The Usability of an Online Tool to Promote the Use of Evidence-Based Smoking Cessation Interventions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Procedure
2.2. Materials
DA Components
- An introduction, which explained the goals and relevance of the DA and summarized the EBSCIs and the other elements of the DA.
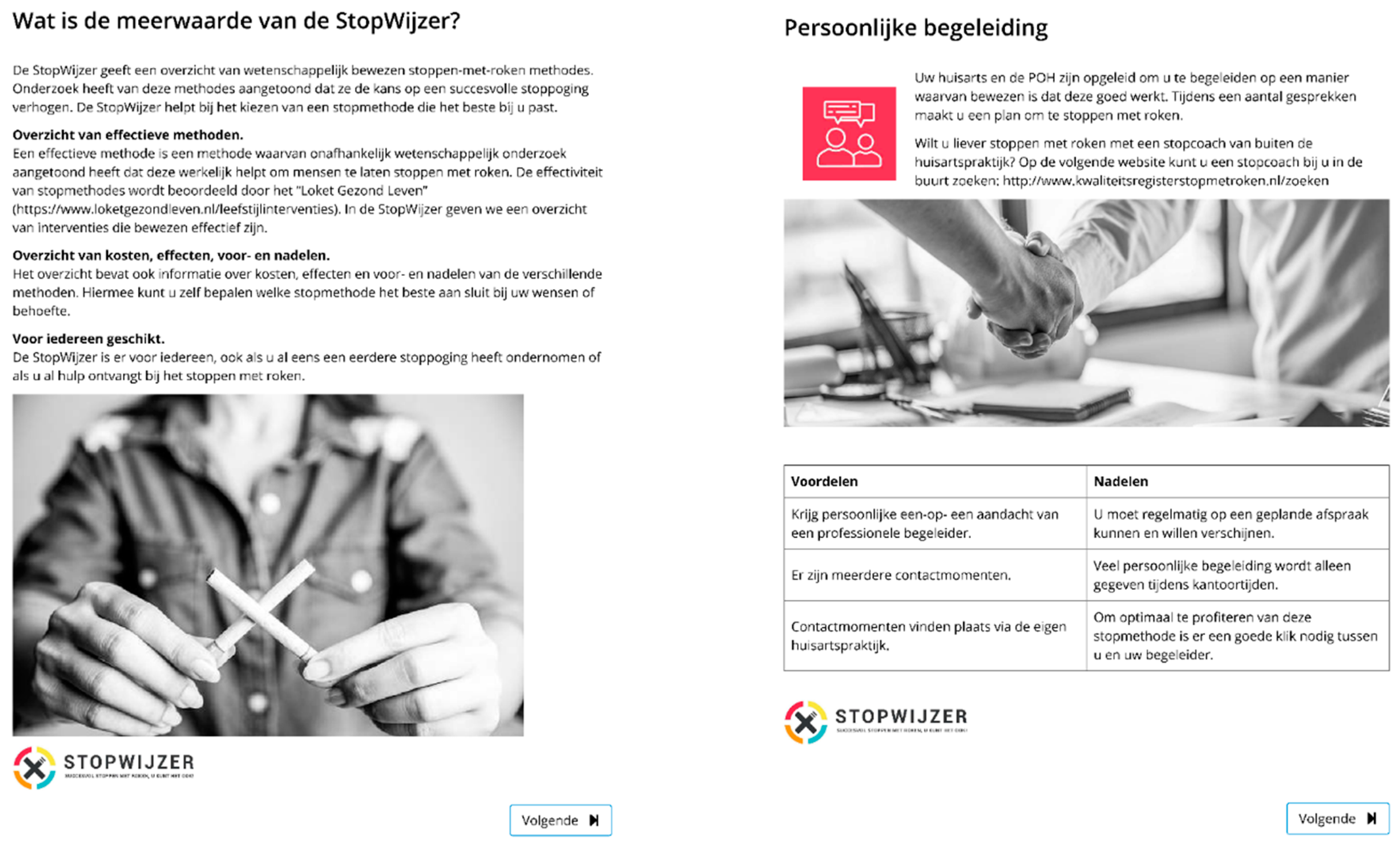
- An overview of the different EBSCIs in the following order: face-to-face counseling; eHealth; counseling via telephone; group counseling; nicotine replacement therapy; pharmacotherapy; non-evidence-based “cessation” methods of acupuncture [29], laser therapy [46], auriculotherapy [47], hypnosis [48] and e-cigarettes (see Figure 1).
- An overview of the possible reimbursements of EBSCIs by health insurers with a calculation tool to help patients understand how much money they could save by quitting smoking.
