Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface
Abstract
1. Introduction
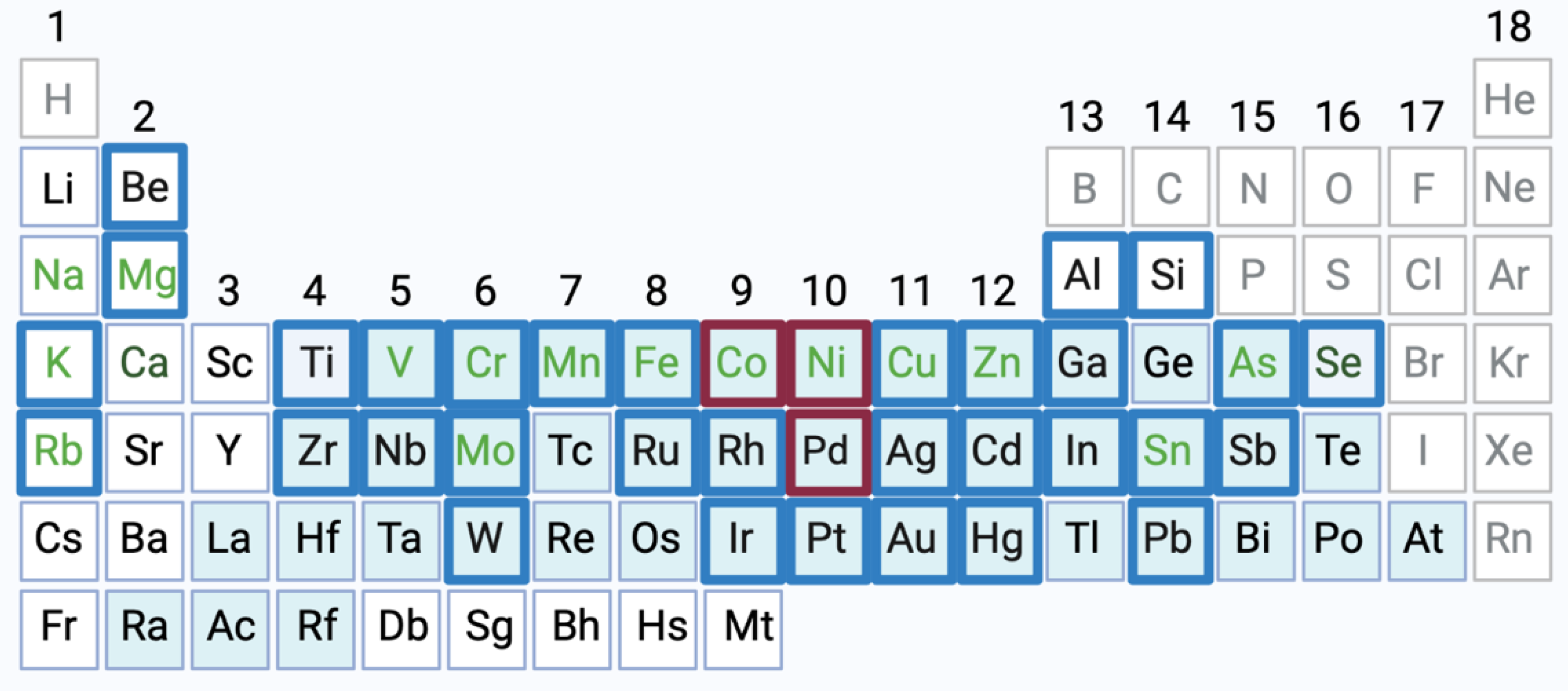
2. Physicochemical Properties, Physiologic Functions and Toxicity of Ni, Co and Pd Ions
3. Metal Allergies
3.1. Exposure, Epidemiology and Regulatory Aspects
3.2. Case Reports
4. Immunological Mechanisms and Diagnostic Approaches
4.1. Pathomechanism of ACD
4.1.1. Sensitization Phase
4.1.2. Elicitation Phase and T Cell Effector Responses
4.2. Diagnosis of Metal Allergies
4.2.1. Patch Testing as the Current Diagnostic Standard
4.2.2. Challenges Associated with Diagnostic In Vitro Tests
4.2.3. Predictive Tests for Sensitizing Properties
5. TCR Antigen Recognition, Metal-Induced T Cell Epitopes and Cross-Reactivity
5.1. General Considerations Regarding Antigen Recognition by TCR
5.1.1. TCR-pMHC Contact Points and General Binding Orientation
5.1.2. Cross-Reactivity due to Conserved Binding Sites
5.1.3. Cross-Reactivity due to Induced Fit
5.2. Metal-Induced T Cell Epitopes
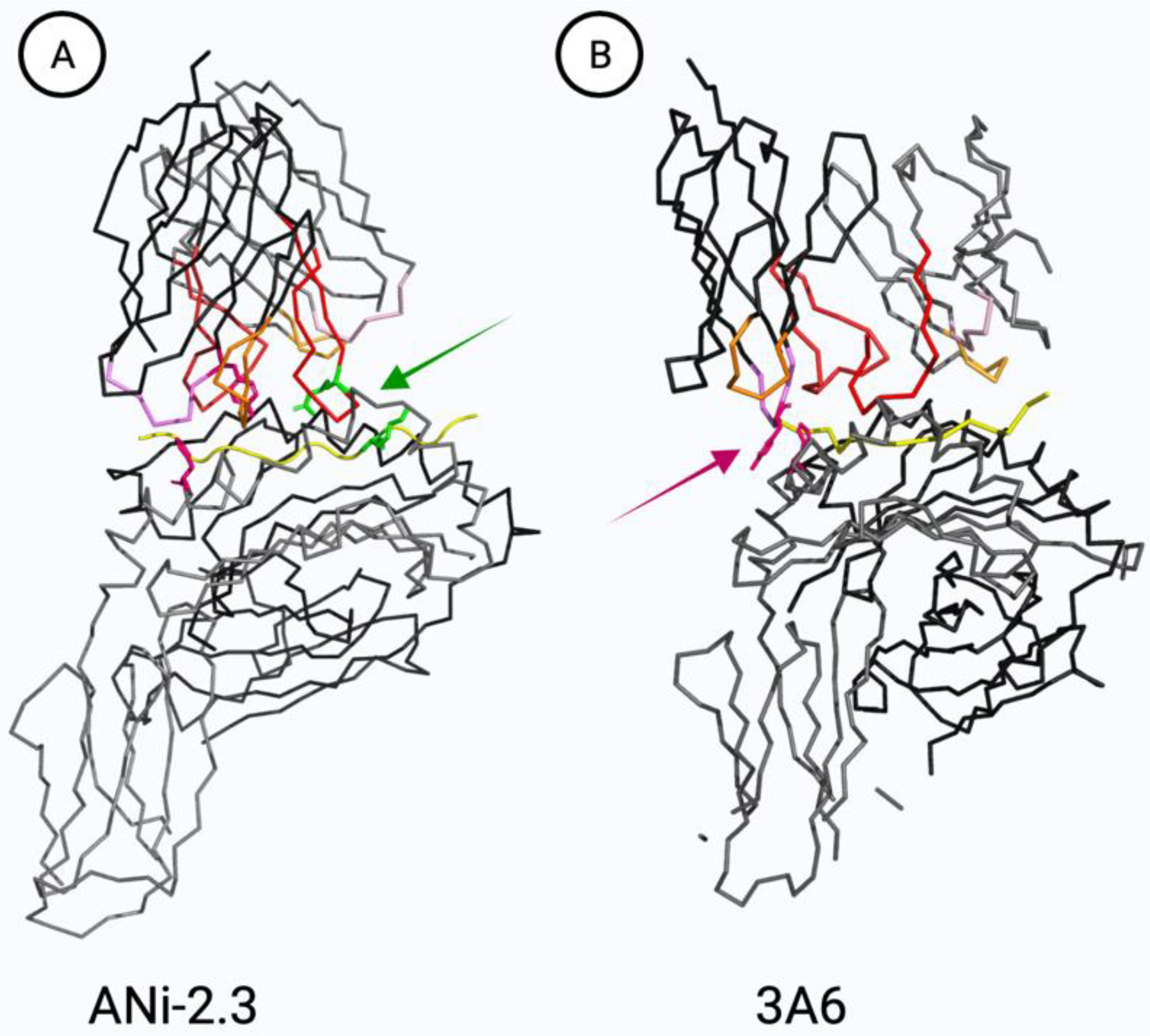
5.3. TCR Cross-Reactivity to Ni, Co, and Pd
5.4. TCR Cross-Reactivity between Other Metal Allergens
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alinaghi, F.; Bennike, N.H.; Egeberg, A.; Thyssen, J.P.; Johansen, J.D. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermat. 2019, 80, 77–85. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Menné, T. Metal Allergy—A Review on Exposures, Penetration, Genetics, Prevalence, and Clinical Implications. Chem. Res. Toxicol. 2010, 23, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Gittler, J.K.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: Implications for contact dermatitis. J. Allergy Clin. Immunol. 2013, 131, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Werfel, T.; Lepoittevin, J.-P.; White, I.R. Contact Allergy—Emerging Allergens and Public Health Impact. Int. J. Environ. Res. Public Health 2020, 17, 2404. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Thyssen, J.P. Metal Allergy: From Dermatitis to Implant and Device Failure; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Thierse, H.-J.; Gamerdinger, K.; Junkes, C.; Guerreiro, N.; Weltzien, H.U. T cell receptor (TCR) interaction with haptens: Metal ions as non-classical haptens. Toxicology 2005, 209, 101–107. [Google Scholar] [CrossRef]
- Schmidt, M.; Goebeler, M. Immunology of metal allergies. J. Dtsch. Dermatol. Ges. 2015, 13, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Kader, H.; Kerstan, A.; Hetta, H.F.; Serfling, E.; Goebeler, M.; Muhammad, K. Intricate Relationship Between Adaptive and Innate Immune System in Allergic Contact Dermatitis. Yale J. Biol. Med. 2020, 93, 699–709. [Google Scholar] [PubMed]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar]
- Masterton, W.L.; Hurley, C.N. Chemistry: Principles and Reactions; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Römpp, R.; Blaß, W. “Schwermetalle”. Thieme Gruppe. 2016. Available online: https://roempp.thieme.de/lexicon/RD-19-01472. (accessed on 25 August 2021).
