Abstract
The low-carbohydrate high-fat (LCHF) diet has recently been subject to attention on account of its reported influences on body composition and physical performance. However, the combined effect of LCHF with high-intensity interval training (HIIT) is unclear. A systematic review and meta-analysis were conducted to explore the effect of the LCHF diet combined with HIIT on human body composition (i.e., body weight (BM), body mass index (BMI), fat mass (FM), body fat percentage (BFP), fat-free mass (FFM)) and maximal oxygen uptake (VO2max). Online libraries (PubMed, Web of Science, EMBASE, Cochrane Library, EBSCO, CNKI, Wan Fang) were used to search initial studies until July 2021, from which 10 out of 2440 studies were included. WMD served as the effect size with a confidence interval value of 95%. The results of meta-analysis showed a significant reduction in BM (WMD = −5.299; 95% CI: −7.223, −3.376, p = 0.000), BMI (WMD = −1.150; 95% CI: −2.225, −0.075, p = 0.036), BFP (WMD = −2.787; 95% CI: −4.738, −0.835, p = 0.005) and a significant increase in VO2max (WMD = 3.311; 95% CI: 1.705, 4.918, p = 0.000), while FM (WMD = −2.221; 95% CI: −4.582, 0.139, p = 0.065) and FFM (WMD = 0.487; 95% CI: −3.512, 4.469, p = 0.814) remained unchanged. In conclusion, the LCHF diet combined with HIIT can reduce weight and fat effectively. This combination is sufficient to prevent muscle mass loss during LCHF, and further enhance VO2max. Further research might be required to clarify the effect of other types of exercise on body composition and physical performance during LCHF.
1. Introduction
Body composition is influenced by nutritional and physical activity intervention. The low-carbohydrate high-fat (LCHF) diet is a re-emerged dietary approach, which is characterized by decreased carbohydrate intake (approximately 50 g/d) and high levels of fat consumption with adequate protein provided [1]. The global prevalence of overweight and obesity has multiplied in recent decades [2]. Obesity is a complex disease and a major risk factor for cardiovascular and metabolic disorders, especially atherosclerosis, type II diabetes, and metabolic syndrome [3], and it increases the risk of death [4]. LCHF has become fashionable because of its potential to induce rapid weight loss, including ketogenic diets (KDs), the Zone diet, the South Beach diet, the Atkins diet, and other carbohydrate-restricted diets (CRD) [5,6]. The purpose of this low-carb, high-fat dietary strategy of LCHF is to increase the utilization of fat as muscle fuel and to keep the body at high levels of circulating ketones for weight loss [7,8]. Some studies revealed that individuals who consumed the LCHF diet experienced greater body weight (BM) and fat mass (FM) loss than those adhering to other dietary interventions, such as a calorie-restricted diet or low-fat diet [9,10,11] [9(RCT),10(RCT).11(RCT)], which highlights the effectiveness of LCHF in managing obesity. In addition to being a dietary solution for obese people, LCHF is also effective in the treatment of type 2 diabetes [12], epilepsy [13], Alzheimer’s disease [14], Parkinson’s disease [14], and in reducing the risk of asthma [15]. In addition, LCHF is also used by exercise enthusiasts to enhance aerobic capacity [16] (e.g., maximum oxygen uptake).
Although LCHF has gained considerable attention in the dietary treatment of chronic diseases such as obesity and type 2 diabetes [12], there is much controversy. LCHF not only may reduce lean mass (LM) and free fat mass (FFM) [17,18,19,20,21] [17 (RCT), 18 (RCT), 19 (NRCT), 20 (NRCT), 21 (RCT)] but also may cause several adverse reactions, including constipation, bad breath, muscle cramps, headache, diarrhea, weakness, and skin rashes [22]. Furthermore, LCHF may lead to liver inflammation, liver fat accumulation, insulin resistance, dyslipidemia, and hypertension, increasing the risk of cardiovascular disease [23]. However, unlike these dietary approaches, exercise interventions, such as high-intensity interval training (HIIT) or resistance training (RT), are known to bring beneficial changes in body function and composition, which imply the combination with exercise may counteract this negative effect. It is now well established from various studies that HIIT or RT improve physical function (e.g., ameliorate insulin resistance [24,25], reduce liver fat accumulation [26,27], decrease cardiovascular disease risk [28,29], improve aerobic capacity [30,31], enhance muscle strength [32], and increase LM and FFM [21,30,33,34] [21 (RCT), 30 (RCT), 33 (RCT), 34 (RCT)]. The benefits of HIIT and RT on LM and FFM are attributed to increased energy expenditure due to muscle contraction, which enhances adipose metabolism in muscle tissue and muscle growth-related factor gene expression [35]. Nevertheless, a recent meta-analysis presented that individuals assigned to a ketogenic diet showed fat-free mass loss, and this was not ameliorated by the combination of resistance training [36]. Thus, whether other exercise forms, such as HIIT, could reverse this negative effect requires attention.