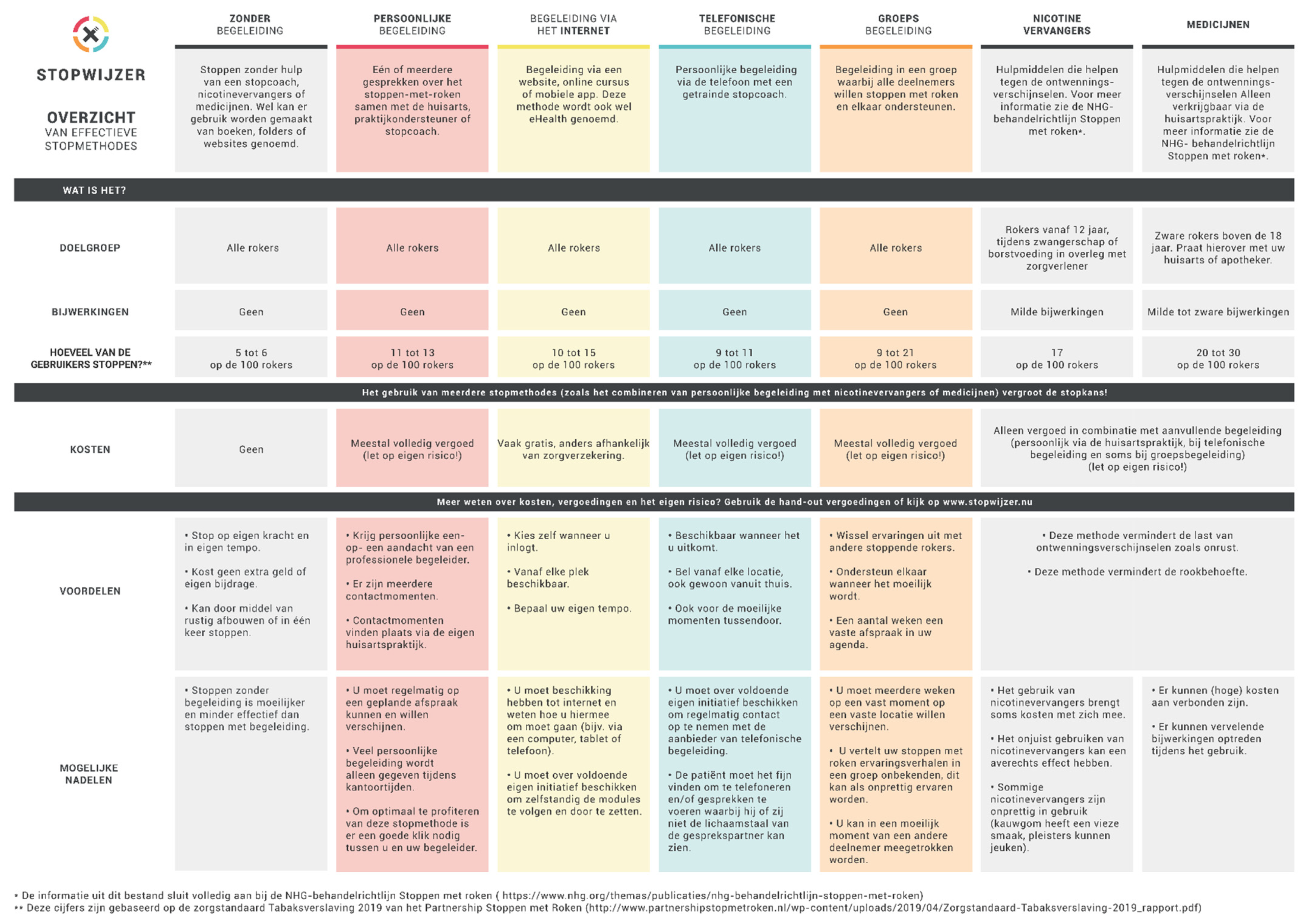
- The website also contained an overview of the options, which could also be downloaded. The overview listed the EBSCIs mentioned above and gave an outline of their target groups, strengths and weaknesses, effectiveness and costs (see Figure 2).
2.3. Measurements
2.3.1. Usability, Program Evaluation and Decisional Conflict
2.3.2. Intention to Use EBSCIs
2.4. Data Analysis
3. Results
3.1. Study Sample Characteristics
3.2. Program Evaluation and Decisional Conflict
3.3. Intention to Use EBSCIs
4. Discussion
4.1. Potential Strengths and Limitations of the Study
4.2. Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Report on the Global Tobacco Epidemic, 2019: Offer Help to Quit Tobacco Use; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014.