- Shahzad, B.; Tanveer, M.; Rehman, A.; Alam Cheema, S.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; Whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Kostenkova, K. Open questions on the biological roles of first-row transition metals. Commun. Chem. 2020, 3, 1–4. [Google Scholar] [CrossRef]
- Sigel, H.; Sigel, A. The bio-relevant metals of the periodic table of the elements. Zeitschrift für Naturforschung B 2019, 74, 461–471. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Gopalakrishnan, A.V. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)—induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J.; Peana, M.F. Metals, autoimmunity, and neuroendocrinology: Is there a connection? Environ. Res. 2020, 187, 109541. [Google Scholar] [CrossRef] [PubMed]
- Heddle, J.; Scott, D.; Unzai, S.; Park, S.-Y.; Tame, J.R.H. Crystal Structures of the Liganded and Unliganded Nickel-binding Protein NikA from Escherichia coli. J. Biol. Chem. 2003, 278, 50322–50329. [Google Scholar] [CrossRef] [PubMed]
- Dudev, M.; Wang, J.; Dudev, T.; Lim, C. Factors Governing the Metal Coordination Number in Metal Complexes from Cambridge Structural Database Analyses. J. Phys. Chem. B 2006, 110, 1889–1895. [Google Scholar] [CrossRef]
- Halcrow, M.; Christou, G. Biomimetic Chemistry of Nickel. Chem. Rev. 1994, 94, 2421–2481. [Google Scholar] [CrossRef]
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517. [Google Scholar] [CrossRef]
- Fernandes, H.S.; Teixeira, C.S.S.; Sousa, S.F.; Cerqueira, N.M.F.S.A. Formation of Unstable and very Reactive Chemical Species Catalyzed by Metalloenzymes: A Mechanistic Overview. Molecules 2019, 24, 2462. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J. Bioinformatics of Metalloproteins and Metalloproteomes. Molecules 2020, 25, 3366. [Google Scholar] [CrossRef]
- Nim, Y.S.; Wong, K.-B. The Maturation Pathway of Nickel Urease. Inorganics 2019, 7, 85. [Google Scholar] [CrossRef]
- Zambelli, B.; Banaszak, K.; Merloni, A.; Kiliszek, A.; Rypniewski, W.; Ciurli, S. Selectivity of Ni(II) and Zn(II) binding to Sporosarcina pasteurii UreE, a metallochaperone in the urease assembly: A calorimetric and crystallographic study. JBIC J. Biol. Inorg. Chem. 2013, 18, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.L.; Mulrooney, S.B.; Hausinger, R.P. Nickel-dependent metalloenzymes. Arch. Biochem. Biophys. 2014, 544, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Giri, N.; Zhang, R.; Yamane, K.; Zhang, Y.; Maroney, M.; Costa, M. Nickel Ions Inhibit Histone Demethylase JMJD1A and DNA Repair Enzyme ABH2 by Replacing the Ferrous Iron in the Catalytic Centers. J. Biol. Chem. 2010, 285, 7374–7383. [Google Scholar] [CrossRef] [PubMed]
- Canaz, E.; Kilinc, M.; Sayar, H.; Kiran, G.; Ozyurek, E. Lead, selenium and nickel concentrations in epithelial ovarian cancer, borderline ovarian tumor and healthy ovarian tissues. J. Trace Elem. Med. Biol. 2017, 43, 217–223. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L.; et al. Immunotoxicity of nickel: Pathological and toxicological effects. Ecotoxicol. Environ. Saf. 2020, 203, 111006. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Nair, N.; Saini, M.R. Embryotoxic and Teratogenic Effects of Nickel in Swiss Albino Mice during Organogenetic Period. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.; Jacquet, P. Embryotoxicity and genotoxicity of nickel. IARC Sci. Publ. 1984, 53, 277–291. [Google Scholar]
- Khrustalev, V.V.; Khrustaleva, T.A.; Poboinev, V.V.; Karchevskaya, C.I.; Shablovskaya, E.A.; Terechova, T.G. Cobalt(ii) cation binding by proteins. Metallomics 2019, 11, 1743–1752. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R. Cobalt Complexes Catalyze Reduction of Nitro Compounds: Mechanistic Studies. Chemistryselect 2017, 2, 8197–8206. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, S. Cobalt proteins. JBIC J. Biol. Inorg. Chem. 1999, 261, 1–9. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total. Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Wahlqvist, F.; Bryngelsson, I.-L.; Westberg, H.; Vihlborg, P.; Andersson, L. Dermal and inhalable cobalt exposure—Uptake of cobalt for workers at Swedish hard metal plants. PLoS ONE 2020, 15, e0237100. [Google Scholar] [CrossRef]
- Lauwerys, R.; Lison, D. Health risks associated with cobalt exposure—An overview. Sci. Total. Environ. 1994, 150, 1–6. [Google Scholar] [CrossRef]
- Green, B.; Griffiths, E.; Almond, S. Neuropsychiatric symptoms following metal-on-metal implant failure with cobalt and chromium toxicity. BMC Psychiatry 2017, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Dhooge, I.; Wuyts, F.L.; Maes, L.K. The ototoxic potential of cobalt from metal-on-metal hip implants: A pilot study on the patient-reported auditory, vestibular, and general neurological outcome. Int. J. Audiol. 2021, 60, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Sun, X.; Yu, G.; Xu, Q.; Li, N.; Xiao, C.; Yin, X.; Cao, K.; Han, J.; He, Q.-Y. Putative cobalt- and nickel-binding proteins and motifs in Streptococcus pneumoniae. Metallomics 2013, 5, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Black, J. Competitive binding of chromium, cobalt and nickel to serum proteins. Biomaterials 1994, 15, 262–268. [Google Scholar] [CrossRef]
- Merget, R.; Rosner, G. Evaluation of the health risk of platinum group metals emitted from automotive catalytic converters. Sci. Total. Environ. 2001, 270, 165–173. [Google Scholar] [CrossRef]
- Jbara, M.; Maity, S.K.; Brik, A. Palladium in the Chemical Synthesis and Modification of Proteins. Angew. Chem. Int. Ed. 2017, 56, 10644–10655. [Google Scholar] [CrossRef]
- Faurschou, A.; Menné, T.; Johansen, J.D.; Thyssen, J.P. Metal allergen of the 21st century-a review on exposure, epidemiology and clinical manifestations of palladium allergy. Contact Dermat. 2011, 64, 185–195. [Google Scholar] [CrossRef]
- Kielhorn, J.; Melber, C.; Keller, D.; Mangelsdorf, I. Palladium—A review of exposure and effects to human health. Int. J. Hyg. Environ. Health 2002, 205, 417–432. [Google Scholar] [CrossRef]
- Yoshida, S.; Sakamoto, H.; Mikami, H.; Onuma, K.; Shoji, T.; Nakagawa, H.; Hasegawa, H.; Amayasu, H. Palladium allergy exacerbating bronchial asthma. J. Allergy Clin. Immunol. 1999, 103, 1211–1212. [Google Scholar] [CrossRef]
- Gebel, T.; Lantzsch, H.; Pleßow, K.; Dunkelberg, H. Genotoxicity of platinum and palladium compounds in human and bacterial cells. Mutat. Res. Toxicol. Environ. Mutagen. 1997, 389, 183–190. [Google Scholar] [CrossRef]
- Roach, K.A.; Stefaniak, A.B.; Roberts, J.R. Metal nanomaterials: Immune effects and implications of physicochemical properties on sensitization, elicitation, and exacerbation of allergic disease. J. Immunotoxicol. 2019, 16, 87–124. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.E.; Meade, B.J. Potential Health Effects Associated with Dermal Exposure to Occupational Chemicals. Environ. Health Insights 2014, 8, EHI.S15258-62. [Google Scholar] [CrossRef]
- Teo, W.Z.W.; Schalock, P.C. Metal Hypersensitivity Reactions to Orthopedic Implants. Dermatol. Ther. 2017, 7, 53–64. [Google Scholar] [CrossRef]
- Haddad, S.F.; Helm, M.M.; Meath, B.; Adams, C.; Packianathan, N.; Uhl, R. Exploring the Incidence, Implications, and Relevance of Metal Allergy to Orthopaedic Surgeons. JAAOS Glob. Res. Rev. 2019, 3, e023. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wan, Y.; Ben, Y.; Fan, S.; Hu, J. Relative importance of different exposure routes of heavy metals for humans living near a municipal solid waste incinerator. Environ. Pollut. 2017, 226, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Nag, R.; O’Rourke, S.M.; Cummins, E. Risk factors and assessment strategies for the evaluation of human or environmental risk from metal(loid)s—A focus on Ireland. Sci. Total Environ. 2022, 802, 149839. [Google Scholar] [CrossRef] [PubMed]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Katta, R.; Schlichte, M. Diet and Dermatitis: Food Triggers. J. Clin. Aesthetic Dermatol. 2014, 7, 30–36. [Google Scholar]
