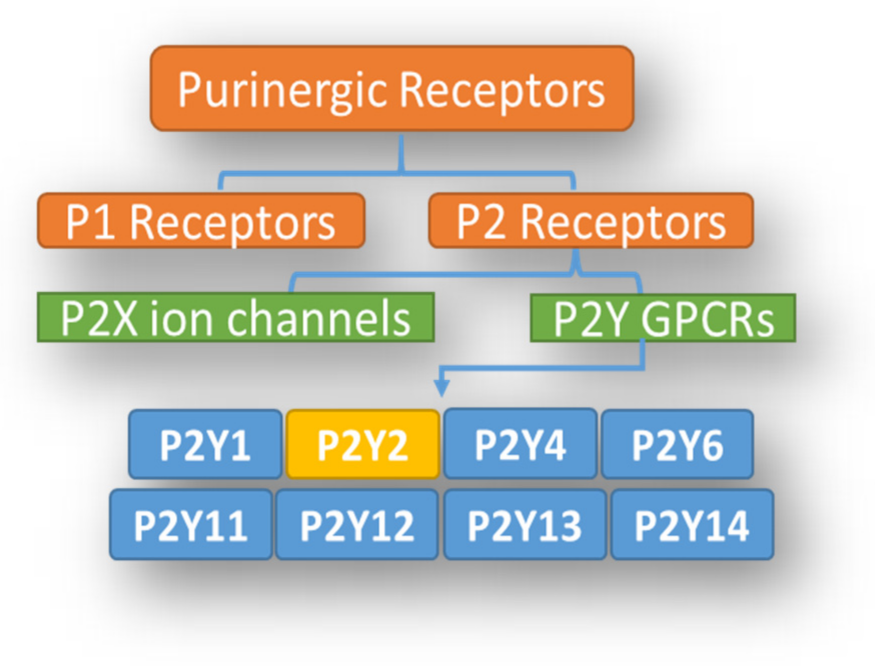
Role of the Purinergic P2Y2 Receptor in Pulmonary Hypertension
Abstract
1. Introduction
2. P2Y2 Expression in Pulmonary Parenchyma, Vasculature, and Inflammatory Cells
3. Deletion, Overexpression, and Disorders of P2Y2
3.1. Global P2Y2 Knockout
3.2. Conditional (Inducible) Knockout of P2Y2
3.3. P2Y2 Overexpression
4. P2Y2 Signaling
5. Physiological Function and Dysfunction of P2Y2
5.1. Control of Vascular Tone (Vasorelaxation or Vasoconstriction)
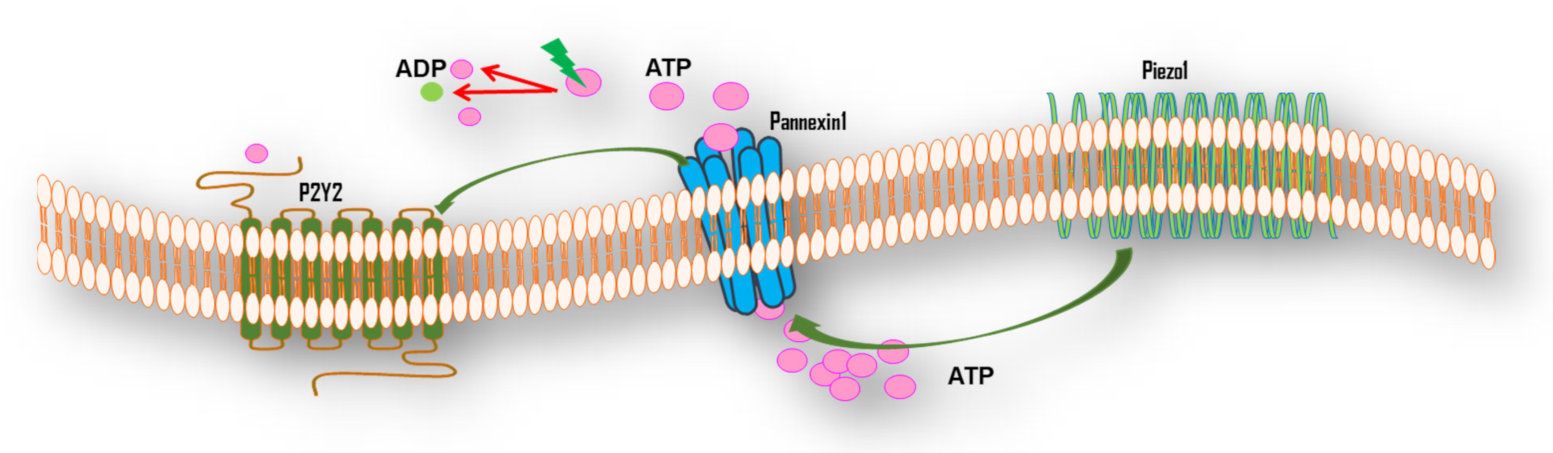
5.2. Mechanotransduction of FSS
5.3. Involvement in Phagocytic Clearance
5.4. Proliferation/Anti-Apoptosis and Vascular Remodeling
5.5. Pulmonary Angiogenesis
5.6. Blood Lung Barrier Dysfunction and Permeability of PAECs
5.7. P2Y2 in Pulmonary Hypertension
6. Pharmacological Importance of P2Y2 as a Potential PH Therapy
6.1. P2Y2 Agonists
6.2. P2Y2 Antagonists
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Galie, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An insider view on the World Symposium on Pulmonary Hypertension. Lancet Respir. Med. 2019, 7, 484–485. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Erb, L.; Weisman, G.A. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip. Rev. Membr. Transp. Signal 2012, 1, 789–803. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Tu, L.; Guignabert, C.; Merkus, D.; Zhou, Z. Purinergic Dysfunction in Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2020, 9, e017404. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, M.; Fonseca, M.; Perez-Zoghbi, J.F. Purinergic receptor stimulation induces calcium oscillations and smooth muscle contraction in small pulmonary veins. J. Physiol. 2018, 596, 2491–2506. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hu, J.; Subedi, K.P.; Lin, A.H.; Paudel, O.; Ran, P.; Sham, J.S. Extracellular Adenosine Diphosphate Ribose Mobilizes Intracellular Ca2+ via Purinergic-Dependent Ca2+ Pathways in Rat Pulmonary Artery Smooth Muscle Cells. Cell. Physiol. Biochem. 2015, 37, 2043–2059. [Google Scholar] [CrossRef]
- Lyubchenko, T.; Woodward, H.; Veo, K.D.; Burns, N.; Nijmeh, H.; Liubchenko, G.A.; Stenmark, K.R.; Gerasimovskaya, E.V. P2Y1 and P2Y13 purinergic receptors mediate Ca2+ signaling and proliferative responses in pulmonary artery vasa vasorum endothelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C266–C275. [Google Scholar] [CrossRef]
- Giannattasio, G.; Ohta, S.; Boyce, J.R.; Xing, W.; Balestrieri, B.; Boyce, J.A. The purinergic G protein-coupled receptor 6 inhibits effector T cell activation in allergic pulmonary inflammation. J. Immunol. 2011, 187, 1486–1495. [Google Scholar] [CrossRef]
- De Proost, I.; Pintelon, I.; Wilkinson, W.J.; Goethals, S.; Brouns, I.; Van Nassauw, L.; Riccardi, D.; Timmermans, J.P.; Kemp, P.J.; Adriaensen, D. Purinergic signaling in the pulmonary neuroepithelial body microenvironment unraveled by live cell imaging. FASEB J. 2009, 23, 1153–1160. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, A.; White, C.W. Purinergic signaling and kinase activation for survival in pulmonary oxidative stress and disease. Free Radic. Biol. Med. 2006, 41, 29–40. [Google Scholar] [CrossRef]
- McMillan, M.R.; Burnstock, G.; Haworth, S.G. Vasoconstriction of intrapulmonary arteries to P2-receptor nucleotides in normal and pulmonary hypertensive newborn piglets. Br. J. Pharmacol. 1999, 128, 549–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCormack, D.G.; Barnes, P.J.; Evans, T.W. Purinoceptors in the pulmonary circulation of the rat and their role in hypoxic vasoconstriction. Br. J. Pharmacol. 1989, 98, 367–372. [Google Scholar] [CrossRef]
- Li, H.H.; Hsu, H.H.; Chang, G.J.; Chen, I.C.; Ho, W.J.; Hsu, P.C.; Chen, W.J.; Pang, J.S.; Huang, C.C.; Lai, Y.J. Prostanoid EP4 agonist L-902,688 activates PPARgamma and attenuates pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L349–L359. [Google Scholar] [CrossRef]
- Lai, Y.J.; Pullamsetti, S.S.; Dony, E.; Weissmann, N.; Butrous, G.; Banat, G.A.; Ghofrani, H.A.; Seeger, W.; Grimminger, F.; Schermuly, R.T. Role of the prostanoid EP4 receptor in iloprost-mediated vasodilatation in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 188–196. [Google Scholar] [CrossRef]
- Olschewski, H.; Rose, F.; Schermuly, R.; Ghofrani, H.A.; Enke, B.; Olschewski, A.; Seeger, W. Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharmacol. Ther. 2004, 102, 139–153. [Google Scholar] [CrossRef]
- Olschewski, H.; Rose, F.; Grunig, E.; Ghofrani, H.A.; Walmrath, D.; Schulz, R.; Schermuly, R.; Grimminger, F.; Seeger, W. Cellular pathophysiology and therapy of pulmonary hypertension. J. Lab. Clin. Med. 2001, 138, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Glusa, E.; Adam, C. Endothelium-dependent relaxation induced by cathepsin G in porcine pulmonary arteries. Br. J. Pharmacol. 2001, 133, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, H.; Ghofrani, H.A.; Walmrath, D.; Schermuly, R.; Temmesfeld-Wollbruck, B.; Grimminger, F.; Seeger, W. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am. J. Respir. Crit. Care Med. 1999, 160, 600–607. [Google Scholar] [CrossRef]
- Walmrath, D.; Schermuly, R.; Pilch, J.; Grimminger, F.; Seeger, W. Effects of inhaled versus intravenous vasodilators in experimental pulmonary hypertension. Eur. Respir. J. 1997, 10, 1084–1092. [Google Scholar] [CrossRef]
- Strielkov, I.; Krause, N.C.; Sommer, N.; Schermuly, R.T.; Ghofrani, H.A.; Grimminger, F.; Gudermann, T.; Dietrich, A.; Weissmann, N. Hypoxic pulmonary vasoconstriction in isolated mouse pulmonary arterial vessels. Exp. Physiol. 2018, 103, 1185–1191. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.Y.; Huang, Y.; Wang, N. Rosiglitazone Attenuated Endothelin-1-Induced Vasoconstriction of Pulmonary Arteries in the Rat Model of Pulmonary Arterial Hypertension via Differential Regulation of ET-1 Receptors. PPAR Res. 2014, 2014, 374075. [Google Scholar] [CrossRef]
- Paddenberg, R.; Tiefenbach, M.; Faulhammer, P.; Goldenberg, A.; Gries, B.; Pfeil, U.; Lips, K.S.; Piruat, J.I.; Lopez-Barneo, J.; Schermuly, R.T.; et al. Mitochondrial complex II is essential for hypoxia-induced pulmonary vasoconstriction of intra- but not of pre-acinar arteries. Cardiovasc. Res. 2012, 93, 702–710. [Google Scholar] [CrossRef]
- MacLean, M.R.; McCulloch, K.M.; Baird, M. Endothelin ETA- and ETB-receptor-mediated vasoconstriction in rat pulmonary arteries and arterioles. J. Cardiovasc. Pharmacol. 1994, 23, 838–845. [Google Scholar] [CrossRef]
- Wang, S.; Iring, A.; Strilic, B.; Albarran Juarez, J.; Kaur, H.; Troidl, K.; Tonack, S.; Burbiel, J.C.; Muller, C.E.; Fleming, I.; et al. P2Y(2) and Gq/G(1)(1) control blood pressure by mediating endothelial mechanotransduction. J. Clin. Investig. 2015, 125, 3077–3086. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K. Understanding dry eye syndrome. Adv. Exp. Med. Biol. 2002, 506, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Bielory, L. Ocular allergy and dry eye syndrome. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; Li, W. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int. J. Mol. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef]