- Polder, J.J.; van Gils, P.F.; Kok, L.; Talhout, R.; Feenstra, T. De rekening van roken. Tijdschr. voor Geneeskd. 2017, 161, D833. [Google Scholar]
- Verdurmen, J.; Monshouwer, K.; Van Laar, M.; Van Bon-Martens, M. Factsheet Continu Onderzoek Rookgewoonten 2013; Trimbos-Instituut: Utrecht, The Netherlands, 2014. [Google Scholar]
- Willemsen, M.C. Tobacco Control Policy in The Netherlands: Between Economy, Public Health, and Ideology; Springer Nature: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Mariolis, P.; Rock, V.; Asman, K.; Merritt, R.; Malarcher, A.; Husten, C.; Pechacek, T. Tobacco use among adults—United States, 2005. Oncol. Times 2006, 28, 42–47. [Google Scholar] [CrossRef]
- Ministerie van Volkgezondheid Welzijn en Sport. Nationaal Preventieakkoord: Naar een Gezonder Nederland; Ministry of Public Health, Welfare and Sport: Den Haag, The Netherlands, 2018.
- Bommele, J.; Willemsen, M. Kerncijfers Roken 2020; Trimbos-instituut: Utrecht, The Netherlands, 2021. [Google Scholar]
- Hughes, J.R.; Keely, J.; Naud, S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004, 99, 29–38. [Google Scholar] [CrossRef]
- Chaiton, M.; Diemert, L.; Cohen, J.E.; Bondy, S.J.; Selby, P.; Philipneri, A.; Schwartz, R. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open 2016, 6, e011045. [Google Scholar] [CrossRef] [Green Version]
- West, R.; Raw, M.; McNeill, A.; Stead, L.; Aveyard, P.; Bitton, J.; Stapleton, J.; McRobbie, H.; Pokhrel, S.; Lester-George, A. Health-care interventions to promote and assist tobacco cessation: A review of efficacy, effectiveness and affordability for use in national guideline development. Addiction 2015, 110, 1388–1403. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, M.E.; Seydel, E.R.; DeVries, H.; Mudde, A.N.; Kok, G.J. Effectiveness of a minimal contact smoking cessation program for Dutch general practitioners: A randomized controlled trial. Prev. Med. 2001, 32, 182–190. [Google Scholar] [CrossRef]
- Stead, L.F.; Buitrago, D.; Preciado, N.; Sanchez, G.; Hartmann-Boyce, J.; Lancaster, T. Physician advice for smoking cessation. Cochrane Database Syst. Rev. 2013, 5, 1–62. [Google Scholar] [CrossRef]
- Aveyard, P.; Begh, R.; Parsons, A.; West, R. Brief opportunistic smoking cessation interventions: A systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction 2012, 107, 1066–1073. [Google Scholar] [CrossRef]
- Papadakis, S.; Cole, A.G.; Reid, R.D.; Coja, M.; Aitken, D.; Mullen, K.A.; Gharib, M.; Pipe, A.L. Increasing Rates of Tobacco Treatment Delivery in Primary Care Practice: Evaluation of the Ottawa Model for Smoking Cessation. Ann. Fam. Med. 2016, 14, 235–243. [Google Scholar] [CrossRef]
- van Rossem, C.; Spigt, M.; Viechtbauer, W.; Lucas, A.E.; van Schayck, O.C.; Kotz, D. Effectiveness of intensive practice nurse counselling versus brief general practitioner advice, both combined with varenicline, for smoking cessation: A randomized pragmatic trial in primary care. Addiction 2017, 112, 2237–2247. [Google Scholar] [CrossRef]
- Verbiest, M.E.; Chavannes, N.H.; Crone, M.R.; Nielen, M.M.; Segaar, D.; Korevaar, J.C.; Assendelft, W.J. An increase in primary care prescriptions of stop-smoking medication as a result of health insurance coverage in the Netherlands: Population based study. Addiction 2013, 108, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, N.; Bolman, C.; van Adrichem, M.; Candel, M.; Muris, J.; de Vries, H. Comparison of text and video computer-tailored interventions for smoking cessation: Randomized controlled trial. J. Med. Int. Res. 2014, 16, e69. [Google Scholar] [CrossRef] [Green Version]
- Te Poel, F.; Bolman, C.; Reubsaet, A.; de Vries, H. Efficacy of a single computer-tailored e-mail for smoking cessation: Results after 6 months. Health Educ. Res. 2009, 24, 930–940. [Google Scholar] [CrossRef] [Green Version]
- Berndt, N.; Bolman, C.; Lechner, L.; Max, W.; Mudde, A.; de Vries, H.; Evers, S. Economic evaluation of a telephone-and face-to-face-delivered counseling intervention for smoking cessation in patients with coronary heart disease. Eur. J. Health Econ. 2016, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- McEwen, A.; West, R.; McRobbie, H. Effectiveness of specialist group treatment for smoking cessation vs. one-to-one treatment in primary care. Addict. Behav. 2006, 31, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Orleans, C.T.; Woolf, S.H.; Rothemich, S.F.; Marks, J.S.; Isham, G.J. The top priority: Building a better system for tobacco-cessation counseling. Am. J. Prev. Med. 2006, 31, 103–106. [Google Scholar] [CrossRef]
