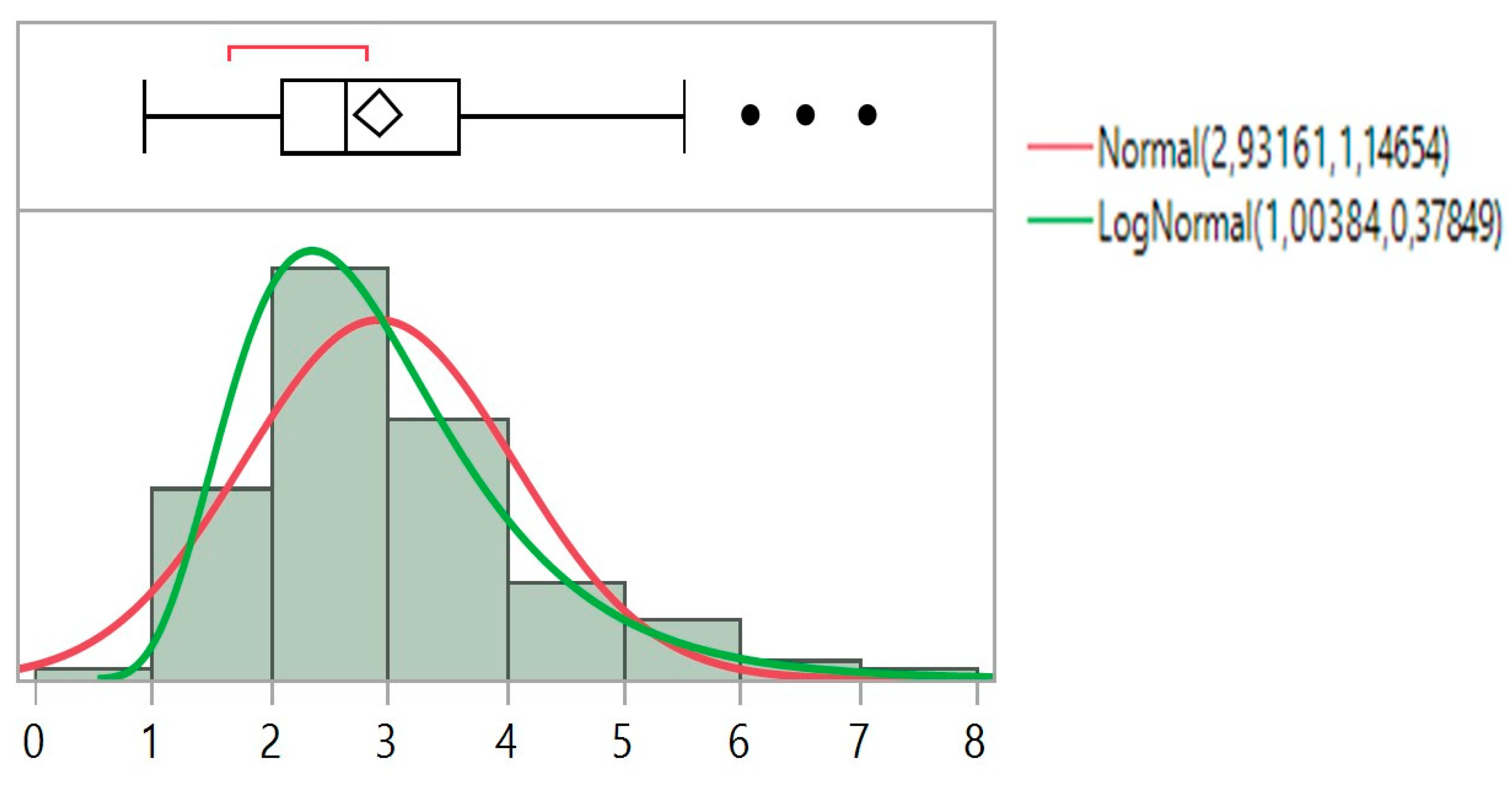
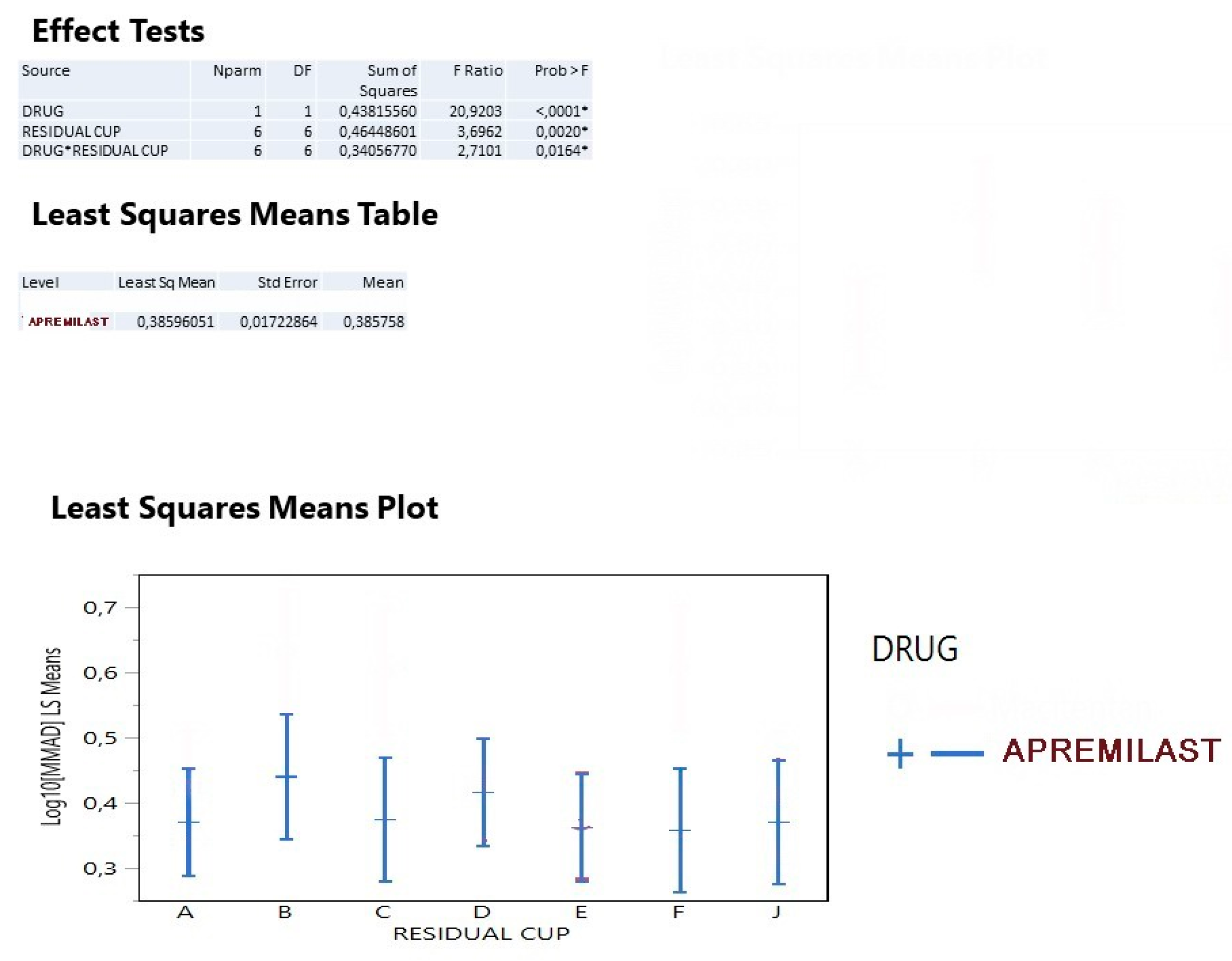
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarogoulidis, P.; Porpodis, K.; Kioumis, I.; Petridis, D.; Lampaki, S.; Spyratos, D.; Papaiwannou, A.; Organtzis, J.; Kontakiotis, T.; Manika, K.; et al. Experimentation with inhaled bronchodilators and corticosteroids. Int. J. Pharm. 2014, 461, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Bloom, C.; Lewis, J.; Marjenberg, Z.; Platz, J.H.; Langham, S. Impact of health technology assessment on prescribing patterns of inhaled fixed-dose combination triple therapy in chronic obstructive pulmonary disease. J. Mark. Access Health Policy 2021, 9, 1929757. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Kioumis, I.; Porpodis, K.; Spyratos, D.; Tsakiridis, K.; Huang, H.; Li, Q.; Turner, J.F.; Browning, R.; Hohenforst-Schmidt, W.; et al. Clinical experimentation with aerosol antibiotics: Current and future methods of administration. Drug Des. Dev. Ther. 2013, 7, 1115–1134. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Kioumis, I.; Ritzoulis, C.; Petridis, D.; Darwiche, K.; Porpodis, K.; Spyratos, D.; Parrish, S.; Browning, R.; Li, Q.; et al. New insights in the production of aerosol antibiotics. Evaluation of the optimal aerosol production system for ampicillin-sulbactam, meropenem, ceftazidime, cefepime and piperacillin-tazobactam. Int. J. Pharm. 2013, 455, 182–188. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Papanas, N.; Kouliatsis, G.; Spyratos, D.; Zarogoulidis, K.; Maltezos, E. Inhaled insulin: Too soon to be forgotten? J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 213–223. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Petridis, D.; Ritzoulis, C.; Li, Q.; Huang, H.; Ning, Y.; Darwiche, K.; Freitag, L.; Zarogoulidis, K. Further experimentation of inhaled; LANTUS, ACTRAPID and HUMULIN with todays’ production systems. Int. J. Pharm. 2013, 458, 39–47. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Darwiche, K.; Krauss, L.; Huang, H.; Zachariadis, G.A.; Katsavou, A.; Hohenforst-Schmidt, W.; Papaiwannou, A.; Vogl, T.J.; Freitag, L.; et al. Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. Future Oncol. 2013, 9, 1307–1313. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Petridis, D.; Ritzoulis, C.; Darwiche, K.; Spyratos, D.; Huang, H.; Goldberg, E.P.; Yarmus, L.; Li, Q.; Freitag, L.; et al. Establishing the optimal nebulization system for paclitaxel, docetaxel, cisplatin, carboplatin and gemcitabine: Back to drawing the residual cup. Int. J. Pharm. 