- Buscher, R.; Hoerning, A.; Patel, H.H.; Zhang, S.; Arthur, D.B.; Grasemann, H.; Ratjen, F.; Insel, P.A. P2Y2 receptor polymorphisms and haplotypes in cystic fibrosis and their impact on Ca2+ influx. Pharmacogenet. Genom. 2006, 16, 199–205. [Google Scholar] [CrossRef]
- Kellerman, D.; Evans, R.; Mathews, D.; Shaffer, C. Inhaled P2Y2 receptor agonists as a treatment for patients with Cystic Fibrosis lung disease. Adv. Drug Deliv. Rev. 2002, 54, 1463–1474. [Google Scholar] [CrossRef]
- Endo, K.I.; Sakamoto, A.; Fujisawa, K. Diquafosol tetrasodium elicits total cholesterol release from rabbit meibomian gland cells via P2Y2 purinergic receptor signalling. Sci. Rep. 2021, 11, 6989. [Google Scholar] [CrossRef]
- Deterding, R.; Retsch-Bogart, G.; Milgram, L.; Gibson, R.; Daines, C.; Zeitlin, P.L.; Milla, C.; Marshall, B.; Lavange, L.; Engels, J.; et al. Safety and tolerability of denufosol tetrasodium inhalation solution, a novel P2Y2 receptor agonist: Results of a phase 1/phase 2 multicenter study in mild to moderate cystic fibrosis. Pediatr. Pulmonol. 2005, 39, 339–348. [Google Scholar] [CrossRef]
- Deterding, R.R.; Lavange, L.M.; Engels, J.M.; Mathews, D.W.; Coquillette, S.J.; Brody, A.S.; Millard, S.P.; Ramsey, B.W.; The Cystic Fibrosis Therapeutics Development Network; The Inspire 08-103 Working Group. Phase 2 randomized safety and efficacy trial of nebulized denufosol tetrasodium in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 362–369. [Google Scholar] [CrossRef]
- Accurso, F.J.; Moss, R.B.; Wilmott, R.W.; Anbar, R.D.; Schaberg, A.E.; Durham, T.A.; Ramsey, B.W.; Group, T.-I.S. Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am. J. Respir. Crit. Care Med. 2011, 183, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Belete, H.A.; Hubmayr, R.D.; Wang, S.; Singh, R.D. The role of purinergic signaling on deformation induced injury and repair responses of alveolar epithelial cells. PLoS ONE 2011, 6, e27469. [Google Scholar] [CrossRef] [PubMed]
- Olotu, C.; Kiefmann, M.; Ronneburg, C.; Lehmensiek, F.; Cuvenhaus, A.; Meidl, V.; Goetz, A.E.; Kiefmann, R. Analysis of purine receptor expression and functionality in alveolar epithelial cells. Purinergic Signal 2020, 16, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, E.; Fauler, M.; Fois, G.; Frick, M. P2 Purinergic Signaling in the Distal Lung in Health and Disease. Int. J. Mol. Sci. 2020, 21, 4973. [Google Scholar] [CrossRef]
- Mitchell, C.; Syed, N.I.; Tengah, A.; Gurney, A.M.; Kennedy, C. Identification of contractile P2Y1, P2Y6, and P2Y12 receptors in rat intrapulmonary artery using selective ligands. J. Pharmacol. Exp. Ther. 2012, 343, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Walsh, M.P.; Jankowski, V.; Jankowski, J.; Zheng, X.L. Up4A stimulates endothelium-independent contraction of isolated rat pulmonary artery. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L733–L738. [Google Scholar] [CrossRef]
- Hartley, S.A.; Kato, K.; Salter, K.J.; Kozlowski, R.Z. Functional evidence for a novel suramin-insensitive pyrimidine receptor in rat small pulmonary arteries. Circ. Res. 1998, 83, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Visovatti, S.H.; Hyman, M.C.; Goonewardena, S.N.; Anyanwu, A.C.; Kanthi, Y.; Robichaud, P.; Wang, J.; Petrovic-Djergovic, D.; Rattan, R.; Burant, C.F.; et al. Purinergic dysregulation in pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H286–H298. [Google Scholar] [CrossRef]
- Konduri, G.G.; Bakhutashvili, I.; Frenn, R.; Chandrasekhar, I.; Jacobs, E.R.; Khanna, A.K. P2Y purine receptor responses and expression in the pulmonary circulation of juvenile rabbits. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H157–H164. [Google Scholar] [CrossRef]
- Batori, R.; Kumar, S.; Bordan, Z.; Cherian-Shaw, M.; Kovacs-Kasa, A.; MacDonald, J.A.; Fulton, D.J.R.; Erdodi, F.; Verin, A.D. Differential mechanisms of adenosine- and ATPgammaS-induced microvascular endothelial barrier strengthening. J. Cell. Physiol. 2019, 234, 5863–5879. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sokabe, T.; Ohura, N.; Nakatsuka, H.; Kamiya, A.; Ando, J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H793–H803. [Google Scholar] [CrossRef]
- Zemskov, E.; Lucas, R.; Verin, A.D.; Umapathy, N.S. P2Y receptors as regulators of lung endothelial barrier integrity. J. Cardiovasc. Dis. Res. 2011, 2, 14–22. [Google Scholar] [CrossRef]
- Helenius, M.H.; Vattulainen, S.; Orcholski, M.; Aho, J.; Komulainen, A.; Taimen, P.; Wang, L.; de Jesus Perez, V.A.; Koskenvuo, J.W.; Alastalo, T.P. Suppression of endothelial CD39/ENTPD1 is associated with pulmonary vascular remodeling in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L1046–L1057. [Google Scholar] [CrossRef] [PubMed]
- Kylhammar, D.; Bune, L.T.; Radegran, G. P2Y(1) and P2Y(1)(2) receptors in hypoxia- and adenosine diphosphate-induced pulmonary vasoconstriction in vivo in the pig. Eur. J. Appl. Physiol. 2014, 114, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Luneburg, N.; Stage, A.; Schmitz, M.; Korbelin, J.; Harbaum, L.; Matuszcak, C.; Mienert, J.; Bokemeyer, C.; Boger, R.H.; et al. The P2-receptor-mediated Ca(2+) signalosome of the human pulmonary endothelium—Implications for pulmonary arterial hypertension. Purinergic Signal 2019, 15, 299–311. [Google Scholar] [CrossRef]
- Chen, B.C.; Lee, C.M.; Lee, Y.T.; Lin, W.W. Characterization of signaling pathways of P2Y and P2U purinoceptors in bovine pulmonary artery endothelial cells. J. Cardiovasc. Pharmacol. 1996, 28, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, A.; Ghosh, M.; Leslie, C.C.; White, C.W. Extracellular ATP-mediated signaling for survival in hyperoxia-induced oxidative stress. J. Biol. Chem. 2004, 279, 16317–16325. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.J.; Farkas, L.; Rahman, T.; Kolb, M.R. ATP stimulates Ca(2+)-waves and gene expression in cultured human pulmonary fibroblasts. Int. J. Biochem. Cell Biol. 2009, 41, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Homolya, L.; Watt, W.C.; Lazarowski, E.R.; Koller, B.H.; Boucher, R.C. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y(2) receptor (−/−) mice. J. Biol. Chem. 1999, 274, 26454–26460. [Google Scholar] [CrossRef]
- Liang, S.; Desai, A.A.; Black, S.M.; Tang, H. Cytokines, Chemokines, and Inflammation in Pulmonary Arterial Hypertension. Adv. Exp. Med. Biol. 2021, 1303, 275–303. [Google Scholar] [CrossRef] [PubMed]
- Klouda, T.; Yuan, K. Inflammation in Pulmonary Arterial Hypertension. Adv. Exp. Med. Biol. 2021, 1303, 351–372. [Google Scholar] [CrossRef]
- Mercurio, V.; Cuomo, A.; Naranjo, M.; Hassoun, P.M. Inflammatory Mechanisms in the Pathogenesis of Pulmonary Arterial Hypertension: Recent Advances. Compr. Physiol. 2021, 11, 1805–1829. [Google Scholar] [CrossRef]
- Huertas, A.; Tu, L.; Humbert, M.; Guignabert, C. Chronic inflammation within the vascular wall in pulmonary arterial hypertension: More than a spectator. Cardiovasc. Res. 2020, 116, 885–893. [Google Scholar] [CrossRef]