- Stead, L.F.; Carroll, A.J.; Lancaster, T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst. Rev. 2017, 3, CD001007. [Google Scholar] [CrossRef]
- Lindson, N.; Chepkin, S.C.; Ye, W.; Fanshawe, T.R.; Bullen, C.; Hartmann-Boyce, J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2019, 4, CD013308. [Google Scholar] [CrossRef]
- Hughes, J.R.; Stead, L.F.; Hartmann-Boyce, J.; Cahill, K.; Lancaster, T. Antidepressants for smoking cessation. Cochrane Database Syst. Rev. 2014, CD000031. [Google Scholar] [CrossRef] [Green Version]
- Cahill, K.; Lindson-Hawley, N.; Thomas, K.H.; Fanshawe, T.R.; Lancaster, T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 2016, CD006103. [Google Scholar] [CrossRef] [Green Version]
- Chavannes, N.; Drenthen, T.; Wind, L.; Van Avendonk, M.; Van den Donk, M.; Verduijn, M. NHG-Behandelrichtlijn Stoppen Met Roken; The Nederlands Huisartsen Genootschap: Utrecht, The Netherlands, 2017. [Google Scholar]
- Stop Smoking Partnership. Guideline Treatment Tobacco Addiction [Richtlijn Behandeling van Tabaksverslaving]; Stop Smoking Partnership: Alphen aan den Rijn, The Netherlands, 2019. [Google Scholar]
- White, A.R.; Rampes, H.; Liu, J.P.; Stead, L.F.; Campbell, J. Acupuncture and related interventions for smoking cessation. Cochrane Database Syst. Rev. 2014, 1, 1–73. [Google Scholar] [CrossRef]
- Friedrichs, A.; Spies, M.; Härter, M.; Buchholz, A. Patient preferences and shared decision making in the treatment of substance use disorders: A systematic review of the literature. PloS ONE 2016, 11, e0145817. [Google Scholar] [CrossRef] [PubMed]
- Hoving, C.; Visser, A.; Mullen, P.D.; van den Borne, B. A history of patient education by health professionals in Europe and North America: From authority to shared decision making education. Patient Educ. Couns. 2010, 78, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Filippidis, F.T.; Laverty, A.A.; Mons, U.; Jimenez-Ruiz, C.; Vardavas, C.I. Changes in smoking cessation assistance in the European Union between 2012 and 2017: Pharmacotherapy versus counselling versus e-cigarettes. Tob. Control 2019, 28, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Borland, R.; Li, L.; Driezen, P.; Wilson, N.; Hammond, D.; Thompson, M.E.; Fong, G.T.; Mons, U.; Willemsen, M.C.; McNeill, A. Cessation assistance reported by smokers in 15 countries participating in the International Tobacco Control (ITC) policy evaluation surveys. Addiction 2012, 107, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Centraal Bureau voor Statistiek. Zorggebruik Verschilt per Opleidingsniveau. Available online: https://www.cbs.nl/nl-nl/nieuws/2014/46/zorggebruik-verschilt-per-opleidingsniveau (accessed on 17 June 2021).
- Springvloet, L.; Van Laar, M. Roken Onder Volwassenen: Kerncijfers 2016; Nationaal Expertisecentrum Tabaksontmoediging: Utrecht, The Netherlands, 2017. [Google Scholar]
- Zwar, N.A.; Richmond, R.L. Role of the general practitioner in smoking cessation. Drug Alcohol Rev. 2006, 25, 21–26. [Google Scholar] [CrossRef]
- Dierick-van Daele, A.T.; Metsemakers, J.F.; Derckx, E.W.; Spreeuwenberg, C.; Vrijhoef, H.J. Nurse practitioners substituting for general practitioners: Randomized controlled trial. J. Adv. Nurs. 2009, 65, 391–401. [Google Scholar] [CrossRef]
- Fiore, M.C.; Bailey, W.C.; Cohen, S.J.; Dorfman, S.F.; Goldstein, M.G.; Gritz, E.R.; Heyman, R.B.; Jaen, C.R.; Kottke, T.E.; Lando, H.A. Treating Tobacco Use and Dependence: Clinical Practice Guideline; US Department of Health and Human Services: Rockville, MD, USA, 2000.
- Zijlstra, D.N.; Hoving, C.; Bolman, C.; Muris, J.W.M.; De Vries, H. Do professional perspectives on evidence-based smoking cessation methods align? A Delphi study among researchers and healthcare professionals. Health Educ. Res. 2021. [Google Scholar] [CrossRef]
- Stacey, D.; Légaré, F.; Lewis, K.; Barry, M.J.; Bennett, C.L.; Eden, K.B.; Holmes-Rovner, M.; Llewellyn-Thomas, H.; Lyddiatt, A.; Thomson, R.; et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2017, 4, 1–287. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, D.; Bolman, C.; Muris, J.; de Vries, H. What went wrong? A randomized controlled trial of a process and effect evaluation of a referral aid for smoking cessation counseling in primary care. Submitted for publication.