- Salik, E.; Løvik, I.; Andersen, K.E.; Bygum, A. Persistent Skin Reactions and Aluminium Hypersensitivity Induced by Childhood Vaccines. Acta Derm. Venereol. 2016, 96, 967–971. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Ofenloch, R.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.-J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of contact allergy in the general population in different European regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Mortz, C.G.; Lauritsen, J.M.; Bindslev-Jensen, C.; Andersen, K.E. Nickel Sensitization in Adolescents and Association with Ear Piercing, Use of Dental Braces and Hand Eczema. Acta Derm. Venereol. 2002, 82, 359–364. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, S.M.; White, I.R.; Kimber, I.; McFadden, J.P.; Tziotzios, C. Contact allergy across the human lifespan. J. Allergy Clin. Immunol. 2020, 145, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-F. Contact sensitization to European baseline series of allergens in university students in Beijing. Contact Dermat. 2010, 62, 371–372. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.-F. Contact sensitization to 34 common contact allergens in university students in Beijing. Contact Dermat. 2015, 73, 323–324. [Google Scholar] [CrossRef] [PubMed]
- DeKoven, J.G.; Warshaw, E.M.; Zug, K.A.; Maibach, H.I.; Belsito, D.V.; Sasseville, D.; Taylor, J.S.; Fowler, J.F.; Mathias, C.G.T.; Marks, J.G.; et al. North American Contact Dermatitis Group Patch Test Results: 2015–2016. Dermatitis 2018, 29, 297–309. [Google Scholar] [CrossRef]
- Filatova, D.; Cherpak, C. Mechanisms of Nickel-Induced Cell Damage in Allergic Contact Dermatitis and Nutritional Intervention Strategies. Endocr. Metab. Immune Disord.—Drug Targets 2020, 20, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Linneberg, A.; Menné, T.; Johansen, J.D. The epidemiology of contact allergy in the general population—Prevalence and main findings. Contact Dermat. 2007, 57, 287–299. [Google Scholar] [CrossRef]
- Uter, W.; Bauer, A.; Fortina, A.B.; Bircher, A.J.; Brans, R.; Buhl, T.; Cooper, S.M.; Czarnecka-Operacz, M.; Dickel, H.; Dugonik, A.; et al. Patch test results with the European baseline series and additions thereof in the ESSCA network, 2015–2018. Contact Dermat. 2021, 84, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.B.; Pelletier, J.L.; Jacob, S.E.; Schneider, L.C. Nickel Allergic Contact Dermatitis: Identification, Treatment, and Prevention. Pediatrics 2020, 145, e20200628. [Google Scholar] [CrossRef]
- Fischer, L.A.; Menné, T.; Johansen, J.D. Dose per unit area—A study of elicitation of nickel allergy. Contact Dermat. 2007, 56, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ahlström, M.G.; Midander, K.; Menné, T.; Lidén, C.; Johansen, J.D.; Julander, A.; Thyssen, J.P. Nickel deposition and penetration into the stratum corneum after short metallic nickel contact: An experimental study. Contact Dermat. 2019, 80, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kasper-Sonnenberg, M.; Sugiri, D.; Wurzler, S.; Ranft, U.; Dickel, H.; Wittsiepe, J.; Hölzer, J.; Lemm, F.; Eberwein, G.; Altmeyer, P.; et al. Prevalence of nickel sensitization and urinary nickel content of children are increased by nickel in ambient air. Environ. Res. 2011, 111, 266–273. [Google Scholar] [CrossRef]
- European Parliament. Commission Directive 2004/96/EC of 27 September 2004 amending Council Directive 76/769/EEC as regards restrictions on the marketing and use of nickel for piercing post assemblies for purpose of adapting its Annex I to technical progress. Off. J. Eur. Union 2004, 301, 51–52. [Google Scholar]
- European Parliament. European Parliament and Council Directive 94/27/EC of 30 June 1994: Amending for the 12th time Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations. Off. J. Eur. Commun. 1994, 188, 1–2. [Google Scholar]
- European Parliament. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the registration, evaluation, authorisation and restriction of chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Union 2006, 396, 1–849. [Google Scholar]
- Ahlström, M.G.; Menné, T.; Thyssen, J.P.; Johansen, J.D. The European nickel regulation and changes since its introduction. Contact Dermat. 2017, 76, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Thierse, H.-J.; Luch, A. Verbraucherschutz und Risikobewertung—Allergieauslösende Substanzen in Verbraucherprodukten. Allergo J. 2019, 28, 22–41. [Google Scholar] [CrossRef]
- European Parliament. Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Off. J. Eur. Union 2004, 23, 3–16. [Google Scholar]
- Erfani, B.; Lidén, C.; Midander, K. Short and frequent skin contact with nickel. Contact Dermat. 2015, 73, 222–230. [Google Scholar] [CrossRef]
- Hegewald, J.; Uter, W.; Pfahlberg, A.; Geier, J.; Schnuch, A.; Ivdk, T. A multifactorial analysis of concurrent patch-test reactions to nickel, cobalt, and chromate. Allergy 2005, 60, 372–378. [Google Scholar] [CrossRef]
- Forte, G.; Petrucci, F.; Bocca, B. Metal allergens of growing significance: Epidemiology, immunotoxicology, strategies for testing and prevention. Inflamm. Allergy—Drug Targets 2008, 7, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Rubins, A.; Romanova, A.; Septe, M.; Maddukuri, S.; Schwartz, R.A.; Rubins, S. Contact dermatitis: Etiologies of the allergic and irritant type. Acta Dermatovenerol. Alp. Pannonica et Adriat. 2020, 29, 181–184. [Google Scholar] [CrossRef]
- European Parliament. Commission Delegated Regulation (EU) 2020/217 of 4 October 2019 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures and correcting that Regulation. Off. J. Eur. Union 2019, L44, 1–14. [Google Scholar]
- European Chemicals Agency. Opinion on an Annex XV Dossier Proposing Restrictions on Skin Sensitising Substances; European Chemicals Agency: Helsinki, Finland, 2020. [Google Scholar]
- González-Ruiz, L.; De Caso, E.V.; Peña-Sánchez, R.; Silvestre-Salvador, J.F.; Peña-Sáncez, R. Delayed hypersensitivity to palladium dichloride: 15-year retrospective study in a skin allergy unit. Contact Dermat. 2019, 81, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Durosaro, O.; el-Azhary, R.A. A 10-year retrospective study on palladium sensitivity. Dermatitis 2009, 20, 208–213. [Google Scholar] [CrossRef]
- Kapp, F.; Summer, B.; Thomas, P. Usefulness of lymphocyte transformation test and in vitro cytokine release in differentiating between independent and cross-reacting nickel/palladium allergy. Immun. Inflamm. Dis. 2020, 8, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Nucera, E.; Chini, R.; Rizzi, A.; Schiavino, D.; Buonomo, A.; Aruanno, A.; Ricci, R.; Mangiola, F.; Campanale, M.C.; Gasbarrini, A.; et al. Eosinophilic oesophagitis (in nickel-allergic patient) regressed after nickel oral desensitization: A case report. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419827771. [Google Scholar] [CrossRef]
- Marcant, P.; Alcaraz, I.; Beauval, N.; Martin de Lassalle, E.; Chantelot, C.; Staumont-Sallé, D. Metal implant allergy: A diagnostic challenge illustrating the limits of the nickel spot test. Contact Dermat. 2021, 85, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bent, S.A.; Berg, T.; Karst, U.; Sperling, M.; Rustemeyer, T. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermat. 2019, 81, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Weiß, K.T.; Schreiver, I.; Siewert, K.; Luch, A.; Haslböck, B.; Berneburg, M.; Bäumler, W. Tattoos–more than just colored skin? Searching for tattoo allergens. JDDG J. Dtsch. Dermatol. Ges. 2021, 19, 657–669. [Google Scholar]