- Myrtek, D.; Muller, T.; Geyer, V.; Derr, N.; Ferrari, D.; Zissel, G.; Durk, T.; Sorichter, S.; Luttmann, W.; Kuepper, M.; et al. Activation of human alveolar macrophages via P2 receptors: Coupling to intracellular Ca2+ increases and cytokine secretion. J. Immunol. 2008, 181, 2181–2188. [Google Scholar] [CrossRef]
- Bowler, J.W.; Bailey, R.J.; North, R.A.; Surprenant, A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br. J. Pharmacol. 2003, 140, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Raible, D.G.; McDermott, L.J.; Pelleg, A.; Schulman, E.S. Effects of purine and pyrimidine nucleotides on intracellular Ca2+ in human eosinophils: Activation of purinergic P2Y receptors. J. Allergy Clin. Immunol. 2001, 107, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Alkayed, F.; Kashimata, M.; Koyama, N.; Hayashi, T.; Tamura, Y.; Azuma, Y. P2Y11 purinoceptor mediates the ATP-enhanced chemotactic response of rat neutrophils. J. Pharmacol. Sci. 2012, 120, 288–295. [Google Scholar] [CrossRef]
- Chen, Y.; Shukla, A.; Namiki, S.; Insel, P.A.; Junger, W.G. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J. Leukoc. Biol. 2004, 76, 245–253. [Google Scholar] [CrossRef]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef]
- Rieg, T.; Bundey, R.A.; Chen, Y.; Deschenes, G.; Junger, W.; Insel, P.A.; Vallon, V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007, 21, 3717–3726. [Google Scholar] [CrossRef]
- Zhang, Y.; Sands, J.M.; Kohan, D.E.; Nelson, R.D.; Martin, C.F.; Carlson, N.G.; Kamerath, C.D.; Ge, Y.; Klein, J.D.; Kishore, B.K. Potential role of purinergic signaling in urinary concentration in inner medulla: Insights from P2Y2 receptor gene knockout mice. Am. J. Physiol. Renal Physiol. 2008, 295, F1715–F1724. [Google Scholar] [CrossRef]
- Cressman, V.L.; Lazarowski, E.; Homolya, L.; Boucher, R.C.; Koller, B.H.; Grubb, B.R. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(-) transport. J. Biol. Chem. 1999, 274, 26461–26468. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.Q.; Wang, Y.Y.; Jiang, Y.P.; Li, L.; Wang, H.J. VSNL1 Promotes Gastric Cancer Cell Proliferation and Migration by Regulating P2X3/P2Y2 Receptors and Is a Clinical Indicator of Poor Prognosis in Gastric Cancer Patients. Gastroenterol. Res. Pract. 2020, 2020, 7241942. [Google Scholar] [CrossRef]
- Homolya, L.; Steinberg, T.H.; Boucher, R.C. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J. Cell Biol. 2000, 150, 1349–1360. [Google Scholar] [CrossRef]
- Winters, S.L.; Davis, C.W.; Boucher, R.C. Mechanosensitivity of mouse tracheal ciliary beat frequency: Roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L614–L624. [Google Scholar] [CrossRef]
- Geary, C.; Akinbi, H.; Korfhagen, T.; Fabre, J.E.; Boucher, R.; Rice, W. Increased susceptibility of purinergic receptor-deficient mice to lung infection with Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L890–L895. [Google Scholar] [CrossRef] [PubMed]
- Vanderstocken, G.; Van de Paar, E.; Robaye, B.; di Pietrantonio, L.; Bondue, B.; Boeynaems, J.M.; Desmecht, D.; Communi, D. Protective role of P2Y2 receptor against lung infection induced by pneumonia virus of mice. PLoS ONE 2012, 7, e50385. [Google Scholar] [CrossRef] [PubMed]
- Vanderstocken, G.; Bondue, B.; Horckmans, M.; Di Pietrantonio, L.; Robaye, B.; Boeynaems, J.M.; Communi, D. P2Y2 receptor regulates VCAM-1 membrane and soluble forms and eosinophil accumulation during lung inflammation. J. Immunol. 2010, 185, 3702–3707. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Chen, Y.; Hirsh, M.I.; Yip, L.; Junger, W.G. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock 2008, 30, 173–177. [Google Scholar] [CrossRef]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Shainberg, A.; Hochhauser, E.; Cheporko, Y.; Tobar, A.; Birk, E.; Pinhas, L.; Leipziger, J.; Don, J.; Porat, E. UTP reduces infarct size and improves mice heart function after myocardial infarct via P2Y2 receptor. Biochem. Pharmacol. 2011, 82, 1126–1133. [Google Scholar] [CrossRef]
- Hochhauser, E.; Cohen, R.; Waldman, M.; Maksin, A.; Isak, A.; Aravot, D.; Jayasekara, P.S.; Muller, C.E.; Jacobson, K.A.; Shainberg, A. P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo. Purinergic Signal 2013, 9, 633–642. [Google Scholar] [CrossRef]
- Qian, S.; Regan, J.N.; Shelton, M.T.; Hoggatt, A.; Mohammad, K.S.; Herring, P.B.; Seye, C.I. The P2Y2 nucleotide receptor is an inhibitor of vascular calcification. Atherosclerosis 2017, 257, 38–46. [Google Scholar] [CrossRef][Green Version]
- Patel, J.J.; Zhu, D.; Opdebeeck, B.; D’Haese, P.; Millan, J.L.; Bourne, L.E.; Wheeler-Jones, C.P.D.; Arnett, T.R.; MacRae, V.E.; Orriss, I.R. Inhibition of arterial medial calcification and bone mineralization by extracellular nucleotides: The same functional effect mediated by different cellular mechanisms. J. Cell. Physiol. 2018, 233, 3230–3243. [Google Scholar] [CrossRef]
- Menzies, R.I.; Unwin, R.J.; Bailey, M.A. Renal P2 receptors and hypertension. Acta Physiol. (Oxf.) 2015, 213, 232–241. [Google Scholar] [CrossRef]
- Guns, P.J.; Van Assche, T.; Fransen, P.; Robaye, B.; Boeynaems, J.M.; Bult, H. Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br. J. Pharmacol. 2006, 147, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, S.; Hoggatt, A.; Tang, H.; Hacker, T.A.; Obukhov, A.G.; Herring, P.B.; Seye, C.I. Endothelial Cell-Specific Deletion of P2Y2 Receptor Promotes Plaque Stability in Atherosclerosis-Susceptible ApoE-Null Mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Agca, C.; Seye, C.; Kashuba Benson, C.M.; Rikka, S.; Chan, A.W.; Weisman, G.A.; Agca, Y. Development of a novel transgenic rat overexpressing the P2Y(2) nucleotide receptor using a lentiviral vector. J. Vasc. Res. 2009, 46, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Agca, Y.; Qian, S.; Agca, C.; Seye, C.I. Direct Evidence for P2Y2 Receptor Involvement in Vascular Response to Injury. J. Vasc. Res. 2016, 53, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Grampp, S.; Goppelt-Struebe, M.; Schreiber, R.; Kunzelmann, K.; Peters, D.J.; Leipziger, J.; Schley, G.; Schodel, J.; Eckardt, K.U.; et al. P2Y2R is a direct target of HIF-1alpha and mediates secretion-dependent cyst growth of renal cyst-forming epithelial cells. Purinergic Signal 2016, 12, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Tak, E.; Jun, D.Y.; Kim, S.H.; Park, G.C.; Lee, J.; Hwang, S.; Song, G.W.; Lee, S.G. Upregulation of P2Y2 nucleotide receptor in human hepatocellular carcinoma cells. J. Int. Med. Res. 2016, 44, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Sathanoori, R.; Rosi, F.; Gu, B.J.; Wiley, J.S.; Muller, C.E.; Olde, B.; Erlinge, D. Shear stress modulates endothelial KLF2 through activation of P2X4. Purinergic Signal 2015, 11, 139–153. [Google Scholar] [CrossRef]
- Sathanoori, R.; Bryl-Gorecka, P.; Muller, C.E.; Erb, L.; Weisman, G.A.; Olde, B.; Erlinge, D. P2Y2 receptor modulates shear stress-induced cell alignment and actin stress fibers in human umbilical vein endothelial cells. Cell. Mol. Life Sci. 2017, 74, 731–746. [Google Scholar] [CrossRef]
- Rodriguez-Zayas, A.E.; Torrado, A.I.; Miranda, J.D. P2Y2 receptor expression is altered in rats after spinal cord injury. Int. J. Dev. Neurosci. 2010, 28, 413–421. [Google Scholar] [CrossRef]
