Recent Malignant Melanoma Epidemiology in Upper Silesia, Poland. A Decade-Long Study Focusing on the Agricultural Sector
Abstract
1. Introduction
1.1. Aim of the Study
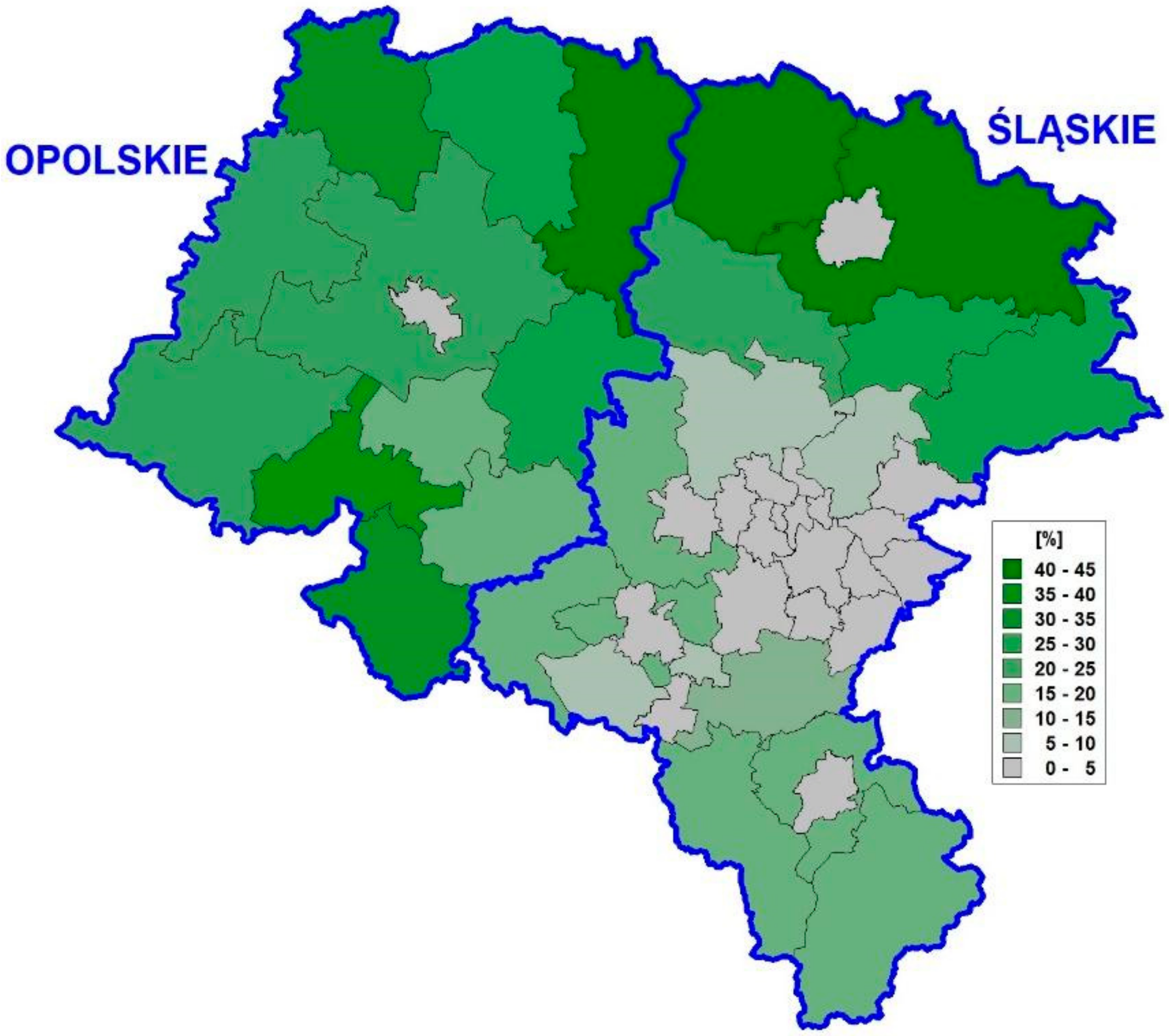
1.2. Upper Silesia
2. Material
3. Methods
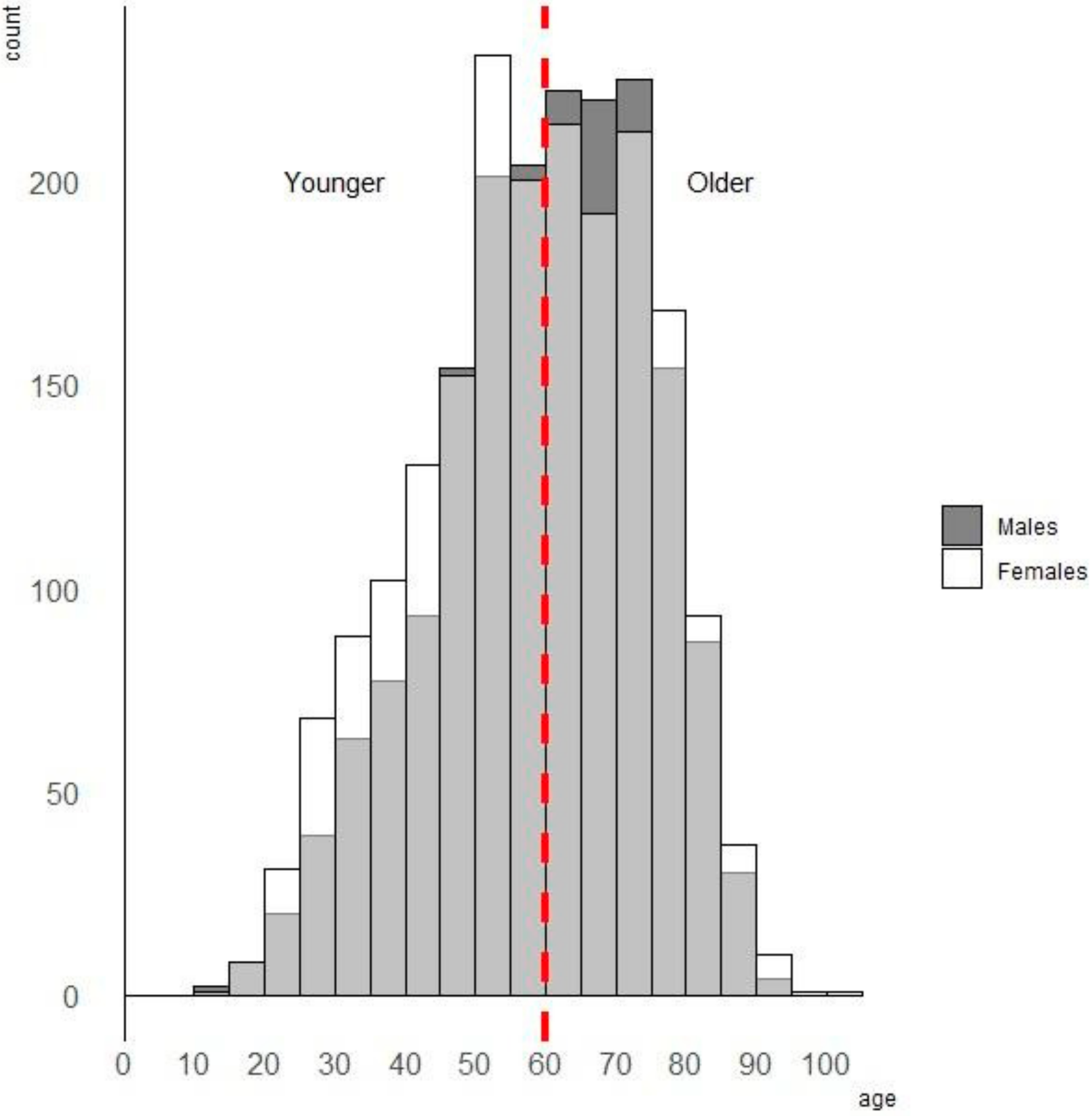
4. Results
5. Discussion
6. Conclusions
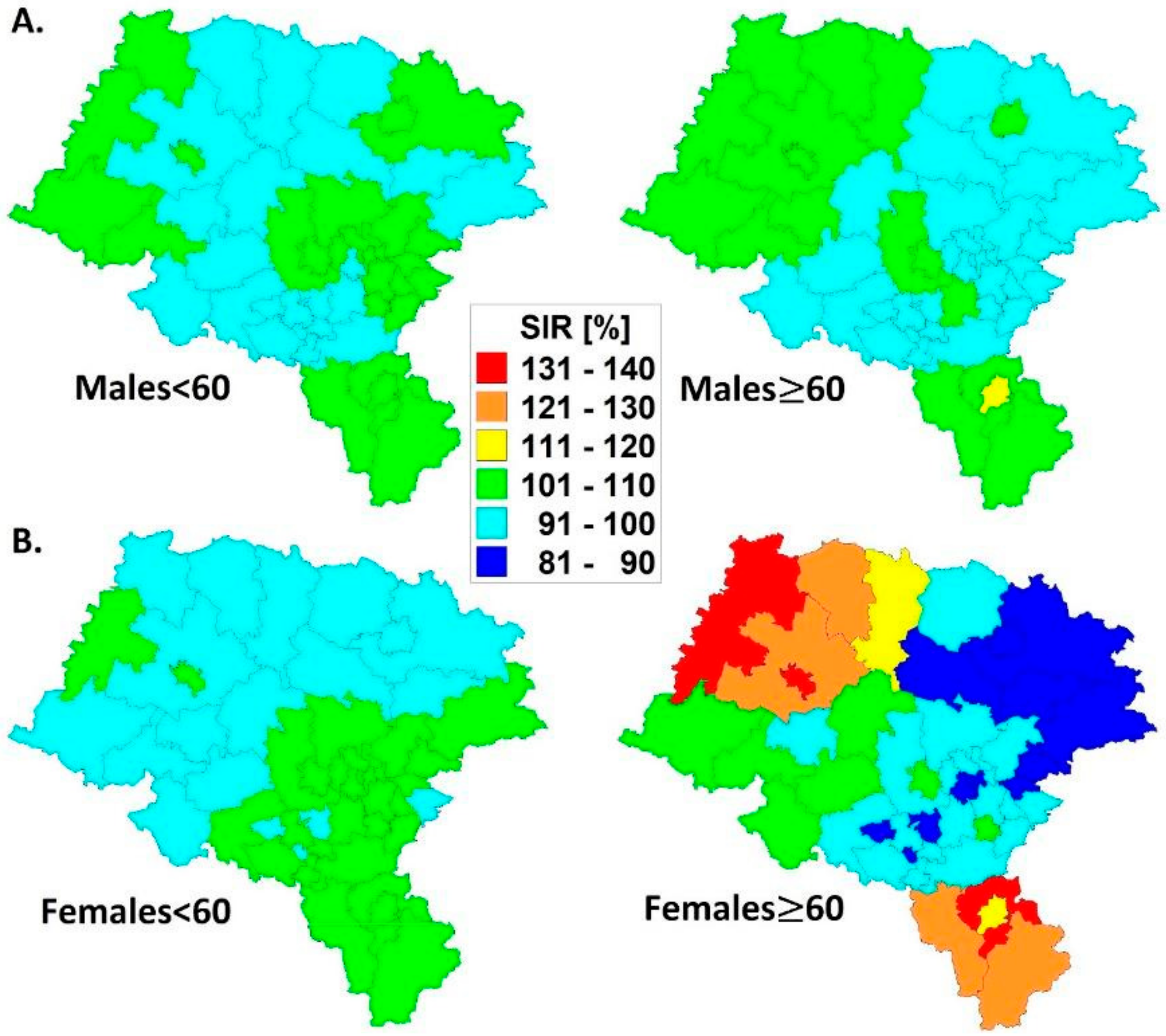
- Spatial malignant melanoma standardized incidence ratios are lower in the younger (<60) age group for both men and women; a more dynamic variation of SIRs is observed in older (≥60) women than in men.
- The decade spatio-temporal growth rates of malignant melanoma are more dynamic in the older (≥60) population inhabiting Upper Silesia; the GRs statistically significantly correlate with the SIR models.
- The five-year disease clusters of malignant melanoma with an elevated relative risk in older (≥60) men and women coincide with each other in space and time; more diverse patterns were established in women.
- There is a ‘migration’ of malignant melanoma risk in the agricultural population from statistically significant negative in younger (<60) men towards positive in older (≥60) women. This could have connections to economic, hormonal or physiological backgrounds.
- The results above indicate that females are at a higher risk of malignant melanoma than males and this could only be proved with spatio-temporal data modeling.
7. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rigel, D.S.; Carucci, J.A. Malignant melanoma: Prevention, early detection, and treatment in the 21st century. CA Cancer J. Clin. 2000, 50, 215–236. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0; IARC CancerBase: Lyon, France, 2012; Volume 11, Available online: https://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012/ (accessed on 30 August 2021).
- Kosary, C.; Altekruse, S.; Ruhl, J.; Lee, R.; Dickie, L. Clinical and prognostic factors for melanoma of the skin using SEER registries: Collaborative stage data collection system, version 1 and version 2. Cancer 2014, 120 (Suppl. S23), 3807–3814. [Google Scholar] [CrossRef]
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. Morb. Mortal Wkly. Rep. 2015, 64, 591–596. [Google Scholar]
- Nikolaou, V.; Stratigos, A.J. Emerging trends in the epidemiology of melanoma. Br. J. Dermatol. 2014, 170, 11–19. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures. 2017. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html/ (accessed on 30 August 2021).
- Jemal, A.; Saraiya, M.; Patel, P.; Cherala, S.; Barnholtz-Sloan, J.; Kim, J.; Wiggins, C.L.; Wingo, P.A. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J. Am. Acad. Dermatol. 2011, 65, S17.e1–S17.e11. [Google Scholar] [CrossRef]
- Shen, W.; Sakamoto, N.; Yang, L. Melanoma-specific mortality and competing mortality in patients with non-metastatic malignant melanoma: A population-based analysis. BMC Cancer 2016, 16, 413. [Google Scholar] [CrossRef]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER Cancer Statistics Review 1975–2013; 2016. Available online: https://seer.cancer.gov/archive/csr/1975_2013/ (accessed on 30 August 2021).
- Erdei, E.; Torres, S. A new understanding in the epidemiology of melanoma. Expert Rev. Anticancer Ther. 2010, 10, 1811–1823. [Google Scholar] [CrossRef]
- Whiteman, D.; Green, A.; Olsen, C. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin cancer: Epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Chang, C.-L.; Schabert, V.F.; Munakata, J.; Donga, P.; Abhyankar, S.; Reyes, C.M.; Yim, Y.M. Comparative healthcare costs in patients with metastatic melanoma in the USA. Melanoma Res. 2015, 25, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Besag, J.; York, J.; Mollié, A. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Congdon, P. Applied Bayesian Modelling; Wiley: Chichester, UK, 2003; pp. 310–317. [Google Scholar]
- Kulldorff, M. A spatial scan statistic. Commun. Stat. Theory Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Spiegelhalter, D.; Thomas, A.; Best, N.; Lunn, D. WinBUGS; Version 1.4.3; Medical Research Council-Biostatistics Unit: Cambridge, UK, 2007; Available online: https://www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-winbugs/ (accessed on 30 August 2021).