2013, 453, 480–487. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Eleftheriadou, E.; Sapardanis, I.; Zarogoulidou, V.; Lithoxopoulou, H.; Kontakiotis, T.; Karamanos, N.; Zachariadis, G.; Mabroudi, M.; Zisimopoulos, A.; et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investig. New Drugs 2012, 30, 1628–1640. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Chatzaki, E.; Porpodis, K.; Domvri, K.; Hohenforst-Schmidt, W.; Goldberg, E.P.; Karamanos, N.; Zarogoulidis, K. Inhaled chemotherapy in lung cancer: Future concept of nanomedicine. Int. J. Nanomed. 2012, 7, 1551–1572. [Google Scholar] [CrossRef]
- Zarogouldis, P.; Karamanos, N.K.; Porpodis, K.; Domvri, K.; Huang, H.; Hohenforst-Schimdt, W.; Goldberg, E.P.; Zarogoulidis, K. Vectors for inhaled gene therapy in lung cancer. Application for nano oncology and safety of bio nanotechnology. Int. J. Mol. Sci. 2012, 13, 10828–10862. [Google Scholar] [CrossRef]
- Baliaka, A.; Zarogoulidis, P.; Domvri, K.; Hohenforst-Schmidt, W.; Sakkas, A.; Huang, H.; Le Pivert, P.; Koliakos, G.; Koliakou, E.; Kouzi-Koliakos, K.; et al. Intratumoral gene therapy versus intravenous gene therapy for distant metastasis control with 2-Diethylaminoethyl-Dextran Methyl Methacrylate Copolymer Non-Viral Vector-p53. Gene Ther. 2013, 21, 158–167. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Hohenforst-Schmidt, W.; Darwiche, K.; Krauss, L.; Sparopoulou, D.; Sakkas, L.; Gschwendtner, A.; Huang, H.; Turner, F.J.; Freitag, L.; et al. 2-diethylaminoethyl-dextran methyl methacrylate copolymer nonviral vector: Still a long way toward the safety of aerosol gene therapy. Gene Ther. 2013, 20, 1022–1028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zisis, C.; Tsakiridis, K.; Kougioumtzi, I.; Zarogoulidis, P.; Darwiche, K.; Machairiotis, N.; Zaric, B.; Katsikogiannis, N.; Kesisis, G.; Stylianaki, A.; et al. The management of the advanced colorectal cancer: Management of the pulmonary metastases. J. Thorac. Dis. 2013, 5 (Suppl. 4), S383–S388. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H. Interview with Dr You Han Bae: Ligand-mediated versus ‘passive’ targeting approaches in nanoparticle oncology research. Ther. Deliv. 2012, 3, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, K.; Zarogoulidis, P.; Karamanos, N.K.; Domvri, K.; Chatzaki, E.; Constantinidis, T.C.; Kakolyris, S.; Zarogoulidis, K. Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: A future dilemma for micro-oncology. Future Oncol. 