- Simonsen, A.B.; Friis, U.F.; Johansen, J.D.; Zachariae, C.; Sloth, J.J.; Thyssen, J.P. Occupational allergic contact dermatitis caused by cobalt in machine oil. Contact Dermat. 2019, 80, 59–61. [Google Scholar] [CrossRef]
- Marsidi, N.; Beijnen, J.H.; Van Zuuren, E.J. Palladium-induced granulomas analysed with inductively coupled plasma mass spectrometry. Contact Dermat. 2018, 79, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.H.; Igyártó, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Helou, D.G.; Martin, S.F.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Römer, S. Nrf2 Involvement in Chemical-Induced Skin Innate Immunity. Front. Immunol. 2019, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.M.; Roberts, D.W.; Patlewicz, G. Is skin penetration a determining factor in skin sensitization potential and potency? Refuting the notion of a LogKow threshold for skin sensitization. J. Appl. Toxicol. 2017, 37, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, T.; Kubo, A.; Yokouchi, M.; Adachi, T.; Kobayashi, T.; Kitashima, D.Y.; Fujii, H.; Clausen, B.; Koyasu, S.; Amagai, M.; et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 2011, 208, 2607–2613. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Jakob, T.; Ring, J.; Udey, M.C. Multistep navigation of Langerhans/ dendritic cells in and out of the skin. J. Allergy Clin. Immunol. 2001, 108, 688–696. [Google Scholar] [CrossRef]
- Vargas, P.; Maiuri, P.; Bretou, M.; Sáez, P.J.; Pierobon, P.; Maurin, M.; Chabaud, M.; Lankar, D.; Obino, D.; Terriac, E.; et al. Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat. Cell Biol. 2016, 18, 43–53. [Google Scholar] [CrossRef]
- E Macatonia, S.; Edwards, A.J.; Knight, S.C. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology 1986, 59, 509–514. [Google Scholar]
- Gaide, O.; Emerson, R.O.; Jiang, X.; Gulati, N.; Nizza, S.T.; Desmarais, C.; Robins, H.; Krueger, J.G.; Clark, R.A.; Kupper, T.S. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 2015, 21, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Hayashi, S.-I. CD4+ Resident Memory T Cells Mediate Long-Term Local Skin Immune Memory of Contact Hypersensitivity in BALB/c Mice. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Bonneville, M.; Chavagnac, C.; Vocanson, M.; Rozieres, A.; Benetiere, J.; Pernet, I.; Denis, A.; Nicolas, J.-F.; Hennino, A. Skin Contact Irritation Conditions the Development and Severity of Allergic Contact Dermatitis. J. Investig. Dermatol. 2007, 127, 1430–1435. [Google Scholar] [CrossRef]
- McKee, A.S.; Fontenot, A.P. Interplay of innate and adaptive immunity in metal-induced hypersensitivity. Curr. Opin. Immunol. 2016, 42, 25–30. [Google Scholar] [CrossRef]
- Li, X.; Zhong, F. Nickel Induces Interleukin-1β Secretion via the NLRP3–ASC–Caspase-1 Pathway. Inflammation 2014, 37, 457–466. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Caicedo, M.S.; Desai, R.; McAllister, K.; Reddy, A.; Jacobs, J.J.; Hallab, N.J. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. J. Orthop. Res. 2009, 27, 847–854. [Google Scholar] [CrossRef]
- Chamaon, K.; Schönfeld, P.; Awiszus, F.; Bertrand, J.; Lohmann, C.H. Ionic cobalt but not metal particles induces ROS generation in immune cells in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1246–1253. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Me-tal induced Oxidative Damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Lu, L.; Vollmer, J.; Moulon, C.; Weltzien, H.U.; Marrack, P.; Kappler, J. Components of the Ligand for a Ni++ Reactive Human T Cell Clone. J. Exp. Med. 2003, 197, 567–574. [Google Scholar] [CrossRef]
- Gamerdinger, K.; Moulon, C.; Karp, D.R.; Van Bergen, J.; Koning, F.; Wild, D.; Pflugfelder, U.; Weltzien, H.U. A New Type of Metal Recognition by Human T Cells: Contact residues for peptide-independent bridging of T cell receptor and major histocompatibility complex by nickel. J. Exp. Med. 2003, 197, 1345–1353. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Riedel, F.; Leddermann, M.; Bacher, P.; Scheffold, A.; Kuhl, H.; Timmermann, B.; Chudakov, D.M.; Molin, S.; Worm, M.; et al. TCRs with segment TRAV9-2 or a CDR3 histidine are overrepresented among nickel-specific CD4+ T cells. Allergy 2020, 75, 2574–2586. [Google Scholar] [CrossRef]
- Höper, T.; Siewert, K.; Dumit, V.I.; von Bergen, M.; Schubert, K.; Haase, A. The Contact Allergen NiSO4 Triggers a Distinct Molecular Response in Primary Human Dendritic Cells Compared to Bacterial LPS. Front. Immunol. 2021, 12, 12. [Google Scholar] [CrossRef]
- Schmidt, M.; Raghavan, B.; Müller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Vennegaard, M.T.; Dyring-Andersen, B.; Skov, L.; Nielsen, M.M.; Schmidt, J.D.; Bzorek, M.; Poulsen, S.S.; Thomsen, A.R.; Woetmann, A.; Thyssen, J.P.; et al. Epicutaneous exposure to nickel induces nickel allergy in mice via a MyD88-dependent and interleukin-1-dependent pathway. Contact Dermat. 2014, 71, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L.; et al. Nickel induces inflammatory activation via NF-κB, MAPKs, IRF3 and NLRP3 inflammasome signaling pathways in macrophages. Aging 2019, 11, 11659–11672. [Google Scholar] [CrossRef]
- Rachmawati, D.; Bontkes, H.J.; Verstege, M.I.; Muris, J.; von Blomberg, B.M.E.; Scheper, R.J.; van Hoogstraten, I.M.W. Transition metal sensing by Toll-like receptor-4: Next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermat. 2013, 68, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.S.; Lisby, S.; Baadsgaard, O.; Volund, A.; Menne, T. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br. J. Dermatol. 2002, 146, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhong, Q.; Tian, T.; Dubin, K.; Athale, S.K.; Kupper, T.S. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell–mediated immunity. Nat. Med. 2010, 16, 224–227. [Google Scholar] [CrossRef]
- Christiansen, E.S.; Andersen, K.E.; Bindslev-Jensen, C.; Halken, S.; Kjaer, H.F.; Eller, E.; Høst, A.; Mortz, C.G. Low patch test reactivity to nickel in unselected adolescents tested repeatedly with nickel in infancy. Pediatr. Allergy Immunol. 2016, 27, 636–639. [Google Scholar] [CrossRef]
- Brasch, J.; Becker, D.; Aberer, W.; Bircher, A.; Kränke, B.; Jung, K.; Przybilla, B.; Biedermann, T.; Werfel, T.; John, S.M.; et al. Leitlinie Kontaktekzem. Allergo J. 2014, 23, 30–43. [Google Scholar] [CrossRef]
- Martin, S.F.; Esser, P.R. Innate Immune Mechanisms in Contact Dermatitis; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–14. [Google Scholar]
- Clark, R.A. Resident memory T cells in human health and disease. Sci. Transl. Med. 2015, 7, 269rv1. [Google Scholar] [CrossRef]
- Gadsbøll, A.-S.Ø.; Jee, M.H.; Funch, A.B.; Alhede, M.; Mraz, V.; Weber, J.F.; Callender, L.A.; Carroll, E.C.; Bjarnsholt, T.; Woetmann, A.; et al. Pathogenic CD8+ Epidermis-Resident Memory T Cells Displace Dendritic Epidermal T Cells in Allergic Dermatitis. J. Investig. Dermatol. 2020, 140, 806–815.e5. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.D.; Ahlström, M.G.; Johansen, J.D.; Dyring-Andersen, B.; Agerbeck, C.; Nielsen, M.M.; Poulsen, S.S.; Woetmann, A.; Ødum, N.; Thomsen, A.R.; et al. Rapid allergen-induced interleukin-17 and interferon-γ secretion by skin-resident memory CD8+T cells. Contact Dermat. 2017, 76, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Kish, D.D.; Li, X.; Fairchild, R.L. CD8 T Cells Producing IL-17 and IFN-γ Initiate the Innate Immune Response Required for Responses to Antigen Skin Challenge. J. Immunol. 2009, 182, 5949–5959. [Google Scholar] [CrossRef] [PubMed]