- Zijlstra, D.N.; Muris, J.W.M.; Bolman, C.; Elling, J.M.; Knapen, V.; de Vries, H. A referral aid for smoking cessation interventions in primary care: Study protocol for a randomized controlled trial. Prim. Health Care Res. Dev. 2021, 22, e22. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H. An integrated approach for understanding health behavior; the I-change model as an example. Psychol. Behav. Sci. Int. J. 2017, 2, 555–585. [Google Scholar] [CrossRef] [Green Version]
- de Ruijter, D.; Candel, M.; Smit, E.S.; de Vries, H.; Hoving, C. The Effectiveness of a Computer-Tailored E-Learning Program for Practice Nurses to Improve Their Adherence to Smoking Cessation Counseling Guidelines: Randomized Controlled Trial. J. Med. Int. Res. 2018, 20, e193. [Google Scholar] [CrossRef]
- Ministerie van Algemene Zaken. Taalniveau B1. Available online: https://www.communicatierijk.nl/vakkennis/rijkswebsites/aanbevolen-richtlijnen/taalniveau-b1 (accessed on 4 June 2021).
- Kerr, C.M.; Lowe, P.B.; Spielholz, N.I. Low level laser for the stimulation of acupoints for smoking cessation: A double blind, placebo controlled randomised trial and semi structured interviews. J. Chin. Med. 2008, 86, 46. [Google Scholar]
- Bier, I.D.; Wilson, J.; Studt, P.; Shakleton, M. Auricular acupuncture, education, and smoking cessation: A randomized, sham-controlled trial. Am. J. Public Health 2002, 92, 1642–1647. [Google Scholar] [CrossRef]
- Carmody, T.P.; Duncan, C.; Simon, J.A.; Solkowitz, S.; Huggins, J.; Lee, S.; Delucchi, K. Hypnosis for smoking cessation: A randomized trial. Nicotine Tob. Res. 2008, 10, 811–818. [Google Scholar]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; FAGERSTROM, K.O. The Fagerström test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- de Vries, H.; Kremers, S.P.; Smeets, T.; Brug, J.; Eijmael, K. The effectiveness of tailored feedback and action plans in an intervention addressing multiple health behaviors. Am. J. Health Promot. 2008, 22, 417–425. [Google Scholar] [CrossRef]
- Dijkstra, A.; De Vries, H.; Bakker, M. Pros and cons of quitting, self-efficacy, and the stages of change in smoking cessation. J. Consult. Clin. Psychol. 1996, 64, 758. [Google Scholar] [CrossRef]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An empirical evaluation of the system usability scale. Intl. J. Hum.–Comput. Interact. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Stanczyk, N.E.; Bolman, C.; Muris, J.W.; de Vries, H. Study protocol of a Dutch smoking cessation e-health program. BMC Public Health 2011, 11, 847. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, A.M. Validation of a decisional conflict scale. Med Decis. Mak. 1995, 15, 25–30. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A. User manual-decisional conflict scale. 2010. Available online: http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf (accessed on 27 July 2021).
- Bangor, A.; Kortum, P.; Miller, J. Determining what individual SUS scores mean: Adding an adjective rating scale. J. Usabil. Stud. 2009, 4, 114–123. [Google Scholar]
- Glanz, K.; Rimer, B.K.; Viswanath, K. Health Behavior and Health Education: Theory, Research, and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- DiClemente, C.C.; Prochaska, J.O.; Fairhurst, S.K.; Velicer, W.F.; Velasquez, M.M.; Rossi, J.S. The process of smoking cessation: An analysis of precontemplation, contemplation, and preparation stages of change. J. Consult. Clin. Psychol. 1991, 59, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.; De Vries, H.; Roijackers, J.; van Breukelen, G. Tailored interventions to communicate stage-matched information to smokers in different motivational stages. J. Consult. Clin. Psychol. 1998, 66, 549–557. [Google Scholar] [CrossRef]