- Kulldorff, M. SaTScan; v9.7; Harvard Medical School: Boston, MA, USA, 2021; Available online: https://www.satscan.org/ (accessed on 30 August 2021).
- Leiser, C.L.; Taddie, M.; Hemmert, R.; Steed, R.R.; Vanderslice, J.A.; Henry, K.; Ambrose, J.; O’Neil, B.; Smith, K.R.; Hanson, H.A. Spatial clusters of cancer incidence: Analyzing 1940 census data linked to 1966–2017 cancer records. Cancer Causes Control 2020, 31, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Tukiendorf, A. Could socio-economic transformation and the resulting psychological stress influence cancer risk in Opole province, Poland? Central Eur. J. Public Health 2005, 13, 125–131. [Google Scholar]
- Strömberg, U.; Parkes, B.L.; Holmén, A.; Peterson, S.; Holmberg, E.; Baigi, A.; Piel, F.B. Disease mapping of early- and late-stage cancer to monitor inequalities in early detection: A study of cutaneous malignant melanoma. Eur. J. Epidemiol. 2020, 35, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.B.; Lagas, J.S.; Broestl, L.; Sponagel, J.; Rockwell, N.; Rhee, G.; Rosen, S.F.; Chen, S.; Klein, R.S.; Imoukhuede, P.; et al. Sex differences in cancer mechanisms. Biol. Sex Differ. 2020, 11, 17. [Google Scholar] [CrossRef]
- Markman, J.L.; Porritt, R.; Wakita, D.; Lane, M.E.; Martinon, D.; Rivas, M.N.; Luu, M.; Posadas, E.M.; Crother, T.R.; Arditi, M. Loss of testosterone impairs anti-tumor neutrophil function. Nat. Commun. 2020, 11, 1613–1615. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Bijon, A.; Mesrine, S.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F. Cutaneous Melanoma and Endogenous Hormonal Factors: A Large French Prospective Study. Am. J. Epidemiol. 2011, 173, 1192–1202. [Google Scholar] [CrossRef]
- Koomen, E.; Joosse, A.; Herings, R.M.C.; Casparie, M.K.; Guchelaar, H.J.; Nijsten, T. Estrogens, oral contraceptives and hormonal replacement therapy increase the incidence of cutaneous melanoma: A population-based case-control study. Ann Oncol. 2009, 20, 358–364. [Google Scholar] [CrossRef]
- Feskanich, D.; Hunter, D.J.; Willett, W.C.; Spiegelman, D.; Stampfer, M.J.; Speizer, F.; Colditz, G. Oral contraceptive use and risk of melanoma in premenopausal women. Br. J. Cancer 1999, 81, 918–923. [Google Scholar] [CrossRef]
- Karagas, M.R.; Zens, M.S.; Stukel, T.; Swerdlow, A.; Rosso, S.; Osterlind, A.; Mack, T.; Kirkpatrick, C.; Holly, E.A.; Green, A.; et al. Pregnancy History and Incidence of Melanoma in Women: A Pooled Analysis. Cancer Causes Control 2006, 17, 11–19. [Google Scholar] [CrossRef]
- Kvåle, G.; Heuch, I.; Nilssen, S. Parity in Relation to Mortality and Cancer Incidence: A Prospective Study of Norwegian Women. Int. J. Epidemiol. 1994, 23, 691–699. [Google Scholar] [CrossRef]