- Bagchi, S.; Liao, Z.; Gonzalez, F.A.; Chorna, N.E.; Seye, C.I.; Weisman, G.A.; Erb, L. The P2Y2 nucleotide receptor interacts with alphav integrins to activate Go and induce cell migration. J. Biol. Chem. 2005, 280, 39050–39057. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Seye, C.I.; Weisman, G.A.; Erb, L. The P2Y2 nucleotide receptor requires interaction with alpha v integrins to access and activate G12. J. Cell Sci. 2007, 120, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shao, C.; Lu, W.; Yan, C.; Yao, Q.; Zhu, M.; Chen, P.; Gu, P.; Fu, Y.; Fan, X. Adenosine triphosphate-induced rabbit corneal endothelial cell proliferation in vitro via the P2Y2-PI3K/Akt signaling axis. Cells Tissues Organs 2014, 199, 131–139. [Google Scholar] [CrossRef] [PubMed]
- El Husseini, D.; Boulanger, M.C.; Mahmut, A.; Bouchareb, R.; Laflamme, M.H.; Fournier, D.; Pibarot, P.; Bosse, Y.; Mathieu, P. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: Implication for calcific aortic valve disease. J. Mol. Cell. Cardiol. 2014, 72, 146–156. [Google Scholar] [CrossRef]
- Wang, T.; Takikawa, Y.; Watanabe, A.; Kakisaka, K.; Oikawa, K.; Miyamoto, Y.; Suzuki, K. Proliferation of mouse liver stem/progenitor cells induced by plasma from patients with acute liver failure is modulated by P2Y2 receptor-mediated JNK activation. J. Gastroenterol. 2014, 49, 1557–1566. [Google Scholar] [CrossRef]
- Cha, H.J.; Jung, M.S.; Ahn do, W.; Choi, J.K.; Ock, M.S.; Kim, K.S.; Yoon, J.H.; Song, E.J.; Song, K.S. Silencing of MUC8 by siRNA increases P2Y(2)-induced airway inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L495–L502. [Google Scholar] [CrossRef]
- Eun, S.Y.; Ko, Y.S.; Park, S.W.; Chang, K.C.; Kim, H.J. IL-1beta enhances vascular smooth muscle cell proliferation and migration via P2Y2 receptor-mediated RAGE expression and HMGB1 release. Vascul. Pharmacol. 2015, 72, 108–117. [Google Scholar] [CrossRef]
- Byun, Y.S.; Yoo, Y.S.; Kwon, J.Y.; Joo, J.S.; Lim, S.A.; Whang, W.J.; Mok, J.W.; Choi, J.S.; Joo, C.K. Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp. Eye Res. 2016, 143, 89–97. [Google Scholar] [CrossRef]
- Albarran-Juarez, J.; Iring, A.; Wang, S.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 2018, 215, 2655–2672. [Google Scholar] [CrossRef]
- Dai, X.; Tohyama, M.; Murakami, M.; Shiraishi, K.; Liu, S.; Mori, H.; Utsunomiya, R.; Maeyama, K.; Sayama, K. House dust mite allergens induce interleukin 33 (IL-33) synthesis and release from keratinocytes via ATP-mediated extracellular signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165719. [Google Scholar] [CrossRef]
- Zaparte, A.; Cappellari, A.R.; Brandao, C.A.; de Souza, J.B.; Borges, T.J.; Kist, L.W.; Bogo, M.R.; Zerbini, L.F.; Ribeiro Pinto, L.F.; Glaser, T.; et al. P2Y2 receptor activation promotes esophageal cancer cells proliferation via ERK1/2 pathway. Eur. J. Pharmacol. 2021, 891, 173687. [Google Scholar] [CrossRef]
- Uchida, Y.; Rutaganira, F.U.; Jullie, D.; Shokat, K.M.; von Zastrow, M. Endosomal Phosphatidylinositol 3-Kinase Is Essential for Canonical GPCR Signaling. Mol. Pharmacol. 2017, 91, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Li, B.I.; Matteson, P.G.; Ababon, M.F.; Nato, A.Q., Jr.; Lin, Y.; Nanda, V.; Matise, T.C.; Millonig, J.H. The orphan GPCR, Gpr161, regulates the retinoic acid and canonical Wnt pathways during neurulation. Dev. Biol. 2015, 402, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, H.; Wang, Z.; Rao, Y.; Wu, C.F.; Kitamoto, T. A novel role for ecdysone in Drosophila conditioned behavior: Linking GPCR-mediated non-canonical steroid action to cAMP signaling in the adult brain. PLoS Genet. 2013, 9, e1003843. [Google Scholar] [CrossRef]
- Favara, D.M.; Liebscher, I.; Jazayeri, A.; Nambiar, M.; Sheldon, H.; Banham, A.H.; Harris, A.L. Elevated expression of the adhesion GPCR ADGRL4/ELTD1 promotes endothelial sprouting angiogenesis without activating canonical GPCR signalling. Sci. Rep. 2021, 11, 8870. [Google Scholar] [CrossRef] [PubMed]
- Erb, L.; Liao, Z.; Seye, C.I.; Weisman, G.A. P2 receptors: Intracellular signaling. Pflugers Arch. 2006, 452, 552–562. [Google Scholar] [CrossRef]
- Liu, J.; Liao, Z.; Camden, J.; Griffin, K.D.; Garrad, R.C.; Santiago-Perez, L.I.; Gonzalez, F.A.; Seye, C.I.; Weisman, G.A.; Erb, L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J. Biol. Chem. 2004, 279, 8212–8218. [Google Scholar] [CrossRef]
- Seye, C.I.; Yu, N.; Gonzalez, F.A.; Erb, L.; Weisman, G.A. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J. Biol. Chem. 2004, 279, 35679–35686. [Google Scholar] [CrossRef]
- Fukai, K.; Nakamura, A.; Hoshino, A.; Nakanishi, N.; Okawa, Y.; Ariyoshi, M.; Kaimoto, S.; Uchihashi, M.; Ono, K.; Tateishi, S.; et al. Pyk2 aggravates hypoxia-induced pulmonary hypertension by activating HIF-1alpha. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H951–H959. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liao, Z.; Cao, C.; Wang, J.; Huxley, V.H.; Baker, O.; Weisman, G.A.; Erb, L. The P2Y2 Receptor Interacts with VE-Cadherin and VEGF Receptor-2 to Regulate Rac1 Activity in Endothelial Cells. J. Biomed. Sci. Eng. 2014, 7, 1105–1121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hein, T.W.; Kuo, L. cAMP-independent dilation of coronary arterioles to adenosine: Role of nitric oxide, G proteins, and K(ATP) channels. Circ. Res. 1999, 85, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Rieg, J.A.; Burt, J.M.; Ruth, P.; Rieg, T. P2Y(2) receptor activation decreases blood pressure via intermediate conductance potassium channels and connexin 37. Acta Physiol. 2015, 213, 628–641. [Google Scholar] [CrossRef]
- Hoffmann, C.; Ziegler, N.; Reiner, S.; Krasel, C.; Lohse, M.J. Agonist-selective, receptor-specific interaction of human P2Y receptors with beta-arrestin-1 and -2. J. Biol. Chem. 2008, 283, 30933–30941. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.E.; Nelson, C.P.; Everitt, D.; Brighton, P.J.; Standen, N.B.; Challiss, R.A.; Willets, J.M. G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling. Cardiovasc. Res. 2011, 89, 193–203. [Google Scholar] [CrossRef]
- Morris, G.E.; Nelson, C.P.; Brighton, P.J.; Standen, N.B.; Challiss, R.A.; Willets, J.M. Arrestins 2 and 3 differentially regulate ETA and P2Y2 receptor-mediated cell signaling and migration in arterial smooth muscle. Am. J. Physiol. Cell Physiol. 2012, 302, C723–C734. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Bakowski, D.; Nelson, C.; Mehta, R.; Almeyda, R.; Bates, G.; Parekh, A.B. Cysteinyl leukotriene type I receptor desensitization sustains Ca2+-dependent gene expression. Nature 2012, 482, 111–115. [Google Scholar] [CrossRef]
- Shenoy, S.K.; Lefkowitz, R.J. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 2011, 32, 521–533. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.X.; Liu, H.; Xu, C.B. Enhanced G-protein coupled receptors-mediated contraction and reduced endothelium-dependent relaxation in hypertension. Eur. J. Pharmacol. 2007, 557, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, Y.X.; Cao, L.; Liu, Y.; Xu, C.B. Heat stress alters G-protein coupled receptor-mediated function and endothelium-dependent relaxation in rat mesenteric artery. Eur. J. Pharmacol. 2008, 588, 280–285. [Google Scholar] [CrossRef]