2013, 9, 505–525. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Giraleli, C.; Karamanos, N.K. Inhaled chemotherapy in lung cancer: Safety concerns of nanocomplexes delivered. Ther. Deliv. 2012, 3, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Kontakiotis, T.; Zarogoulidis, K. Inhaled gene therapy in lung cancer: “As for the future, our task is not to foresee it, but to enable it. ” Ther. Deliv. 2012, 3, 919–921. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Petridis, D.; Ritzoulis, C.; Darwiche, K.; Kioumis, I.; Porpodis, K.; Spyratos, D.; Hohenforst-Schmidt, W.; Yarmus, L.; Huang, H.; et al. Internal mouthpiece designs as a future perspective for enhanced aerosol deposition. Comparative results for aerosol chemotherapy and aerosol antibiotics. Int. J. Pharm. 2013, 456, 325–331. [Google Scholar] [CrossRef]
- Ferron, G.A.; Kerrebijn, K.F.; Weber, J. Properties of aerosols produced with three nebulizers. Am. Rev. Respir. Dis. 1976, 114, 899–908. [Google Scholar] [PubMed]
- Kendrick, A.H.; Smith, E.C.; Wilson, R.S. Selecting and using nebuliser equipment. Thorax 1997, 52 (Suppl 2), S92–S101. [Google Scholar] [CrossRef]
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Darwiche, K.; Yarmus, L.; Spyratos, D.; Secen, N.; Hohenforst-Schmidt, W.; Katsikogiannis, N.; Huang, H.; Gschwendtner, A.; Zarogoulidis, K. Defense mechanisms of the respiratory system and aerosol production systems. Med. Chem. 2014, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, K.; Zarogoulidis, P.; Baehner, K.; Welter, S.; Tetzner, R.; Wohlschlaeger, J.; Theegarten, D.; Nakajima, T.; Freitag, L. Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2866–2870. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Darwiche, K.; Huang, H.; Spyratos, D.; Yarmus, L.; Li, Q.; Kakolyris, S.; Syrigos, K.; Zarogoulidis, K. Time recall; future concept of chronomodulating chemotherapy for cancer. Curr. Pharm. Biotechnol. 2013, 14, 632–642. [Google Scholar] [CrossRef]
- Boukovinas, I.; Tsakiridis, K.; Zarogoulidis, P.; Machairiotis, N.; Katsikogiannis, N.; Kougioumtzi, I.; Zarogoulidis, K. Neo-adjuvant chemotherapy in early stage non-small cell lung cancer. J. Thorac. Dis. 2013, 5, S446–S448. [Google Scholar] [CrossRef]
- Barnes, N.; Calverley, P.M.; Kaplan, A.; Rabe, K.F. Chronic obstructive pulmonary disease and exacerbations: Clinician insights from the global Hidden Depths of COPD survey. Curr. Med. Res. Opin. 2013, 13, 54. [Google Scholar] [CrossRef]