- Soetens, K.C.; Vandelanotte, C.; de Vries, H.; Mummery, K.W. Using online computer tailoring to promote physical activity: A randomized trial of text, video, and combined intervention delivery modes. J. Health Commun. 2014, 19, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Fagerlin, A.; Pignone, M.; Abhyankar, P.; Col, N.; Feldman-Stewart, D.; Gavaruzzi, T.; Kryworuchko, J.; Levin, C.A.; Pieterse, A.H.; Reyna, V. Clarifying values: An updated review. BMC Med. Inform. Decis. Mak. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Elwyn, G.; O’Connor, A.; Stacey, D.; Volk, R.; Edwards, A.G.; Coulter, A.; Thomas, R.; Barratt, A.; Barry, M.; Bernstein, S. International Patient Decision Aids Standards (IPDAS) Collaboration. Developing a quality criteria framework for patient decision aid: Online international Delphi consensus process. Br. Med. J. 2006, 333, 417–419. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.L.; Wijnen, B.; de Vries, H. A Review of the Theoretical Basis, Effects, and Cost Effectiveness of Online Smoking Cessation Interventions in the Netherlands: A Mixed-Methods Approach. J. Med. Int. Res. 2017, 19, e230. [Google Scholar] [CrossRef]
- Taylor, G.M.J.; Dalili, M.N.; Semwal, M.; Civljak, M.; Sheikh, A.; Car, J. Internet-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2017, 9, CD007078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanczyk, N.E.; Smit, E.S.; Schulz, D.N.; de Vries, H.; Bolman, C.; Muris, J.W.; Evers, S.M. An economic evaluation of a video- and text-based computer-tailored intervention for smoking cessation: A cost-effectiveness and cost-utility analysis of a randomized controlled trial. PLoS ONE 2014, 9, e110117. [Google Scholar] [CrossRef] [PubMed]
- Elfeddali, I.; Bolman, C.; Candel, M.J.; Wiers, R.W.; de Vries, H. Preventing smoking relapse via Web-based computer-tailored feedback: A randomized controlled trial. J. Med. Int. Res. 2012, 14, e109. [Google Scholar] [CrossRef] [Green Version]
- Smit, E.S.; Evers, S.M.; de Vries, H.; Hoving, C. Cost-effectiveness and cost-utility of Internet-based computer tailoring for smoking cessation. J. Med. Int. Res. 2013, 15, e57. [Google Scholar] [CrossRef] [PubMed]
- de Josselin de Jong, S.; Candel, M.; Segaar, D.; Cremers, H.P.; de Vries, H. Efficacy of a Web-based computer-tailored smoking prevention intervention for Dutch adolescents: Randomized controlled trial. J. Med. Int. Res. 2014, 16, e82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, E.S.; Candel, M.J.; Hoving, C.; de Vries, H. Results of the PAS Study: A Randomized Controlled Trial Evaluating the Effectiveness of a Web-Based Multiple Tailored Smoking Cessation Program Combined With Tailored Counseling by Practice Nurses. Health Commun. 2016, 31, 1165–1173. [Google Scholar] [CrossRef] [Green Version]
- Willemsen, M.C.; Wiebing, M.; Van Emst, A.; Zeeman, G. Helping smokers to decide on the use of efficacious smoking cessation methods: A randomized controlled trial of a decision aid. Addiction 2006, 101, 441–449. [Google Scholar] [CrossRef]
- Bouter, L.M.; Dongen, M.C.J.M.; Zielhuis, G.A. Epidemiologisch Onderzoek: Opzet en Interpretatie; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Gultzow, T.; Smit, E.S.; Hudales, R.; Knapen, V.; Rademakers, J.; Dirksen, C.D.; Hoving, C. An Autonomy-Supportive Online Decision Aid to Assist Smokers in Choosing Evidence-Based Cessation Assistance: Development Process and Protocol of a Randomized Controlled Trial. JMIR Res. Protoc. 2020, 9, e21772. [Google Scholar] [CrossRef] [PubMed]
- Altendorf, M.; Hoving, C.; Van Weert, J.C.; Smit, E.S. Effectiveness of Message Frame-Tailoring in a Web-Based Smoking Cessation Program: Randomized Controlled Trial. J. Med. Int. Res. 2020, 22, e17251. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.L.; Schwabe, I.; Walthouwer, M.J.L.; Oenema, A.; Lechner, L.; de Vries, H. Effectiveness of a Video-Versus Text-Based Computer-Tailored Intervention for Obesity Prevention after One Year: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2017, 14, 1275. [Google Scholar] [CrossRef] [Green Version]
- Elling, J.M.; De Vries, H. Influence of Animation-Versus Text-Based Delivery of a Web-Based Computer-Tailored Smoking Cessation Intervention on User Perceptions. Eur. J. Health Commun. 2021, 2, 1–23. [Google Scholar] [CrossRef]