- Jin, H.; Ko, Y.S.; Park, S.W.; Kim, H.J. P2Y2R activation by ATP induces oxLDL-mediated inflammasome activation through modulation of mitochondrial damage in human endothelial cells. Free Radic. Biol. Med. 2019, 136, 109–117. [Google Scholar] [CrossRef]
- Schuchardt, M.; Prufer, J.; Prufer, N.; Wiedon, A.; Huang, T.; Chebli, M.; Jankowski, V.; Jankowski, J.; Schafer-Korting, M.; Zidek, W.; et al. The endothelium-derived contracting factor uridine adenosine tetraphosphate induces P2Y(2)-mediated pro-inflammatory signaling by monocyte chemoattractant protein-1 formation. J. Mol. Med. 2011, 89, 799–810. [Google Scholar] [CrossRef]
- Nichols, K.K.; Yerxa, B.; Kellerman, D.J. Diquafosol tetrasodium: A novel dry eye therapy. Expert Opin. Investig. Drugs 2004, 13, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Diquafosol ophthalmic solution 3%: A review of its use in dry eye. Drugs 2015, 75, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Yerxa, B.R.; Sabater, J.R.; Davis, C.W.; Stutts, M.J.; Lang-Furr, M.; Picher, M.; Jones, A.C.; Cowlen, M.; Dougherty, R.; Boyer, J.; et al. Pharmacology of INS37217 [P(1)-(uridine 5′)-P(4)- (2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y(2) receptor agonist for the treatment of cystic fibrosis. J. Pharmacol. Exp. Ther. 2002, 302, 871–880. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, T.I.T.; Soares, V.E.M.; Wruck, J.; Dos Anjos, F.; de Resende, E.S.D.T.; de Oliveira Maciel, S.F.V.; Bagatini, M.D. Hyperinflammation and airway surface liquid dehydration in cystic fibrosis: Purinergic system as therapeutic target. Inflamm. Res. 2021, 70, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Iyinikkel, J.; Murray, F. GPCRs in pulmonary arterial hypertension: Tipping the balance. Br. J. Pharmacol. 2018, 175, 3063–3079. [Google Scholar] [CrossRef]
- Janssens, R.; Paindavoine, P.; Parmentier, M.; Boeynaems, J.M. Human P2Y2 receptor polymorphism: Identification and pharmacological characterization of two allelic variants. Br. J. Pharmacol. 1999, 127, 709–716. [Google Scholar] [CrossRef][Green Version]
- Hasan, D.; Satalin, J.; van der Zee, P.; Kollisch-Singule, M.; Blankman, P.; Shono, A.; Somhorst, P.; den Uil, C.; Meeder, H.; Kotani, T.; et al. Excessive Extracellular ATP Desensitizes P2Y2 and P2X4 ATP Receptors Provoking Surfactant Impairment Ending in Ventilation-Induced Lung Injury. Int. J. Mol. Sci. 2018, 19, 1185. [Google Scholar] [CrossRef]
- Dekker, R.J.; van Soest, S.; Fontijn, R.D.; Salamanca, S.; de Groot, P.G.; VanBavel, E.; Pannekoek, H.; Horrevoets, A.J. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 2002, 100, 1689–1698. [Google Scholar] [CrossRef]
- Groenendijk, B.C.; Van der Heiden, K.; Hierck, B.P.; Poelmann, R.E. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology 2007, 22, 380–389. [Google Scholar] [CrossRef]
- Kinderlerer, A.R.; Ali, F.; Johns, M.; Lidington, E.A.; Leung, V.; Boyle, J.J.; Hamdulay, S.S.; Evans, P.C.; Haskard, D.O.; Mason, J.C. KLF2-dependent, shear stress-induced expression of CD59: A novel cytoprotective mechanism against complement-mediated injury in the vasculature. J. Biol. Chem. 2008, 283, 14636–14644. [Google Scholar] [CrossRef]
- Doddaballapur, A.; Michalik, K.M.; Manavski, Y.; Lucas, T.; Houtkooper, R.H.; You, X.; Chen, W.; Zeiher, A.M.; Potente, M.; Dimmeler, S.; et al. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 137–145. [Google Scholar] [CrossRef]
- Ko, H.; Carter, R.L.; Cosyn, L.; Petrelli, R.; de Castro, S.; Besada, P.; Zhou, Y.; Cappellacci, L.; Franchetti, P.; Grifantini, M.; et al. Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists. Bioorg. Med. Chem. 2008, 16, 6319–6332. [Google Scholar] [CrossRef]
- Alsaqati, M.; Chan, S.L.; Ralevic, V. Investigation of the functional expression of purine and pyrimidine receptors in porcine isolated pancreatic arteries. Purinergic Signal 2014, 10, 241–249. [Google Scholar] [CrossRef]
- Certal, M.; Vinhas, A.; Pinheiro, A.R.; Ferreirinha, F.; Barros-Barbosa, A.R.; Silva, I.; Costa, M.A.; Correia-de-Sa, P. Calcium signaling and the novel anti-proliferative effect of the UTP-sensitive P2Y11 receptor in rat cardiac myofibroblasts. Cell Calcium 2015, 58, 518–533. [Google Scholar] [CrossRef]
- Linan-Rico, A.; Ochoa-Cortes, F.; Zuleta-Alarcon, A.; Alhaj, M.; Tili, E.; Enneking, J.; Harzman, A.; Grants, I.; Bergese, S.; Christofi, F.L. UTP—Gated Signaling Pathways of 5-HT Release from BON Cells as a Model of Human Enterochromaffin Cells. Front. Pharmacol. 2017, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Hevia, M.J.; Castro, P.; Pinto, K.; Reyna-Jeldes, M.; Rodriguez-Tirado, F.; Robles-Planells, C.; Ramirez-Rivera, S.; Madariaga, J.A.; Gutierrez, F.; Lopez, J.; et al. Differential Effects of Purinergic Signaling in Gastric Cancer-Derived Cells Through P2Y and P2X Receptors. Front. Pharmacol. 2019, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Obara, Y.; Sugama, J.; Kotani, A.; Koike, N.; Ohkubo, S.; Nakahata, N. P2Y2 receptor-Gq/11 signaling at lipid rafts is required for UTP-induced cell migration in NG 108-15 cells. J. Pharmacol. Exp. Ther. 2010, 334, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Feletou, M.; Weston, A.H. Endothelium-derived hyperpolarising factors and associated pathways: A synopsis. Pflugers Arch. 2010, 459, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Cohen, R.A.; Vanhoutte, P.M.; Verbeuren, T.J. TP receptors and oxidative stress hand in hand from endothelial dysfunction to atherosclerosis. Adv. Pharmacol. 2010, 60, 85–106. [Google Scholar] [CrossRef]
- Feletou, M.; Huang, Y.; Vanhoutte, P.M. Vasoconstrictor prostanoids. Pflugers Arch. 2010, 459, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Kohler, R.; Vanhoutte, P.M. Endothelium-derived vasoactive factors and hypertension: Possible roles in pathogenesis and as treatment targets. Curr. Hypertens. Rep. 2010, 12, 267–275. [Google Scholar] [CrossRef]
- Liang, C.F.; Au, A.L.; Leung, S.W.; Ng, K.F.; Feletou, M.; Kwan, Y.W.; Man, R.Y.; Vanhoutte, P.M. Endothelium-derived nitric oxide inhibits the relaxation of the porcine coronary artery to natriuretic peptides by desensitizing big conductance calcium-activated potassium channels of vascular smooth muscle. J. Pharmacol. Exp. Ther. 2010, 334, 223–231. [Google Scholar] [CrossRef]
- Vayssettes-Courchay, C.; Ragonnet, C.; Camenen, G.; Feletou, M.; Cordi, A.A.; Lavielle, G.; Verbeuren, T.J. Role of thromboxane TP and angiotensin AT1 receptors in lipopolysaccharide-induced arterial dysfunction in the rabbit: An in vivo study. Eur. J. Pharmacol. 2010, 634, 113–120. [Google Scholar] [CrossRef]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef]
- Rieg, T.; Gerasimova, M.; Boyer, J.L.; Insel, P.A.; Vallon, V. P2Y(2) receptor activation decreases blood pressure and increases renal Na(+) excretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R510–R518. [Google Scholar] [CrossRef]