- Lambe, M.; Thörn, M.; Sparén, P.; Bergström, R.; Adami, H.O. Malignant melanoma: Reduced risk associated with early childbearing and multiparity. Melanoma Res. 1996, 6, 147–153. [Google Scholar] [CrossRef]
- Gavin, A.; Boyle, R.; Donnelly, D.; Donnelly, C.; Gordon, S.; McElwee, G.; O’Hagan, A. Trends in skin cancer knowledge, sun protection practices and behaviours in the Northern Ireland population. Eur. J. Public Health 2012, 22, 408–412. [Google Scholar] [CrossRef]
- Arisi, M.; Zane, C.; Caravello, S.; Rovati, C.; Zanca, A.; Venturini, M.; Calzavara-Pinton, P. Sun Exposure and Melanoma, Certainties and Weaknesses of the Present Knowledge. Front. Med. 2018, 5, 235. [Google Scholar] [CrossRef]
- Lemarchand, C.; Tual, S.; Levêque-Morlais, N.; Perrier, S.; Belot, A.; Velten, M.; Guizard, A.-V.; Marcotullio, E.; Monnereau, A.; Clin, B.; et al. Cancer incidence in the AGRICAN cohort study (2005–2011). Cancer Epidemiol. 2017, 49, 175–185. [Google Scholar] [CrossRef]
- Kachuri, L.; Harris, M.A.; MacLeod, J.S.; Tjepkema, M.; Peters, P.A.; Demers, P.A. Cancer risks in a population-based study of 70,570 agricultural workers: Results from the Canadian census health and Environment cohort (CanCHEC). BMC Cancer 2017, 17, 343. [Google Scholar] [CrossRef]
- Settimi, L.; Comba, P.; Carrieri, P.; Boffetta, P.; Magnani, C.; Terracini, B.; Andrion, A.; Bosia, S.; Ciapini, C.; De Santis, M.; et al. Cancer risk among female agricultural workers: A multi-center case-control study. Am. J. Ind. Med. 1999, 36, 135–141. [Google Scholar] [CrossRef]
- Rushton, L.; Hutchings, S. The burden of occupationally-related cutaneous malignant melanoma in Britain due to solar radiation. Br. J. Cancer 2017, 116, 536–539. [Google Scholar] [CrossRef][Green Version]
- Trakatelli, M.; Barkitzi, K.; Apap, C.; Majewski, S.; De Vries, E.; EPIDERM Group; Coebergh, J.W.; Apalla, Z.; Ioannides, D.; Kalabalikis, D. Skin cancer risk in outdoor workers: A European multicenter case-control study. J. Eur. Acad Dermatol. Venereol. 2016, 30 (Suppl. S3), 5–11. [Google Scholar] [CrossRef]
- Zink, A.; Tizek, L.; Schielein, M.; Böhner, A.; Biedermann, T.; Wildner, M. Different outdoor professions have different risks—A cross-sectional study comparing non-melanoma skin cancer risk among farmers, gardeners and mountain guides. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1695–1701. [Google Scholar] [CrossRef]
- Dennis, L.K.; Lynch, C.F.; Sandler, D.P.; Alavanja, M.C. Pesticide Use and Cutaneous Melanoma in Pesticide Applicators in the Agricultural Heath Study. Environ. Health Perspect. 2010, 118, 812–817. [Google Scholar] [CrossRef]
- Haluza, D.; Simic, S.; Moshammer, H. Temporal and Spatial Melanoma Trends in Austria: An Ecological Study. Int. J. Environ. Res. Public Health 2014, 11, 734–748. [Google Scholar] [CrossRef]