- Kapsenberg, M.L.; Res, P.; Bos, J.D.; Schootemijer, A.; Teunissen, M.B.M.; Van Schooten, W. Nickel-specific T lymphocyte clones derived from allergic nickel-contact dermatitis lesions in man: Heterogeneity based on requirement of dendritic antigen-presenting cell subsets. Eur. J. Immunol. 1987, 17, 861–865. [Google Scholar] [CrossRef]
- Gawkrodger, D.J.; Carr, M.M.; McVittie, E.; Guy, K.; Hunter, J.A.A. Keratinocyte Expression of MHC Class II Antigens in Allergic Sensitization and Challenge Reactions and in Irritant Contact Dermatitis. J. Investig. Dermatol. 1987, 88, 11–16. [Google Scholar] [CrossRef]
- Cavani, A.; Mei, D.; Corinti, S.; Girolomoni, G.; Guerra, E.; Giani, M.; Pirrotta, L.; Puddu, P. Patients with Allergic Contact Dermatitis to Nickel and Nonallergic Individuals Display Different Nickel-Specific T Cell Responses. Evidence for the Presence of Effector CD8+ and Regulatory CD4+ T Cells. J. Investig. Dermatol. 1998, 111, 621–628. [Google Scholar] [CrossRef]
- Kawano, M.; Nakayama, M.; Aoshima, Y.; Nakamura, K.; Ono, M.; Nishiya, T.; Nakamura, S.; Takeda, Y.; Dobashi, A.; Takahashi, A.; et al. NKG2D+ IFN-γ+ CD8+ T Cells Are Responsible for Palladium Allergy. PLoS ONE 2014, 9, e86810. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Suto, Y.; Ito, K.; Hashimoto, W.; Nishiya, T.; Ueda, K.; Narushima, T.; Takahashi, T.; Ogasawara, K. TRAV7-2*02 Expressing CD8+ T Cells Are Responsible for Palladium Allergy. Int. J. Mol. Sci. 2017, 18, 1162. [Google Scholar] [CrossRef] [PubMed]
- Vocanson, M.; Hennino, A.; Chavagnac, C.; Saint-Mezard, P.; Dubois, B.; Kaiserlian, D.; Nicolas, J.-F. Contribution of CD4+and CD8+T-cells in contact hypersensitivity and allergic contact dermatitis. Expert Rev. Clin. Immunol. 2005, 1, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cavani, A.; Nasorri, F.; Ottaviani, C.; Sebastiani, S.; De Pità, O.; Girolomoni, G. Human CD25+ Regulatory T Cells Maintain Immune Tolerance to Nickel in Healthy, Nonallergic Individuals. J. Immunol. 2003, 171, 5760–5768. [Google Scholar] [CrossRef] [PubMed]
- Moed, H.; Von Blomberg, B.M.E.; Bruynzeel, D.P.; Scheper, R.J.; Gibbs, S.; Rustemeyer, T. Regulation of nickel-induced T-cell responsiveness by CD4+CD25+cells in contact allergic patients and healthy individuals. Contact Dermat. 2005, 53, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Wolfl, M.; Kuball, J.; Ho, W.Y.; Nguyen, H.; Manley, T.J.; Bleakley, M.; Greenberg, P.D. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 2007, 110, 201–210. [Google Scholar] [CrossRef]
- Moed, H.; Boorsma, D.; Stoof, T.; Von Blomberg, B.; Bruynzeel, D.; Scheper, R.; Gibbs, S.; Rustemeyer, T. Nickel-responding T cells are CD4+ CLA+ CD45RO+ and express chemokine receptors CXCR3, CCR4 and CCR10. Br. J. Dermatol. 2004, 151, 32–41. [Google Scholar] [CrossRef]
- Sinigaglia, F.; Scheidegger, D.; Garotta, G.; Scheper, R.; Pletscher, M.; Lanzavecchia, A. Isolation and characterization of Ni-specific T cell clones from patients with Ni-contact dermatitis. J. Immunol. 1985, 135, 3929–3932. [Google Scholar] [PubMed]
- Minang, J.T.; Areström, I.; Troye-Blomberg, M.; Lundeberg, L.; Ahlborg, N. Nickel, cobalt, chromium, palladium and gold induce a mixed Th1- and Th2-type cytokine response in vitro in subjects with contact allergy to the respective metals. Clin. Exp. Immunol. 2006, 146, 417–426. [Google Scholar] [CrossRef]
- Minang, J.T.; Troye-Blomberg, M.; Lundeberg, L.; Ahlborg, N. Nickel Elicits Concomitant and Correlated in vitro Production of Th1-, Th2-Type and Regulatory Cytokines in Subjects with Contact Allergy to Nickel. Scand. J. Immunol. 2005, 62, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Minang, J.T.; Areström, I.; Zuber, B.; Jönsson, G.; Troye-Blomberg, M.; Ahlborg, N. Nickel-induced IL-10 down-regulates Th1- but not Th2-type cytokine responses to the contact allergen nickel. Clin. Exp. Immunol. 2006, 143, 494–502. [Google Scholar] [CrossRef]
- Muris, J.; Feilzer, A.J.; Kleverlaan, C.J.; Rustemeyer, T.; van Hoogstraten, I.M.W.; Scheper, R.J.; von Blomberg, B.M.E. Palladium-induced Th2 cytokine responses reflect skin test reactivity. Allergy 2012, 67, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.; Shemer, A.; da Rosa, J.C.; Rozenblit, M.; Fuentes-Duculan, J.; Gittler, J.K.; Finney, R.; Czarnowicki, T.; Zheng, X.; Xu, H.; et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J. Allergy Clin. Immunol. 2014, 134, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, V.; Palamara, F.; Cordiali-Fei, P.; Vento, A.; Aiello, A.; Picardo, M.; Ensoli, F.; Cristaudo, A. Nickel, palladium and rhodium induced IFN-gamma and IL-10 production as assessed by in vitro ELISpot-analysis in contact dermatitis patients. BMC Immunol. 2008, 9, 19. [Google Scholar] [CrossRef]
- Todberg, T.; Zachariae, C.; Krustrup, D.; Skov, L. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermat. 2018, 78, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Ruge, I.F.; Skov, L.; Zachariae, C.; Thyssen, J.P. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermat. 2020, 83, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J.; Becker, D.; Aberer, W.; Bircher, A.; Kränke, B.; Jung, K.; Przybilla, B.; Biedermann, T.; Werfel, T.; John, S.M.; et al. Guideline contact dermatitis. Allergo J. Int. 2014, 23, 126–138. [Google Scholar] [CrossRef]
- Bourke, J.; Coulson, I.; English, J. Guidelines for the management of contact dermatitis: An update. Br. J. Dermatol. 2009, 160, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.J.; Bruze, M.; Cannavó, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A.; et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—Recommendations on best practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.; Gonçalo, M.; Aerts, O.; Badulici, S.; Bennike, N.H.; Bruynzeel, D.; Dickel, H.; Garcia-Abujeta, J.L.; Giménez-Arnau, A.M.; Hamman, C.; et al. The European baseline series and recommended additions: 2019. Contact Dermat. 2019, 80, 1–4. [Google Scholar] [CrossRef]
- Davis, M.D.; Bhate, K.; Rohlinger, A.L.; Farmer, S.A.; Richardson, D.M.; Weaver, A.L. Delayed patch test reading after 5 days: The Mayo Clinic experience. J. Am. Acad. Dermatol. 2008, 59, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, C.; Isaksson, M.; Möller, H.; Axéll, T.; Liedholm, R.; Bruze, M. The necessity of a test reading after 1 week to detect late positive patch test reactions in patients with oral lichen lesions. Clin. Oral Investig. 2014, 18, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, C.C.A.; Ofenloch, R.; Dittmar, D.; Schuttelaar, M.L.A. New positive patch test reactions on day 7—The additional value of the day 7 patch test reading. Contact Dermat. 2019, 81, 280–287. [Google Scholar] [CrossRef]
- Lindelöf, B. Regional variations of patch test response in nickel-sensitive patients. Contact Dermat. 1992, 26, 202–203. [Google Scholar] [CrossRef]
- Schittenhelm, A.; Stockinger, W. Anaphylaxiestudien bei Mensch und Tier—IV. Mitteilung. Über die Idiosynkrasie gegen Nickel (“Nickelkrätze”) und ihre Beziehung zur Anaphylaxie. Z. Für Die Gesamte Exp. Med. 1925, 45, 58–74. [Google Scholar] [CrossRef]
- Minné, T.; Calvin, G. Concentration threshold of non-occluded nickel exposure in nickel-sensitive individuals and controls with and without surfactant. Contact Dermat. 1993, 29, 180–184. [Google Scholar] [CrossRef]
- Muris, J.; Kleverlaan, C.J.; Feilzer, A.J.; Rustemeyer, T. Sodium tetrachloropalladate (Na2[PdCl4]) as an improved test salt for palladium allergy patch testing. Contact Dermat. 2007, 58, 42–46. [Google Scholar] [CrossRef]