- Velasquez, S.; Eugenin, E.A. Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front. Physiol. 2014, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zheng, X.; Qin, X.; Dou, D.; Xu, H.; Raj, J.U.; Gao, Y. Protein kinase G regulates the basal tension and plays a major role in nitrovasodilator-induced relaxation of porcine coronary veins. Br. J. Pharmacol. 2007, 152, 1060–1069. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Y.; Wang, Y.; Ma, X.; Huang, Y.; Li, R.A.; Wan, S.; Yao, X. Nitric oxide and protein kinase G act on TRPC1 to inhibit 11,12-EET-induced vascular relaxation. Cardiovasc. Res. 2014, 104, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Amirjanians, M.; Egemnazarov, B.; Sydykov, A.; Kojonazarov, B.; Brandes, R.; Luitel, H.; Pradhan, K.; Stasch, J.P.; Redlich, G.; Weissmann, N.; et al. Chronic intratracheal application of the soluble guanylyl cyclase stimulator BAY 41-8543 ameliorates experimental pulmonary hypertension. Oncotarget 2017, 8, 29613–29624. [Google Scholar] [CrossRef]
- Deaglio, S.; Robson, S.C. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv. Pharmacol. 2011, 61, 301–332. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759. [Google Scholar] [CrossRef]
- Hyman, A.J.; Tumova, S.; Beech, D.J. Piezo1 Channels in Vascular Development and the Sensing of Shear Stress. Curr. Top. Membr. 2017, 79, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Beech, D.J. Endothelial Piezo1 channels as sensors of exercise. J. Physiol. 2018, 596, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Rafikova, O.; Al Ghouleh, I.; Rafikov, R. Focus on Early Events: Pathogenesis of Pulmonary Arterial Hypertension Development. Antioxid. Redox Signal. 2019, 31, 933–953. [Google Scholar] [CrossRef]
- Yun, J.H.; Henson, P.M.; Tuder, R.M. Phagocytic clearance of apoptotic cells: Role in lung disease. Expert Rev. Respir. Med. 2008, 2, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Dolgachev, V.; Panicker, S.; Balijepalli, S.; McCandless, L.K.; Yin, Y.; Swamy, S.; Suresh, M.V.; Delano, M.J.; Hemmila, M.R.; Raghavendran, K.; et al. Electroporation-mediated delivery of FER gene enhances innate immune response and improves survival in a murine model of pneumonia. Gene Ther. 2018, 25, 359–375. [Google Scholar] [CrossRef]
- Bucheimer, R.E.; Linden, J. Purinergic regulation of epithelial transport. J. Physiol. 2004, 555, 311–321. [Google Scholar] [CrossRef]
- Griesenbach, U.; Geddes, D.M.; Alton, E.W. Advances in cystic fibrosis gene therapy. Curr. Opin. Pulm. Med. 2004, 10, 542–546. [Google Scholar] [CrossRef]
- Davies, J.C.; Alton, E.W. Airway gene therapy. Adv. Genet. 2005, 54, 291–314. [Google Scholar] [CrossRef]
- Tate, S.; Elborn, S. Progress towards gene therapy for cystic fibrosis. Expert Opin. Drug Deliv. 2005, 2, 269–280. [Google Scholar] [CrossRef]
- Ruan, J.; Hirai, H.; Yang, D.; Ma, L.; Hou, X.; Jiang, H.; Wei, H.; Rajagopalan, C.; Mou, H.; Wang, G.; et al. Efficient Gene Editing at Major CFTR Mutation Loci. Mol. Ther. Nucleic Acids 2019, 16, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Wang, L.Y.; Qu, H.N.; Yu, L.H.; Burnstock, G.; Ni, X.; Xu, M.; Ma, B. P2Y2 receptor-mediated modulation of estrogen-induced proliferation of breast cancer cells. Mol. Cell. Endocrinol. 2011, 338, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ji, Y.G.; Lee, D.H. Uridine triphosphate increases proliferation of human cancerous pancreatic duct epithelial cells by activating P2Y2 receptor. Pancreas 2013, 42, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Tackett, B.C.; Sun, H.; Mei, Y.; Maynard, J.P.; Cheruvu, S.; Mani, A.; Hernandez-Garcia, A.; Vigneswaran, N.; Karpen, S.J.; Thevananther, S. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G1073–G1087. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Voelkel, N.F. Angiogenesis and pulmonary hypertension: A unique process in a unique disease. Antioxid. Redox Signal. 2002, 4, 833–843. [Google Scholar] [CrossRef]
- Wolf, D.; Tseng, N.; Seedorf, G.; Roe, G.; Abman, S.H.; Gien, J. Endothelin-1 decreases endothelial PPARgamma signaling and impairs angiogenesis after chronic intrauterine pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L361–L371. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa-Shindo, Y.; Sakurai, T.; Kamiyoshi, A.; Kawate, H.; Iinuma, N.; Yoshizawa, T.; Koyama, T.; Fukuchi, J.; Iimuro, S.; Moriyama, N.; et al. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J. Clin. Investig. 2008, 118, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Wyder, L.; Suply, T.; Ricoux, B.; Billy, E.; Schnell, C.; Baumgarten, B.U.; Maira, S.M.; Koelbing, C.; Ferretti, M.; Kinzel, B.; et al. Reduced pathological angiogenesis and tumor growth in mice lacking GPR4, a proton sensing receptor. Angiogenesis 2011, 14, 533–544. [Google Scholar] [CrossRef]
- Hanumegowda, C.; Farkas, L.; Kolb, M. Angiogenesis in pulmonary fibrosis: Too much or not enough? Chest 2012, 142, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Sheu, T.J.; Menon, P.; Pang, J.; Ho, H.C.; Shi, S.; Xie, C.; Smolock, E.; Yan, C.; Zuscik, M.J.; et al. Impaired angiogenesis during fracture healing in GPCR kinase 2 interacting protein-1 (GIT1) knock out mice. PLoS ONE 2014, 9, e89127. [Google Scholar] [CrossRef] [PubMed]
- Kir, D.; Schnettler, E.; Modi, S.; Ramakrishnan, S. Regulation of angiogenesis by microRNAs in cardiovascular diseases. Angiogenesis 2018, 21, 699–710. [Google Scholar] [CrossRef]
- Kang, H.; Hong, Z.; Zhong, M.; Klomp, J.; Bayless, K.J.; Mehta, D.; Karginov, A.V.; Hu, G.; Malik, A.B. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am. J. Physiol. Cell Physiol. 2019, 316, C92–C103. [Google Scholar] [CrossRef]
- Gogiraju, R.; Bochenek, M.L.; Schafer, K. Angiogenic Endothelial Cell Signaling in Cardiac Hypertrophy and Heart Failure. Front. Cardiovasc. Med. 2019, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; Hacker, M.R.; Rhee, J.S.; Mitchell, J.; Mahmood, F.; et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485, 333–338. [Google Scholar] [CrossRef]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef]
- McEnaney, R.M.; Shukla, A.; Madigan, M.C.; Sachdev, U.; Tzeng, E. P2Y2 nucleotide receptor mediates arteriogenesis in a murine model of hind limb ischemia. J. Vasc. Surg. 2016, 63, 216–225. [Google Scholar] [CrossRef][Green Version]
- Strassheim, D.; Verin, A.; Batori, R.; Nijmeh, H.; Burns, N.; Kovacs-Kasa, A.; Umapathy, N.S.; Kotamarthi, J.; Gokhale, Y.S.; Karoor, V.; et al. P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 6855. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Pappas, P.J.; Hobson, R.W., II; Boric, M.P.; Sessa, W.C.; Duran, W.N. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J. Physiol. 2006, 574, 275–281. [Google Scholar] [CrossRef]
- Fujihara, T.; Murakami, T.; Fujita, H.; Nakamura, M.; Nakata, K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Investig. Ophthalmol. Vis. Sci. 2001, 42, 96–100. [Google Scholar]
- Umapathy, N.S.; Fan, Z.; Zemskov, E.A.; Alieva, I.B.; Black, S.M.; Verin, A.D. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vascul. Pharmacol. 2010, 52, 199–206. [Google Scholar] [CrossRef]