- Orkić, Ž.; Puntarić, D.; Vidosavljević, D.; Puntarić, I.; Puntarić, E.; Gvozdić, V.; Mayer, D.; Vidosavljević, M.; Muller Vranješ, A. Climatic factors and epidemiologic characteristics of head and neck skin malignancies in Osijek Baranja County, Croatia. Cent. Eur. J. Public Health 2015, 23, 275–285. [Google Scholar] [CrossRef]
- Paolino, G.; Moliterni, E.; Didona, D.; Garelli, V.; Corsetti, P.; Lopez, T.; Richetta, A.G.; Cantisani, C.; Bottoni, U.; Calvieri, S. Clinicopathological features, vitamin D serological levels and prognosis in cutaneous melanoma of shield-sites: An update. Med. Oncol. 2015, 32, 451. [Google Scholar] [CrossRef]

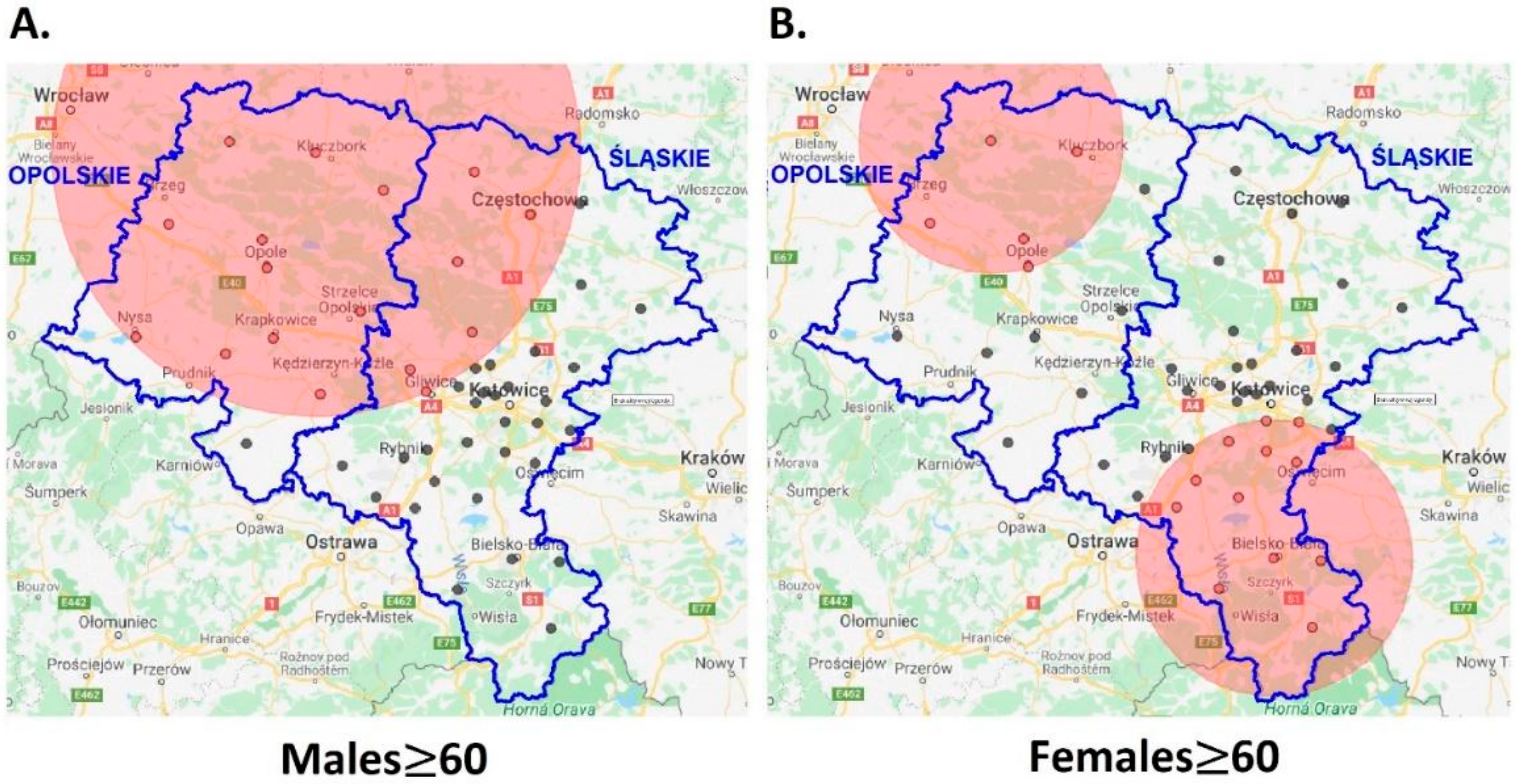
| Population | N, E Coordinates | Radius (km) | Timeframe | RR(CI95%) | p-Value |
|---|---|---|---|---|---|
| Males | 50.9846, 18.1490 | 83.82 | 2009–2013 | 1.45(1.10–1.91) | 0.008 |
| Females | 49.8133, 19.0398 | 44.83 | 2008–2012 | 1.51(1.09–2.10) | 0.014 |
| 51.0174, 17.7593 | 41.90 | 2008–2012 | 1.84(1.07–3.17) | 0.028 |
| Population | SIR | 95%CI | p-Value |
|---|---|---|---|
| Males <60 | 0.994 | (0.988,0.999) | 0.0189 |
| Males ≥60 | 0.998 | (0.993,1.003) | 0.2014 |
| Females <60 | 0.997 | (0.991,1.002) | 0.0991 |
| Females ≥60 | 1.004 | (0.999,1.009) | 0.0663 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukiendorf, A.; Kamińska-Winciorek, G.; Lancé, M.D.; Olszak-Wąsik, K.; Szczepanowski, Z.; Kulik-Parobczy, I.; Wolny-Rokicka, E.I. Recent Malignant Melanoma Epidemiology in Upper Silesia, Poland. A Decade-Long Study Focusing on the Agricultural Sector. Int. J. Environ. Res. Public Health 2021, 18, 10863. https://doi.org/10.3390/ijerph182010863
Tukiendorf A, Kamińska-Winciorek G, Lancé MD, Olszak-Wąsik K, Szczepanowski Z, Kulik-Parobczy I, Wolny-Rokicka EI. Recent Malignant Melanoma Epidemiology in Upper Silesia, Poland. A Decade-Long Study Focusing on the Agricultural Sector. International Journal of Environmental Research and Public Health. 2021; 18(20):10863. https://doi.org/10.3390/ijerph182010863
Chicago/Turabian StyleTukiendorf, Andrzej, Grażyna Kamińska-Winciorek, Marcus Daniel Lancé, Katarzyna Olszak-Wąsik, Zbigniew Szczepanowski, Iwona Kulik-Parobczy, and Edyta Idalia Wolny-Rokicka. 2021. "Recent Malignant Melanoma Epidemiology in Upper Silesia, Poland. A Decade-Long Study Focusing on the Agricultural Sector" International Journal of Environmental Research and Public Health 18, no. 20: 10863. https://doi.org/10.3390/ijerph182010863
APA StyleTukiendorf, A., Kamińska-Winciorek, G., Lancé, M. D., Olszak-Wąsik, K., Szczepanowski, Z., Kulik-Parobczy, I., & Wolny-Rokicka, E. I. (2021). Recent Malignant Melanoma Epidemiology in Upper Silesia, Poland. A Decade-Long Study Focusing on the Agricultural Sector. International Journal of Environmental Research and Public Health, 18(20), 10863. https://doi.org/10.3390/ijerph182010863