- Llewellyn-Thomas, H.A.; Crump, R.T. Decision support for patients: Values clarification and preference elicitation. Med. Care Res. Rev. 2013, 70, 50S–79S. [Google Scholar] [CrossRef] [PubMed]
- Berndt, N.C.; Bolman, C.; de Vries, H.; Segaar, D.; van Boven, I.; Lechner, L. Smoking cessation treatment practices: Recommendations for improved adoption on cardiology wards. J. Cardiovasc. Nurs. 2013, 28, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.S.; Hoving, C.; Cox, V.C.; de Vries, H. Influence of recruitment strategy on the reach and effect of a web-based multiple tailored smoking cessation intervention among Dutch adult smokers. Health Educ. Res. 2012, 27, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Siemer, L.; Pieterse, M.E.; Brusse-Keizer, M.G.J.; Postel, M.G.; Ben Allouch, S.; Sanderman, R. Study protocol for a non-inferiority trial of a blended smoking cessation treatment versus face-to-face treatment (LiveSmokefree-Study). BMC Public Health 2016, 16, 1187. [Google Scholar] [CrossRef] [Green Version]
- Wentzel, J.; van der Vaart, R.; Bohlmeijer, E.T.; van Gemert-Pijnen, J.E. Mixing Online and Face-to-Face Therapy: How to Benefit From Blended Care in Mental Health Care. JMIR Ment. Health 2016, 3, e9. [Google Scholar] [CrossRef] [Green Version]
- Bricca, A.; Swithenbank, Z.; Scott, N.; Treweek, S.; Johnston, M.; Black, N.; Hartmann-Boyce, J.; West, R.; Michie, S.; de Bruin, M. Predictors of recruitment and retention in randomized controlled trials of behavioural smoking cessation interventions: A systematic review and meta-regression analysis. Addiction 2021. [Google Scholar] [CrossRef] [PubMed]
| Example Questions | Cronbach’s α | |
|---|---|---|
| Program evaluation scale constructs | ||
| Attention | The DA held my attention | 0.81 |
| Comprehension | In my opinion, the DA is clear | 0.81 1 |
| Adaptation | The DA applied to me personally | 0.44 2 |
| Appreciation | The DA is interesting | 0.81 |
| Processing | The DA contains good tips on the best way to quit smoking | 0.87 |
| Dose infliction | The DA provides a nice overview of the available evidence-based smoking cessation methods | 0.46 2 |
| Persuasion | The DA is convincing | 0.80 |
| Complete scale | – | 0.93 |
| Decisional conflict scale constructs | ||
| By using the DA, … | ||
| Uncertainty | I know what the best choice is for me | 0.84 |
| Informed | I know which options are available to me | 0.85 |
| Value clarity | I am clear about which benefits matter the most to me | 0.84 |
| Support | I have enough support to make a choice | 0.75 |
| Effective decision | I am satisfied with my choice | 0.78 |
| Complete scale | – | 0.94 |
| Study Sample Characteristics | Total (n = 497) | MU (n = 393) | GU (n = 104) | t | χ2 | p |
|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 41.23 (13.9) | 41.06 (13.9) | 41.96 (12.6) | −0.597 | 0.551 | |
| Female, n (%) | 225 (45.3) | 172 (43.8) | 53 (51.5) | 1.947 | 0.163 | |
| Educational level, n (%) | 0.174 | 0.916 | ||||
| Low | 59 (11.9) | 47 (12) | 11 (10.7) | |||
| Medium | 229 (46.1) | 180 (45.8) | 49 (47.6) | |||
| High | 209 (42.1) | 166 (42.2) | 43 (41.7) | |||
| FTND score 1, mean (SD) | 4.24 (2.4) | 4.32 (2.5) | 3.94 (2.4) | 1.412 | 0.159 | |
| Number of cigarettes smoked/day, mean (SD) | 12.51 (7.7) | 12.56 (7.8) | 12.38 (7.1) | 0.215 | 0.830 | |
| Use of e-cigarettes, n (%) | 4.421 | 0.219 | ||||
| No | 306 (61.6) | 246 (62.6) | 59 (57.3) | |||
| Yes, without nicotine | 40 (8.0) | 35 (8.9) | 5 (4.9) | |||
| Yes, with nicotine | 144 (29.0) | 107 (27.2) | 37 (35.9) | |||
| Yes, do not know whether with or without nicotine | 7 (1.4) | 5 (1.3) | 2 (1.9) | |||
| Previous quit attempt undertaken, n (%) | 309 (62.2) | 248 (63.1) | 61 (59.2) | 0.414 | 0.520 | |
| Intention to quit 2 | 3.97 (0.9) | 3.88 (0.9) | 4.29 (0.8) | −4.334 | 0.000 | |
| Readiness to quit 3 | 3.19 (1.3) | 3.20 (1.9) | 3.12 (1.4) | 0.579 | 0.563 |