- Konduri, G.G.; Forman, K.; Mital, S. Characterization of purine receptors in fetal lamb pulmonary circulation. Pediatr. Res. 2000, 47, 114–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Konduri, G.G.; Woodard, L.L. Selective pulmonary vasodilation by low-dose infusion of adenosine triphosphate in newborn lambs. J. Pediatr. 1991, 119, 94–102. [Google Scholar] [CrossRef]
- Kaapa, P.; Jahnukainen, T.; Gronlund, J.; Rautanen, M.; Halkola, L.; Valimaki, I. Adenosine triphosphate treatment for meconium aspiration-induced pulmonary hypertension in pigs. Acta Physiol. Scand. 1997, 160, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.R. Oxygen-induced pulmonary vasodilation is mediated by adenosine triphosphate in newborn lambs. J. Cardiovasc. Pharmacol. 1997, 30, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Paidas, C.N.; Dudgeon, D.L.; Haller, J.A., Jr.; Clemens, M.G. Adenosine triphosphate: A potential therapy for hypoxic pulmonary hypertension. J. Pediatr. Surg. 1988, 23, 1154–1160. [Google Scholar] [CrossRef]
- Brook, M.M.; Fineman, J.R.; Bolinger, A.M.; Wong, A.F.; Heymann, M.A.; Soifer, S.J. Use of ATP-MgCl2 in the evaluation and treatment of children with pulmonary hypertension secondary to congenital heart defects. Circulation 1994, 90, 1287–1293. [Google Scholar] [CrossRef]
- Crooke, A.; Guzmán-Aranguez, A.; Peral, A.; Abdurrahman, M.K.A.; Pintor, J. Nucleotides in ocular secretions: Their role in ocular physiology. Pharmacol. Ther. 2008, 119, 55–73. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Boeynaems, J.M. P2Y nucleotide receptors: Promise of therapeutic applications. Drug Discov. Today 2010, 15, 570–578. [Google Scholar] [CrossRef]
- Weisman, G.A.; Woods, L.T.; Erb, L.; Seye, C.I. P2Y receptors in the mammalian nervous system: Pharmacology, ligands and therapeutic potential. CNS Neurol. Disord. Drug Targets 2012, 11, 722–738. [Google Scholar] [CrossRef]
- Pintor, J.; Carracedo, G.; Alonso, C.M.; Bautista, A.; Peral, A. Presence of diadenosine polyphosphates in human tears. Pflügers Archiv. 2002, 443, 432–436. [Google Scholar] [CrossRef]
- Burnstock, G. Introductory overview of purinergic signalling. Front. Biosci. 2011, 3, 896–900. [Google Scholar] [CrossRef]
- Brunschweiger, A.; Muller, C.E. P2 receptors activated by uracil nucleotides—An update. Curr. Med. Chem. 2006, 13, 289–312. [Google Scholar] [CrossRef]
- El-Tayeb, A.; Qi, A.; Muller, C.E. Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J. Med. Chem. 2006, 49, 7076–7087. [Google Scholar] [CrossRef]
- Rafehi, M.; Burbiel, J.C.; Attah, I.Y.; Abdelrahman, A.; Muller, C.E. Synthesis, characterization, and in vitro evaluation of the selective P2Y2 receptor antagonist AR-C118925. Purinergic Signal. 2017, 13, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Troadec, J.D.; Thirion, S.; Petturiti, D.; Bohn, M.T.; Poujeol, P. ATP acting on P2Y receptors triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes (pituicytes). Pflugers Arch. 1999, 437, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Fujihara, T.; Nakamura, M.; Nakata, K. P2Y(2) receptor stimulation increases tear fluid secretion in rabbits. Curr. Eye Res. 2000, 21, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Fujihara, T.; Nakamura, M.; Nakata, K. P2Y(2) receptor elicits PAS-positive glycoprotein secretion from rabbit conjunctival goblet cells in vivo. J. Ocul. Pharmacol. Ther. 2003, 19, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jumblatt, J.E.; Jumblatt, M.M. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp. Eye Res. 1998, 67, 341–346. [Google Scholar] [CrossRef]
- Goss, C.H.; McKone, E.F.; Mathews, D.; Kerr, D.; Wanger, J.S.; Millard, S.P.; Cystic Fibrosis Therapeutics Development, N. Experience using centralized spirometry in the phase 2 randomized, placebo-controlled, double-blind trial of denufosol in patients with mild to moderate cystic fibrosis. J. Cyst. Fibros. 2008, 7, 147–153. [Google Scholar] [CrossRef][Green Version]
- Kellerman, D.; Rossi Mospan, A.; Engels, J.; Schaberg, A.; Gorden, J.; Smiley, L. Denufosol: A review of studies with inhaled P2Y(2) agonists that led to Phase 3. Pulm. Pharmacol. Ther. 2008, 21, 600–607. [Google Scholar] [CrossRef]
- Ratjen, F.; Durham, T.; Navratil, T.; Schaberg, A.; Accurso, F.J.; Wainwright, C.; Barnes, M.; Moss, R.B.; Group, T.-S.I. Long term effects of denufosol tetrasodium in patients with cystic fibrosis. J. Cyst. Fibros. 2012, 11, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B. Pitfalls of drug development: Lessons learned from trials of denufosol in cystic fibrosis. J. Pediatr. 2013, 162, 676–680. [Google Scholar] [CrossRef]
- Muoboghare, M.O.; Drummond, R.M.; Kennedy, C. Characterisation of P2Y2 receptors in human vascular endothelial cells using AR-C118925XX, a competitive and selective P2Y2 antagonist. Br. J. Pharmacol. 2019, 176, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Cheng, K.S.; Wong, K.L.; Shiao, L.R.; Leung, Y.M.; Chang, L.Y. ARC 118925XX stimulates cation influx in bEND.3 endothelial cells. Fundam. Clin. Pharmacol. 2019, 33, 604–611. [Google Scholar] [CrossRef]
- Kemp, P.A.; Sugar, R.A.; Jackson, A.D. Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004, 31, 446–455. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Costanzi, S.; Ohno, M.; Joshi, B.V.; Besada, P.; Xu, B.; Tchilibon, S. Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Curr. Top. Med. Chem. 2004, 4, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, P.; Ko, G.Y.; Spinrath, A.; Raulf, A.; von Kugelgen, I.; Wolff, S.C.; Nicholas, R.A.; Kostenis, E.; Holtje, H.D.; Muller, C.E. Key determinants of nucleotide-activated G protein-coupled P2Y(2) receptor function revealed by chemical and pharmacological experiments, mutagenesis and homology modeling. J. Med. Chem. 2009, 52, 2762–2775. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Watt, W.C.; Stutts, M.J.; Boucher, R.C.; Harden, T.K. Pharmacological selectivity of the cloned human P2U-purinoceptor: Potent activation by diadenosine tetraphosphate. Br. J. Pharmacol. 1995, 116, 1619–1627. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Jarvis, M.F.; Williams, M. Purine and pyrimidine (P2) receptors as drug targets. J. Med. Chem. 2002, 45, 4057–4093. [Google Scholar] [CrossRef]
- Kim, H.S.; Ravi, R.G.; Marquez, V.E.; Maddileti, S.; Wihlborg, A.K.; Erlinge, D.; Malmsjo, M.; Boyer, J.L.; Harden, T.K.; Jacobson, K.A. Methanocarba modification of uracil and adenine nucleotides: High potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors. J. Med. Chem. 2002, 45, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, M.; Muller, C.E. Tools and drugs for uracil nucleotide-activated P2Y receptors. Pharmacol. Ther. 2018, 190, 24–80. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tostes, R.C.; Webb, R.C. Uridine adenosine tetraphosphate-induced contraction is increased in renal but not pulmonary arteries from DOCA-salt hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H409–H417. [Google Scholar] [CrossRef]
- Martin-Gil, A.; de Lara, M.J.; Crooke, A.; Santano, C.; Peral, A.; Pintor, J. Silencing of P2Y(2) receptors reduces intraocular pressure in New Zealand rabbits. Br. J. Pharmacol. 2012, 165, 1163–1172. [Google Scholar] [CrossRef]