- Patel, M.; Pilcher, J.; Pritchard, A.; Perrin, K.; Travers, J.; Shaw, D.; Holt, S.; Harwood, M.; Black, P.; Weatherall, M.; et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: A randomised controlled trial. Lancet Respir. Med. 2013, 1, 32–42. [Google Scholar] [CrossRef]
- Bosquillon, C. Drug transporters in the lung—Do they play a role in the biopharmaceutics of inhaled drugs? J. Pharm. Sci. 2010, 99, 2240–2255. [Google Scholar] [CrossRef]
- Niven, R.W.; Ip, A.Y.; Mittelman, S.; Prestrelski, S.J.; Arakawa, T. Some factors associated with the ultrasonic nebulization of proteins. Pharm. Res. 1995, 12, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Finlay, W.H. (Ed.) Chapter 4—Particle size changes due to evaporation or condensation. In The Mechanisms of Inhaled Pharmaceutical Aerosols; Academic Press: London, UK, 2001; pp. 47–91. [Google Scholar]
- Finlay, W.H. (Ed.) Chapter 8—Jet nebulizers. In The Mechanisms of Inhaled Pharmaceutical Aerosols; Academic Press: London, UK, 2001; pp. 175–220. [Google Scholar]
- O’Callaghan, C.; Barry, P.W. The science of nebulised drug delivery. Thorax 1997, 52 (Suppl. 2), S31–S44. [Google Scholar] [CrossRef] [PubMed]
- Steckel, H.; Eskandar, F. Factors affecting aerosol performance during nebulization with jet and ultrasonic nebulizers. Eur. J. Pharm. Sci. 2003, 19, 443–455. [Google Scholar] [CrossRef]
- Hickey, A.J. (Ed.) Pharmaceutical Inhalation Aerosol Technology; Marcer Decker: New York, NY, USA, 1992; pp. 155–185. [Google Scholar]
- Wells, A.F.; Edwards, C.J.; Kivitz, A.J.; Bird, P.; Guerette, B.; Delev, N.; Paris, M.; Teng, L.; Aelion, J.A. Apremilast monotherapy for long-term treatment of active psoriatic arthritis in DMARD-naive patients. Rheumatology 2021. [Google Scholar] [CrossRef]
- Distel, J.; Cazzaniga, S.; Seyed Jafari, S.M.; Emelianov, V.; Schlapbach, C.; Yawalkar, N.; Heidemeyer, K. Long-Term Effectiveness and Drug Survival of Apremilast in Treating Psoriasis: A Real-World Experience. Dermatology 2021. [Google Scholar] [CrossRef]
- Ferguson, G.T.; Kerwin, E.M.; Rheault, T.; Bengtsson, T.; Rickard, K. A Dose-Ranging Study of the Novel Inhaled Dual PDE 3 and 4 Inhibitor Ensifentrine in Patients with COPD Receiving Maintenance Tiotropium Therapy. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Rickard, K.; Rheault, T.; Bengtsson, T.; Singh, D. Symptom Improvement Following Treatment with the Inhaled Dual Phosphodiesterase 3 and 4 Inhibitor Ensifentrine in Patients with Moderate to Severe COPD—A Detailed Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2199–2206. [Google Scholar] [CrossRef]