- Muris, J.; Goossens, A.; Gonçalo, M.; Bircher, A.J.; Giménez-Arnau, A.; Foti, C.; Rustemeyer, T.; Feilzer, A.J.; Kleverlaan, C.J. Sensitization to palladium and nickel in Europe and the relationship with oral disease and dental alloys. Contact Dermat. 2015, 72, 286–296. [Google Scholar] [CrossRef]
- De Groot, A.C. Patch testing. Test Conc. Veh. 2008, 4350, 1–1257. [Google Scholar]
- Gollhausen, R.; Przybilla, B.; Ring, J. Reproducibility of patch tests. J. Am. Acad. Dermatol. 1989, 21, 1196–1202. [Google Scholar] [CrossRef]
- Brasch, J.; Henseler, T.; Aberer, W.; Bäuerle, G.; Frosch, P.J.; Fuchs, T.; Fünfstück, V.; Kaiser, G.; Lischka, G.G.; Pilz, B.; et al. Reproducibility of patch tests: A multicenter study of synchronous left- versus right-sided patch tests by the German Contact Dermatitis Research Group. J. Am. Acad. Dermatol. 1994, 31, 584–591. [Google Scholar] [CrossRef]
- Bourke, J.F.; Batta, K.; Prais, L.; Abdullah, A.; Foulds, I.S. The reproducibility of patch tests. Br. J. Dermatol. 1999, 140, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Ale, S.I.; Maibach, H.I. Reproducibility of patch test results: A concurrent right-versus-left study using TRUE Testtm. Contact Dermat. 2004, 50, 304–312. [Google Scholar] [CrossRef]
- Schaeffer, A.C.V.; Andersen, K.E.; Bindslev-Jensen, C.; Mortz, C.G. The reproducibility of nickel, cobalt and chromate sensitization in patients tested at least twice in the period 1992-2014 with TRUE Test®. Contact Dermat. 2016, 75, 111–113. [Google Scholar] [CrossRef]
- Dittmar, D.; Ofenloch, R.F.; Schuttelaar, M.L.A. Persistence of contact allergy: A retrospective analysis. Contact Dermat. 2018, 78, 143–150. [Google Scholar] [CrossRef]
- Hindsén, M.; Bruze, M.; Christensen, O.B. Individual variation in nickel patch test reactivity. Am. J. Contact Dermat. 1999, 10, 62–67. [Google Scholar] [CrossRef]
- Schalock, P.C.; Crawford, G.; Nedorost, S.; Scheinman, P.L.; Atwater, A.R.; Mowad, C.; Brod, B.; Ehrlich, A.; Watsky, K.L.; Sasseville, D.; et al. Patch Testing for Evaluation of Hypersensitivity to Implanted Metal Devices: A Perspective From the American Contact Dermatitis Society. Dermatitis 2016, 27, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, K.A. Allergy to Surgical Implants. Clin. Rev. Allergy Immunol. 2019, 56, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Hindsén, M.; Spiren, A.; Bruze, M. Cross-reactivity between nickel and palladium demonstrated by systemic administration of nickel. Contact Dermat. 2005, 53, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.; Lewis, M.; Hansen, S.G.; I Strelow, L.; Nelson, J.A.; Sexton, G.J.; Hanifin, J.M.; Slifka, M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003, 9, 1131–1137. [Google Scholar] [CrossRef]
- Marraco, S.A.F.; Soneson, C.; Cagnon, L.; Gannon, P.O.; Allard, M.; Maillard, S.A.; Montandon, N.; Rufer, N.; Waldvogel, S.; Delorenzi, M.; et al. Long-lasting stem cell–like memory CD8 + T cells with a naïve-like profile upon yellow fever vaccination. Sci. Transl. Med. 2015, 7, 282ra48. [Google Scholar] [CrossRef]
- Williams, M.; Bevan, M.J. Effector and Memory CTL Differentiation. Annu. Rev. Immunol. 2007, 25, 171–192. [Google Scholar] [CrossRef]
- Schoon, J.; Ort, M.J.; Huesker, K.; Geißler, S.; Rakow, A. Diagnosis of Metal Hypersensitivity in Total Knee Arthroplasty: A Case Report. Front. Immunol. 2019, 10, 2758. [Google Scholar] [CrossRef]
- Saggau, C.; Scheffold, A.; Bacher, P. Flow Cytometric Characterization of Human Antigen-Reactive T-Helper Cells. Methods Mol. Biol. 2021, 2285, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Oppel, E.; Thomas, P.; Summer, B. Evaluation of lymphocyte transformation tests as compared with patch tests in nickel allergy diagnosis. Contact Dermat. 2017, 76, 228–234. [Google Scholar] [CrossRef]
- Von Blomberg-Van Der Flier, M.; Van Der Burg, C.K.H.; Pos, O.; Van De Plassche-Boers, E.M.; Bruynzeel, D.P.; Garotta, G.; Scheper, R.J. In Vitro Studies in Nickel Allergy: Diagnostic Value of a Dual Parameter Analysis. J. Investig. Dermatol. 1987, 88, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Lisby, S.; Hansen, L.H.; Skov, L.; Menné, T.; Baadsgaard, O. Nickel-induced activation of T cells in individuals with negative patch test to nickel sulphate. Arch. Dermatol. Res. 1999, 291, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Bechara, R.; Pollastro, S.; Azoury, M.E.; Szely, N.; Maillère, B.; de Vries, N.; Pallardy, M. Identification and Characterization of Circulating Naïve CD4+ and CD8+ T Cells Recognizing Nickel. Front. Immunol. 2019, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Blom, L.H.; Elrefaii, S.A.; Zachariae, C.; Thyssen, J.P.; Poulsen, L.K.; Johansen, J.D. Memory T helper cells identify patients with nickel, cobalt, and chromium metal allergy. Contact Dermat. 2021, 85, 7–16. [Google Scholar] [CrossRef]
- Moed, H.; von Blomberg, M.; Bruynzeel, D.P.; Scheper, R.; Gibbs, S.; Rustemeyer, T. Improved detection of allergen-specific T-cell responses in allergic contact dermatitis through the addition of ’cytokine cocktails’. Exp. Dermatol. 2005, 14, 634–640. [Google Scholar] [CrossRef]
- Al-Tawil, N.G.; Marcusson, J.A.; Möller, E. HLA-class II restriction of the proliferative T lymphocyte responses to nickel, cobalt and chromium compounds. Tissue Antigens 2008, 25, 163–172. [Google Scholar] [CrossRef]
- Muris, J.; Kleverlaan, C.J.; Feilzer, A.J.; Valentine-Thon, E. Reactivity to sodium tetrachloropalladate (Na2[PdCl4]) compared to PdCl2and NiCl2in lymphocyte proliferation tests. Allergy 2009, 64, 1152–1156. [Google Scholar] [CrossRef]
- Cristaudo, A.; Bordignon, V.; Petrucci, F.; Caimi, S.; De Rocco, M.; Picardo, M.; Fei, P.C.; Ensoli, F. Release of Palladium from Biomechanical Prostheses in Body Fluids Can Induce or Support PD-Specific IFNγ T Cell Responses and the Clinical Setting of a Palladium Hypersensitivity. Int. J. Immunopathol. Pharmacol. 2009, 22, 605–614. [Google Scholar] [CrossRef]
- Kimber, I.; Quirke, S.; Beck, M. Attempts to identify the causative allergen in cases of allergic contact dermatitis using an in vitro lymphocyte transformation test. Toxicol. In Vitro 1990, 4, 302–306. [Google Scholar] [CrossRef]
- Cederbrant, K.; Anderson, C.; Andersson, T.; Marcusson-Ståhl, M.; Hultman, P. Cytokine Production, Lymphocyte Proliferation and T-Cell Receptor Vβ Expression in Primary Peripheral Blood Mononuclear Cell Cultures from Nickel-Allergic Individuals. Int. Arch. Allergy Immunol. 2003, 132, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Moulon, C.; Vollmer, J.; Weltzien, H.-U. Characterization of processing requirements and metal cross-reactivities in T cell clones from patients with allergic contact dermatitis to nickel. Eur. J. Immunol. 1995, 25, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Hentschel, M.; Kapp, A.; Renz, H. Dichotomy of blood- and skin-derived IL-4-producing allergen-specific T cells and restricted V beta repertoire in nickel-mediated contact dermatitis. J. Immunol. 1997, 158, 2500–2505. [Google Scholar]
- Thomas, P.; Braathen, L.R.; Doerig, M.; Auboeck, J.; Nestle, F.; Werfel, T.; Willert, H.G. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy 2009, 64, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, K.J.; Groot, K.; Van Hoogstraten, I.M.; Von Blomberg, M.E.; Scheper, R.J. Successful Induction of Allergic Contact Dermatitis to Mercury and Chromium in Mice. Int. Arch. Allergy Immunol. 1991, 96, 179–183. [Google Scholar] [CrossRef]
- Mandervelt, C.; Clottens, F.; Demedts, M.; Nemery, B. Assessment of the sensitization potential of five metal salts in the murine local lymph node assay. Toxicology 1997, 120, 65–73. [Google Scholar] [CrossRef]