| Total (n = 497) | MU (n = 393) | GU (n = 104) | t | p | |
|---|---|---|---|---|---|
| Program evaluation scale 1 | 2.42 (0.4) | 3.47 (0.6) | 4.27 (0.5) | −12.674 | 0.000 |
| Attention subscale | 3.47 (0.8) | 3.30 (0.8) | 4.12 (0.7) | −9.835 | 0.000 |
| Comprehension subscale | 3.94 (0.7) | 3.77 (0.7) | 4.59 (0.6) | −11.301 | 0.000 |
| Comprehension: difficult | 3.79 (1.0) | 3.60 (0.9) | 4.53 (0.8) | −9.191 | 0.000 |
| Adaptation: fitted situation | 3.45 (0.9) | 3.32 (0.9) | 3.97 (0.8) | −6.581 | 0.000 |
| Adaptation: lacked information | 3.19 (1.0) | 3.05 (0.9) | 3.72 (1.0) | −6.605 | 0.000 |
| Adaptation: too general | 3.50 (1.0) | 3.36 (0.9) | 4.05 (1.0) | −6.435 | 0.000 |
| Appreciation subscale | 3.59 (0.8) | 3.43 (0.8) | 4.20 (0.6) | −9.386 | 0.000 |
| Process subscale | 3.53 (0.8) | 3.37 (0.7) | 4.16 (0.6) | −9.741 | 0.000 |
| Dose subscale | 3.76 (0.8) | 3.59 (0.8) | 4.46 (0.5) | −11.126 | 0.000 |
| Dose: much information | 3.77 (0.8) | 2.69 (1.0) | 3.57 (1.2) | −7.518 | 0.000 |
| Persuasion subscale | 3.74 (0.7) | 3.57 (0.7) | 4.38 (0.5) | −9.051 | 0.000 |
| Recommendation 2 | 3.75 (0.9) | 3.55 (0.8) | 4.52 (0.6) | −10.606 | 0.000 |
| Mark (1–10) | 7.27 (1.3) | 8.56 (0.9) | −11.531 | 0.000 |
| Total (n = 497) | MU (n = 393) | GU (n = 104) | t | p | |
|---|---|---|---|---|---|
| Decisional conflict scale, mean (SD) 1 | 31.73 (14.8) | 35.56 (13.3) | 17.13 (10.6) | 13.060 | 0.000 |
| Uncertainty subscale | 34.17 (18.0) | 38.13 (16.7) | 19.09 (14.2) | 10.583 | 0.000 |
| Informed subscale | 29.60 (17.9) | 34.01 (16.2) | 12.78 (13.5) | 12.224 | 0.000 |
| Value clarity subscale | 32.88 (17.9) | 36.70 (16.4) | 18.28 (15.7) | 10.218 | 0.000 |
| Support subscale | 32.34 (17.6) | 36.28 (16.7) | 17.31 (12.3) | 10.792 | 0.000 |
| Effective decision subscale | 30.18 (15.0) | 33.40 (13.9) | 17.90 (12.7) | 10.255 | 0.000 |
| MU (n = 393) | GU (n = 104) | Comparison of Changes between the MU Group and the GU Group | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Intention to use EBSCIs, mean amount (SD) 1 | 1.47 (1.1) | 1.59 (1.1) * | 1.89 (1.4) | 1.91 (1.1) | NS |
| Behavioral counseling, % (n) | |||||
| GP | 10.2 (40) | 9.9 (39) | 17.5 (18) | 17.5 (18) | NS |
| PN | 15.8 (62) | 18.3 (72) | 24.3 (25) | 23.3 (24) | NS |
| Stop coach | 12.7 (50) | 16.0 (63) | 15.5 (16) | 15.5 (16) | NS |
| eHealth | 9.9 (39) | 16.3 (64) ** | 12.6 (13) | 30.1 (31) ** | Δ GU > Δ MU * |
| In groups | 7.1 (28) | 8.1 (32) | 1.9 (2) | 4.9 (5) | NS |
| Via telephone | 7.6 (30) | 9.9 (39) | 6.8 (7) | 10.7 (11) | NS |
| NRT | 25.2 (99) | 24.9 (98) | 47.6 (49) | 40.8 (42) | NS |
| Pharmacotherapy | 13.0 (51) | 15.8 (62) | 25.2 (26) | 23.3 (24) | NS |
| NEBSCI 2 | 8.7 (34) | 7.9 (31) | 11.7 (12) | 8.7 (9) | NS |
| None | 37.2 (146) | 31.8 (125) ** | 26.2 (27) | 16.5 (17) * | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zijlstra, D.N.; Bolman, C.A.W.; Muris, J.W.M.; de Vries, H. The Usability of an Online Tool to Promote the Use of Evidence-Based Smoking Cessation Interventions. Int. J. Environ. Res. Public Health 2021, 18, 10836. https://doi.org/10.3390/ijerph182010836
Zijlstra DN, Bolman CAW, Muris JWM, de Vries H. The Usability of an Online Tool to Promote the Use of Evidence-Based Smoking Cessation Interventions. International Journal of Environmental Research and Public Health. 2021; 18(20):10836. https://doi.org/10.3390/ijerph182010836
Chicago/Turabian StyleZijlstra, Daniëlle N., Catherine A. W. Bolman, Jean W. M. Muris, and Hein de Vries. 2021. "The Usability of an Online Tool to Promote the Use of Evidence-Based Smoking Cessation Interventions" International Journal of Environmental Research and Public Health 18, no. 20: 10836. https://doi.org/10.3390/ijerph182010836
APA StyleZijlstra, D. N., Bolman, C. A. W., Muris, J. W. M., & de Vries, H. (2021). The Usability of an Online Tool to Promote the Use of Evidence-Based Smoking Cessation Interventions. International Journal of Environmental Research and Public Health, 18(20), 10836. https://doi.org/10.3390/ijerph182010836