- Degagne, E.; Degrandmaison, J.; Grbic, D.M.; Vinette, V.; Arguin, G.; Gendron, F.P. P2Y2 receptor promotes intestinal microtubule stabilization and mucosal re-epithelization in experimental colitis. J. Cell. Physiol. 2013, 228, 99–109. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, C. Purinergic signaling negatively regulates activity of an olfactory receptor in an odorant-dependent manner. Neuroscience 2014, 275, 89–101. [Google Scholar] [CrossRef]
- Bouchareb, R.; Cote, N.; Marie Chloe, B.; Le Quang, K.; El Husseini, D.; Asselin, J.; Hadji, F.; Lachance, D.; Shayhidin, E.E.; Mahmut, A.; et al. Carbonic anhydrase XII in valve interstitial cells promotes the regression of calcific aortic valve stenosis. J. Mol. Cell. Cardiol. 2015, 82, 104–115. [Google Scholar] [CrossRef]
- Yitzhaki, S.; Hochhauser, E.; Porat, E.; Shainberg, A. Uridine-5′-triphosphate (UTP) maintains cardiac mitochondrial function following chemical and hypoxic stress. J. Mol. Cell. Cardiol. 2007, 43, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Pintor, J.; Miras-Portugal, M.T. Ca(2+)-stores mobilization by diadenosine tetraphosphate, Ap4A, through a putative P2Y purinoceptor in adrenal chromaffin cells. Br. J. Pharmacol. 1992, 106, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Barnes, A.; Camacho, J.; Paterson, C.; Boughtflower, R.; Cousens, D.; Marshall, F. Activity of diadenosine polyphosphates at P2Y receptors stably expressed in 1321N1 cells. Eur. J. Pharmacol. 2001, 430, 203–210. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Fricks, I.; Kendall Harden, T.; Jacobson, K.A. Molecular dynamics simulation of the P2Y14 receptor. Ligand docking and identification of a putative binding site of the distal hexose moiety. Bioorg. Med. Chem. Lett. 2007, 17, 761–766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Tayeb, A.; Qi, A.; Nicholas, R.A.; Muller, C.E. Structural modifications of UMP, UDP, and UTP leading to subtype-selective agonists for P2Y2, P2Y4, and P2Y6 receptors. J. Med. Chem. 2011, 54, 2878–2890. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Muller, C.E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016, 104, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, H.; Gu, Y.; Sun, P.; Sun, J.; Yu, H.; Zheng, H.; Chen, D. P2Y2 promotes fibroblasts activation and skeletal muscle fibrosis through AKT, ERK, and PKC. BMC Musculoskelet. Disord. 2021, 22, 680. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Watt, W.C.; Stutts, M.J.; Brown, H.A.; Boucher, R.C.; Harden, T.K. Enzymatic synthesis of UTP gamma S, a potent hydrolysis resistant agonist of P2U-purinoceptors. Br. J. Pharmacol. 1996, 117, 203–209. [Google Scholar] [CrossRef]
- Pendergast, W.; Yerxa, B.R.; Douglass, J.G., III; Shaver, S.R.; Dougherty, R.W.; Redick, C.C.; Sims, I.F.; Rideout, J.L. Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5′-polyphosphates. Bioorg. Med. Chem. Lett. 2001, 11, 157–160. [Google Scholar] [CrossRef]
- Murakami, T.; Fujihara, T.; Horibe, Y.; Nakamura, M. Diquafosol elicits increases in net Cl- transport through P2Y2 receptor stimulation in rabbit conjunctiva. Ophthalmic Res. 2004, 36, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Godinez, C.; Carracedo, G.; Pintor, J. Diquafosol Delivery from Silicone Hydrogel Contact Lenses: Improved Effect on Tear Secretion. J. Ocul. Pharmacol. Ther. 2018, 34, 170–176. [Google Scholar] [CrossRef]
- Mironova, E.; Suliman, F.; Stockand, J.D. Renal Na(+) excretion consequent to pharmacogenetic activation of Gq-DREADD in principal cells. Am. J. Physiol. Renal Physiol. 2019, 316, F758–F767. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Ko, H.; Cosyn, L.; Maddileti, S.; Besada, P.; Fricks, I.; Costanzi, S.; Harden, T.K.; Calenbergh, S.V.; Jacobson, K.A. Molecular modeling of the human P2Y2 receptor and design of a selective agonist, 2′-amino-2′-deoxy-2-thiouridine 5′-triphosphate. J. Med. Chem. 2007, 50, 1166–1176. [Google Scholar] [CrossRef]
- Kim, J.C.; Woo, S.H. Shear stress induces a longitudinal Ca(2+) wave via autocrine activation of P2Y1 purinergic signalling in rat atrial myocytes. J. Physiol. 2015, 593, 5091–5109. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Xu, J.; Wen, G.; Jin, H.; Liu, X.; Yang, Y.; Ji, B.; Jiang, Y.; Song, P.; Dong, H.; et al. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J. Biol. Chem. 2014, 289, 19137–19149. [Google Scholar] [CrossRef] [PubMed]

| Cell Type | Species | Detection Level | Ref. |
|---|---|---|---|
| PAECs | Human | mRNA | [47,48] |
| Bovine | Agonist/activity | [52] | |
| Rabbits | mRNA and agonist/activity | [45] | |
| LMVECs | Human | mRNA | [48,53] |
| PASMCs | Rat | Agonist/activity | [43] |
| PFs | Human | mRNA | [54] |
| Mouse | mRNA | [55] |
| Ligand | Action | Target | Ref. |
|---|---|---|---|
| ATP | Agonist | P2Y1, P2Y11, P2Y12, P2Y2, P2Y4, P2Y6, P2Y13 | [71,209,210,211] |
| UTP | Agonist | P2Y1, P2Y11, P2Y2, P2Y4, P2Y6 | [204,211,212] |
| 2-thioUTP | Agonist | P2Y2, P2Y4, P2Y6 | [194,213,214,215,216,217] |
| 4-thio-UTP | Agonist | P2Y2, P2Y4 | [193] |
| UTPγS | Agonist | P2Y2 | [209,218] |
| 5BrUTP | Agonist | P2Y2, P2Y6 | [209] |
| Ap4A | Agonist | P2Y2, P2Y4, P2Y12, P2Y13 | [93,127,209,219,220] |
| PSB1114 | Agonist | P2Y2, P2Y4, P2Y6 | [194,212,221,222,223,224] |
| Denufosol (INS37217) | Agonist | P2Y2, P2Y4, P2Y6 | [35,36,37,146,200,201,202,203] |
| Diquafosol (INS365) | Agonist | P2Y1, P2Y2, P2Y4, P2Y6 | [34,98,122,123,124,225,226,227,228] |
| INS45973 | Agonist | P2Y2, P2Y4 | [146,229] |
| MRS2698 | Agonist | P2Y2, P2Y4, P2Y6 | [212,230] |
| MRS2768 | Agonist | P2Y2 | [78,133,134,135,136,137,164,229] |
| AR-C126313 | Antagonist | P2Y2 | [207,230] |
| Reactive blue-2 | Antagonist | P2Y2, P2Y4, P2Y6, P2Y11, P2Y13 | [127,135] |
| AR-C118925XX | Antagonist | P2Y2 | [34,206] |
| PSB-416 | Antagonist | P2Y2 | [208] |
| Suramin | Antagonist | P2Y1, P2Y11, P2Y12, P2Y2, P2Y4, P2Y6, P2Y1, P2Y13 | [6,7,44,98,127,135,164,206,231,232] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shihan, M.; Novoyatleva, T.; Lehmeyer, T.; Sydykov, A.; Schermuly, R.T. Role of the Purinergic P2Y2 Receptor in Pulmonary Hypertension. Int. J. Environ. Res. Public Health 2021, 18, 11009. https://doi.org/10.3390/ijerph182111009
Shihan M, Novoyatleva T, Lehmeyer T, Sydykov A, Schermuly RT. Role of the Purinergic P2Y2 Receptor in Pulmonary Hypertension. International Journal of Environmental Research and Public Health. 2021; 18(21):11009. https://doi.org/10.3390/ijerph182111009
Chicago/Turabian StyleShihan, Mazen, Tatyana Novoyatleva, Thilo Lehmeyer, Akylbek Sydykov, and Ralph T. Schermuly. 2021. "Role of the Purinergic P2Y2 Receptor in Pulmonary Hypertension" International Journal of Environmental Research and Public Health 18, no. 21: 11009. https://doi.org/10.3390/ijerph182111009
APA StyleShihan, M., Novoyatleva, T., Lehmeyer, T., Sydykov, A., & Schermuly, R. T. (2021). Role of the Purinergic P2Y2 Receptor in Pulmonary Hypertension. International Journal of Environmental Research and Public Health, 18(21), 11009. https://doi.org/10.3390/ijerph182111009