- Singh, D.; Martinez, F.J.; Watz, H.; Bengtsson, T.; Maurer, B.T. A dose-ranging study of the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in COPD. Respir. Res. 2020, 21, 47. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Rogliani, P.; Matera, M.G. Ensifentrine (RPL554): An investigational PDE3/4 inhibitor for the treatment of COPD. Expert Opin. Investig. Drugs 2019, 28, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.; Calzetta, L.; Matera, M.G. Ensifentrine (RPL554): An inhaled ‘bifunctional’ dual PDE3/4 inhibitor for the treatment of asthma and chronic obstructive pulmonary disease. Pharm. Pat. Anal. 2018, 7, 249–257. [Google Scholar] [CrossRef]
| Source | Nparm | DF | Sum of Squares | F Ratio | Prob > F |
|---|---|---|---|---|---|
| DRUG | 1 | 1 | 0.01397550 | 1.1912 | 0.2895 |
| NEBULIZER | 2 | 2 | 0.04570143 | 1.9477 | 0.1715 |
| LOADING | 1 | 1 | 0.01473125 | 1.2556 | 0.2772 |
| MOUTHPIECE | 1 | 1 | 0.02564812 | 2.1862 | 0.1565 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarogoulidis, P.; Kosmidis, C.; Kougkas, N.; Lallas, A.; Petridis, D.; Hohenforst-Schmidt, W.; Huang, H.; Freitag, L.; Sardeli, C. Modification of Apremilast from Pills to Aerosol a Future Concept. Int. J. Environ. Res. Public Health 2021, 18, 11590. https://doi.org/10.3390/ijerph182111590
Zarogoulidis P, Kosmidis C, Kougkas N, Lallas A, Petridis D, Hohenforst-Schmidt W, Huang H, Freitag L, Sardeli C. Modification of Apremilast from Pills to Aerosol a Future Concept. International Journal of Environmental Research and Public Health. 2021; 18(21):11590. https://doi.org/10.3390/ijerph182111590
Chicago/Turabian StyleZarogoulidis, Paul, Christoforos Kosmidis, Nikolaos Kougkas, Aimilios Lallas, Dimitris Petridis, Wolfgang Hohenforst-Schmidt, Haidong Huang, Lutz Freitag, and Chrisanthi Sardeli. 2021. "Modification of Apremilast from Pills to Aerosol a Future Concept" International Journal of Environmental Research and Public Health 18, no. 21: 11590. https://doi.org/10.3390/ijerph182111590
APA StyleZarogoulidis, P., Kosmidis, C., Kougkas, N., Lallas, A., Petridis, D., Hohenforst-Schmidt, W., Huang, H., Freitag, L., & Sardeli, C. (2021). Modification of Apremilast from Pills to Aerosol a Future Concept. International Journal of Environmental Research and Public Health, 18(21), 11590. https://doi.org/10.3390/ijerph182111590