- Basketter, D.A.; Lea, L.J.; Cooper, K.J.; A Ryan, C.; Gerberick, G.F.; Dearman, R.J.; Kimber, I. Identification of metal allergens in the local lymph node assay. Arch. Phys. Med. Rehabil. 1999, 10, 207–212. [Google Scholar] [CrossRef]
- Kligman, A.M. The Identification of Contact Allergens by Human Assay III. The Maximization Test: A Procedure for Screening and Rating Contact Sensitizers. J. Investig. Dermatol. 1989, 92, 151S–152S. [Google Scholar] [CrossRef]
- Basketter, D. Nickel: Intrinsic Skin Sensitization Potency and Relation to Prevalence of Contact Allergy. Dermatitis 2021, 32, 71–77. [Google Scholar] [CrossRef]
- Schuttelaar, M.L.A.; Ofenloch, R.F.; Bruze, M.; Cazzaniga, S.; Elsner, P.; Gonçalo, M.; Naldi, L.; Svensson, Å.; Diepgen, T.L. Prevalence of contact allergy to metals in the European general population with a focus on nickel and piercings: The EDEN Fragrance Study. Contact Dermat. 2018, 79, 1–9. [Google Scholar] [CrossRef]
- Gibbs, S.; Kosten, I.; Veldhuizen, R.; Spiekstra, S.; Corsini, E.; Roggen, E.L.; Rustemeyer, T.; Feilzer, A.J.; Kleverlaan, C.J. Assessment of metal sensitizer potency with the reconstructed human epidermis IL-18 assay. Toxicology 2018, 393, 62–72. [Google Scholar] [CrossRef]
- Matzinger, P.; Bevan, M.J. Why do so many lymphocytes respond to major histocompatibility antigens? Cell. Immunol. 1977, 29, 1–5. [Google Scholar] [CrossRef]
- Jerne, N.K. The natural-selection theory of antibody formation. Proc. Natl. Acad. Sci. USA 1955, 41, 849–857. [Google Scholar] [CrossRef]
- Burnet, F.M. A Modification of Jerne’s Theory of Antibody Production using the Concept of Clonal Selection. CA Cancer J. Clin. 1976, 26, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 1998, 19, 395–404. [Google Scholar] [CrossRef]
- Sewell, A.K. Why must T cells be cross-reactive? Nat. Rev. Immunol. 2012, 12, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Arstila, T.P.; Casrouge, A.; Baron, V.; Even, J.; Kanellopoulos, J.; Kourilsky, P. A Direct Estimate of the Human αβ T Cell Receptor Diversity. Science 1999, 286, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Robins, H.S.; Campregher, P.V.; Srivastava, S.K.; Wacher, A.; Turtle, C.J.; Kahsai, O.; Riddell, S.R.; Warren, E.; Carlson, C.S. Comprehensive assessment of T-cell receptor β-chain diversity in αβ T cells. Blood 2009, 114, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- Wooldridge, L.; Ekeruche-Makinde, J.; Berg, H.A.V.D.; Skowera, A.; Miles, J.J.; Tan, M.P.; Dolton, G.; Clement, M.; Llewellyn-Lacey, S.; Price, D.A.; et al. A Single Autoimmune T Cell Receptor Recognizes More Than a Million Different Peptides. J. Biol. Chem. 2012, 287, 1168–1177. [Google Scholar] [CrossRef]
- Garboczi, D.N.; Ghosh, P.; Utz, U.; Fan, Q.R.; Biddison, W.E.; Wiley, D.C. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nat. Cell Biol. 1996, 384, 134–141. [Google Scholar] [CrossRef]
- Leem, J.; De Oliveira, S.H.P.; Krawczyk, K.; Deane, C.M. STCRDab: The structural T-cell receptor database. Nucleic Acids Res. 2018, 46, D406–D412. [Google Scholar] [CrossRef] [PubMed]
- Clayton, G.M.; Wang, Y.; Crawford, F.; Novikov, A.; Wimberly, B.T.; Kieft, J.S.; Falta, M.T.; Bowerman, N.A.; Marrack, P.; Fontenot, A.P.; et al. Structural Basis of Chronic Beryllium Disease: Linking Allergic Hypersensitivity and Autoimmunity. Cell 2014, 158, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Crawford, F.; Marrack, P.; Kappler, J.W.; Dai, S. T-cell receptor (TCR) interaction with peptides that mimic nickel offers insight into nickel contact allergy. Proc. Natl. Acad. Sci. USA 2012, 109, 18517–18522. [Google Scholar] [CrossRef]
- Rudolph, M.G.; Stanfield, R.L.; Wilson, I.A. How tcrs bind mhcs, peptides, and coreceptors. Annu. Rev. Immunol. 2006, 24, 419–466. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.C.; Adams, J.J.; Feng, D.; Ely, L.K. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nat. Immunol. 2009, 10, 143–147. [Google Scholar] [CrossRef]
- Feng, D.; Bond, C.J.; Ely, L.K.; A Maynard, J.; Garcia, K.C. Structural evidence for a germline-encoded T cell receptor–major histocompatibility complex interaction ’codon’. Nat. Immunol. 2007, 8, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, M.; Mendoza, J.; Sethi, D.K.; Dong, S.; Glanville, J.; Dobbins, J.; Özkan, E.; Davis, M.M.; Wucherpfennig, K.W.; Garcia, K.C. Deconstructing the Peptide-MHC Specificity of T Cell Recognition. Cell 2014, 157, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.; Bulek, A.M.; Dolton, G.; Schauenberg, A.J.; Szomolay, B.; Rittase, W.; Trimby, A.; Jothikumar, P.; Fuller, A.; Skowera, A.; et al. Hotspot autoimmune T cell receptor binding underlies pathogen and insulin peptide cross-reactivity. J. Clin. Investig. 2016, 126, 2191–2204. [Google Scholar] [CrossRef]
- Reiser, J.-B.; Grégoire, C.; Darnault, C.; Mosser, T.; Guimezanes, A.; Schmitt-Verhulst, A.-M.; Fontecilla-Camps, J.C.; Mazza, G.; Malissen, B.; Housset, D. A T Cell Receptor CDR3β Loop Undergoes Conformational Changes of Unprecedented Magnitude Upon Binding to a Peptide/MHC Class I Complex. Immunity 2002, 16, 345–354. [Google Scholar] [CrossRef]
- Siewert, K.; Malotka, J.; Kawakami, N.; Wekerle, H.; Hohlfeld, R.; Dornmair, K. Unbiased identification of target antigens of CD8+ T cells with combinatorial libraries coding for short peptides. Nat. Med. 2012, 18, 824–828. [Google Scholar] [CrossRef]
- Arakawa, A.; Siewert, K.; Stöhr, J.; Besgen, P.; Kim, S.-M.; Rühl, G.; Nickel, J.; Vollmer, S.; Thomas, P.; Krebs, S.; et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J. Exp. Med. 2015, 212, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Mazza, C.; Auphan-Anezin, N.; Grégoire, C.; Guimezanes, A.; Kellenberger, C.; Roussel, A.; Kearney, A.; Van Der Merwe, P.A.; Schmitt-Verhulst, A.-M.; Malissen, B. How much can a T-cell antigen receptor adapt to structurally distinct antigenic peptides? EMBO J. 2007, 26, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.C.; Degano, M.; Pease, L.R.; Huang, M.; Peterson, P.A.; Teyton, L.; Wilson, I.A. Structural Basis of Plasticity in T Cell Receptor Recognition of a Self Peptide-MHC Antigen. Science 1998, 279, 1166–1172. [Google Scholar] [CrossRef]
- Baker, B.M.; Scott, D.R.; Blevins, S.J.; Hawse, W.F. Structural and dynamic control of T-cell receptor specificity, cross-reactivity, and binding mechanism. Immunol. Rev. 2012, 250, 10–31. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-H.; Baker, B.M.; Garboczi, D.N.; E Biddison, W.; Wiley, D.C. Four A6-TCR/Peptide/HLA-A2 Structures that Generate Very Different T Cell Signals Are Nearly Identical. Immunity 1999, 11, 45–56. [Google Scholar] [CrossRef]
- Armstrong, K.M.; Piepenbrink, K.H.; Baker, B.M. Conformational changes and flexibility in T-cell receptor recognition of peptide–MHC complexes. Biochem. J. 2008, 415, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Kjer-Nielsen, L.; Clements, C.S.; Purcell, A.; Brooks, A.; Whisstock, J.; Burrows, S.; McCluskey, J.; Rossjohn, J. A Structural Basis for the Selection of Dominant αβ T Cell Receptors in Antiviral Immunity. Immunity 2003, 18, 53–64. [Google Scholar] [CrossRef]
- Borbulevych, O.Y.; Piepenbrink, K.H.; Gloor, B.E.; Scott, D.R.; Sommese, R.F.; Cole, D.K.; Sewell, A.K.; Baker, B.M. T Cell Receptor Cross-reactivity Directed by Antigen-Dependent Tuning of Peptide-MHC Molecular Flexibility. Immunity 2009, 31, 885–896. [Google Scholar] [CrossRef]
- Lang, H.; Jacobsen, H.; Ikemizu, S.; Andersson, C.; Harlos, K.; Madsen, L.; Hjorth, P.; Sondergaard, L.; Svejgaard, A.; Wucherpfennig, K.; et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol. 2002, 3, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, W.A.; Chen, Z.; Gras, S.; Archbold, J.; Tynan, F.E.; Clements, C.S.; Bharadwaj, M.; Kjer-Nielsen, L.; Saunders, P.M.; Wilce, M.C.; et al. T Cell Allorecognition via Molecular Mimicry. Immunity 2009, 31, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Harkiolaki, M.; Holmes, S.L.; Svendsen, P.; Gregersen, J.W.; Jensen, L.T.; McMahon, R.; Friese, M.A.; van Boxel, G.; Etzensperger, R.; Tzartos, J.S.; et al. T Cell-Mediated Autoimmune Disease due to Low-Affinity Crossreactivity to Common Microbial Peptides. Immunity 2009, 30, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lübke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021, 22, 74–85. [Google Scholar] [CrossRef]
- Bacher, P.; Rosati, E.; Esser, D.; Martini, G.R.; Saggau, C.; Schiminsky, E.; Dargvainiene, J.; Schröder, I.; Wieters, I.; Khodamoradi, Y.; et al. Low-Avidity CD4+ T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity 2020, 53, 1258–1271.e5. [Google Scholar] [CrossRef]
- Pichler, W.J. Pharmacological interaction of drugs with antigen-specific immune receptors: The p-i concept. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 301–305. [Google Scholar] [CrossRef]
- Moulon, C.; Wild, D.; Weltzien, H.U.; Dormoy, A. MHC-Dependent and -Independent Activation of Human Nickel-Specific CD8+ Cytotoxic T Cells from Allergic Donors11This work was presented in part at the 26th Annual Meeting of the European Society of Dermatologic Research, Amsterdam, September 19–22, 1996. J. Investig. Dermatol. 1998, 111, 360–366. [Google Scholar] [CrossRef][Green Version]
- Thierse, H.-J.; Moulon, C.; Allespach, Y.; Zimmermann, B.; Doetze, A.; Kuppig, S.; Wild, D.; Herberg, F.; Weltzien, H.U. Metal-Protein Complex-Mediated Transport and Delivery of Ni2+ to TCR/MHC Contact Sites in Nickel-Specific Human T Cell Activation. J. Immunol. 2004, 172, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Falta, M.T.; Pinilla, C.; Mack, D.G.; Tinega, A.N.; Crawford, F.; Giulianotti, M.; Santos, R.; Clayton, G.M.; Wang, Y.; Zhang, X.; et al. Identification of beryllium-dependent peptides recognized by CD4+ T cells in chronic beryllium disease. J. Exp. Med. 2013, 210, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, P.; Labhardt, A.; Sinigaglia, F. Selective interaction of Ni with an MHC-bound peptide. EMBO J. 1991, 10, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, P.; A Spinas, G.; Sinigaglia, F. Gold-specific T cells in rheumatoid arthritis patients treated with gold. J. Clin. Investig. 1992, 89, 254–258. [Google Scholar] [CrossRef]
- De Wall, S.L.; Painter, C.; Stone, J.D.; Bandaranayake, R.; Wiley, D.C.; Mitchison, T.J.; Stern, L.J.; Dedecker, B.S. Noble metals strip peptides from class II MHC proteins. Nat. Chem. Biol. 2006, 2, 197–201. [Google Scholar] [CrossRef]
- Nasorri, F.; Sebastiani, S.; Mariani, V.; Girolomoni, G.; Cavani, A.; De Pità, O.; Puddu, P. Activation of Nickel-Specific CD4+ T Lymphocytes in the Absence of Professional Antigen-Presenting Cells. J. Investig. Dermatol. 2002, 118, 172–179. [Google Scholar] [CrossRef]
- Shigematsu, H.; Kumagai, K.; Suzuki, M.; Eguchi, T.; Matsubara, R.; Nakasone, Y.; Nasu, K.; Yoshizawa, T.; Ichikawa, H.; Mori, T.; et al. Cross-Reactivity of Palladium in a Murine Model of Metal-induced Allergic Contact Dermatitis. Int. J. Mol. Sci. 2020, 21, 4061. [Google Scholar] [CrossRef]
- Griem, P.; Panthel, K.; Kalbacher, H.; Gleichmann, E. Alteration of a model antigen by Au(III) leads to T cell sensitization to cryptic peptides. Eur. J. Immunol. 1996, 26, 279–287. [Google Scholar] [CrossRef]
- Griem, P.; von Vultée, C.; Panthel, K.; Best, S.L.; Sadler, P.J.; Shaw III, C.F. T cell cross-reactivity to heavy metals: Identical cryptic peptides may be presented from protein exposed to different metals. Eur. J. Immunol. 1998, 28, 1941–1947. [Google Scholar] [CrossRef]
- Moulon, C.; Choleva, Y.; Thierse, H.-J.; Wild, D.; Weltzien, H.U. T Cell Receptor Transfection Shows Non-HLA-Restricted Recognition of Nickel by CD8+ Human T Cells to be Mediated by αβ T Cell Receptors. J. Investig. Dermatol. 2003, 121, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Comstedt, L.R.; Dahlin, J.; Bruze, M.; Åkesson, A.; Hindsén, M.; Pontén, A.; Isaksson, M.; Svedman, C. Prevalence of contact allergy to metals: Nickel, palladium, and cobalt in Southern Sweden from 1995–2016. Contact Dermat. 2020, 82, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Gawkrodger, D.J.; Lewis, F.M.; Shah, M. Contact sensitivity to nickel and other metals in jewelry reactors. J. Am. Acad. Dermatol. 2000, 43, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lagrelius, M.; Wahlgren, C.-F.; Matura, M.; Kull, I.; Lidén, C. High prevalence of contact allergy in adolescence: Results from the population-based BAMSE birth cohort. Contact Dermat. 2015, 74, 44–51. [Google Scholar] [CrossRef]
- Lisi, P.; Brunelli, L.; Stingeni, L. Co-sensitivity between cobalt and other transition metals. Contact Dermat. 2003, 48, 172–173. [Google Scholar] [CrossRef]
- Kanerva, L.; Kerosuo, H.; Kullaa, A.; Kerosuo, E. Allergic patch test reactions to palladium chloride in schoolchildren. Contact Dermat. 1996, 34, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Santucci, B.; Cannistraci, C.; Cristaudo, A.; Picardo, M. Multiple sensitivities to transition metals: The nickel palladium reactions. Contact Dermat. 1996, 35, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Kinbara, M.; Nagai, Y.; Takano-Yamamoto, T.; Sugawara, S.; Endo, Y. Cross-reactivity among some metals in a murine metal allergy model. Br. J. Dermatol. 2011, 165, 1022–1029. [Google Scholar] [CrossRef]
- Santucci, B.; Cristaudo, A.; Cannistraci, C.; Picardo, M. Interaction of palladium ions with the skin. Exp. Dermatol. 1995, 4, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Pistoor, F.H.M.; Kapsenberg, M.L.; Bos, J.D.; Meinardi, M.M.H.M.; E Von Blomberg, M.; Scheper, R.J.; Mary, B. Cross-Reactivity of Human Nickel-Reactive T-Lymphocyte Clones with Copper and Palladium. J. Investig. Dermatol. 1995, 105, 92–95. [Google Scholar] [CrossRef]
- Löfström, A.; Wigzell, H. Antigen specific human T cell lines specific for cobalt chloride. Acta Derm. Venereol. 1986, 66, 200–206. [Google Scholar]
- Thomssen, H.; Hoffmann, B.; Schank, M.; Höhler, T.; Thabe, H.; Büschenfelde, K.H.M.Z.; Märker-Hermann, E. Cobalt-specific T lymphocytes in synovial tissue after an allergic reaction to a cobalt alloy joint prosthesis. J. Rheumatol. 2001, 28, 254. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedel, F.; Aparicio-Soto, M.; Curato, C.; Thierse, H.-J.; Siewert, K.; Luch, A. Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. Int. J. Environ. Res. Public Health 2021, 18, 10867. https://doi.org/10.3390/ijerph182010867
Riedel F, Aparicio-Soto M, Curato C, Thierse H-J, Siewert K, Luch A. Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. International Journal of Environmental Research and Public Health. 2021; 18(20):10867. https://doi.org/10.3390/ijerph182010867
Chicago/Turabian StyleRiedel, Franziska, Marina Aparicio-Soto, Caterina Curato, Hermann-Josef Thierse, Katherina Siewert, and Andreas Luch. 2021. "Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface" International Journal of Environmental Research and Public Health 18, no. 20: 10867. https://doi.org/10.3390/ijerph182010867
APA StyleRiedel, F., Aparicio-Soto, M., Curato, C., Thierse, H.-J., Siewert, K., & Luch, A. (2021). Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. International Journal of Environmental Research and Public Health, 18(20), 10867. https://doi.org/10.3390/ijerph182010867