In addition to the anti-obesity effect of LCHF, it has also become a popular nutritional strategy for athletes. Carbohydrates, present in muscles and liver as glycogen, have been confirmed as the primary fuel source for high-intensity exercise, and they are important for maintaining long-lasting exercise performance [37]. Traditional exercise nutrition guidelines propose high levels of carbohydrate consumption to increase skeletal muscle and liver glycogen content, thereby improving endurance performance [38,39]. However, the body’s glycogen storage capacity is limited (approximately 100 g in the liver and 300–700 g in the muscle) [40], which may lead to glycogen depletion during prolonged exercise, causing body fatigue. Therefore, the dietary approach (i.e., LCHF), which could increase endogenous fat oxidation and reduce the body’s dependence on glycogen during prolonged exercise [8], is receiving much attention. Dostal et al. show a greater increase in total time to exhaustion in the LCHF diet group than those in the habitual diet group [41]. However, the positive of LCHF on physical performance is debated. Several lines of evidence suggest that maximal oxygen uptake (VO2max) or peak oxygen uptake (VO2peak) were unchanged or decreased after consuming an LCHF diet [16,42,43,44]. HIIT, on the other hand, has also been proven to promote physical performance. Data from several studies verified that HIIT improves VO2max among healthy and overweight/obese adults [44,45,46] [45 (RCT), 46 (RCT)]. However, the combined effect of LCHF and HIIT on VO2max is still unknown.
Therefore, this study intended to conduct a systematic review and meta-analysis to explore the effect of the LCHF diet combined with HIIT on human body composition (i.e., BM, body mass index (BMI), FM, body fat percentage (BFP), and FFM) and VO2max. We hypothesized that LCHF combined with HIIT would be effective in improving body composition and aerobic capacity. To our knowledge, this is the first systematic meta-analysis to investigate the combined effects of LCHF and HIIT on human body composition and aerobic capacity.
2. Materials and Methods
2.1. Literature Search
Literature search, study selection, data extraction, and data analysis were performed according to PRISMA (Priority Reporting Entries for Reviews and Meta-Analyses). To find relevant prospective studies, a systematic literature search was conducted in electronic databases, including PubMed, Web of Science, EMBASE, Cochrane Library, EBSCO, CNKI, and Wan Fang, from build to July 2021. Our search strategy included the following keywords: “ketogenic diet”, “KDs”, “low carbohydrate diet”, “keto-adaptation”, “carbohydrate-restricted diet”, “high intensity interval training”, “sprint interval training”, “intermittent training”, and “aerobic interval training HIIT”. In addition, we reviewed the reference lists of previous systematic reviews and meta-analyses in the area.
2.2. Inclusion and Exclusion Criteria
Studies were considered eligible for inclusion if they met all of the following criteria: (a) study in adults (≥18 years); (b) dietary intervention was a low-carbohydrate high-fat diet (carbohydrate intake <50 g/d); (c) exercise intervention was high-intensity intermittent exercise; (d) duration of intervention was more than two weeks; (e) included outcomes: BM, BMI, FFM, BFP, FM, and VO2max; and (f) trial design was a crossover or parallel randomized controlled trial (RCT). Exclusion criteria were: (a) not meeting inclusion criteria; (b) combined with other types of dietary interventions; (c) full text not available; and (d) animal, review, and experimental studies.
2.3. Quality Assessments
Study quality was assessed using a “risk of bias” approach, as recommended by the Cochrane risk of bias tool [47]. This method classifies bias in randomized studies as “low”, “high” or “unclear” based on the presence of seven processes (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases).
2.4. Data Extraction
Literature screening and data extraction were performed independently by two researchers (JH and ZW), and a third researcher (LB) assisted in resolving disagreements when they occurred. The following data were extracted from the included studies: (a) type of study; (b) population; (c) age of the population; (d) type of intervention; (e) duration of intervention; (f) measurement tools; and (g) outcome. Outcomes were meta-analyzed using the mean and standard deviation changes between the baseline and final values for each outcome, converting median values to means and 1st–3rd quartiles to standard deviations, respectively.
2.5. Statistical Analysis
Meta-analysis was completed using stata15.0 (College Station, TX, USA). WMD was used as the effect size with a confidence interval value of 95%. Q test with I2 test was used to investigate whether there was heterogeneity among the studies, and a fixed-effects model was applied because p > 0.10 and I2 < 50% indicated better homogeneity between studies. To test for possible bias in the included studies, their funnel plot symmetry as well as the Begg’s rank correlation test were examined.
3. Results
3.1. Study Selection
A total of 2440 articles were yielded through online libraries, and 433 duplicate articles were removed. After accessing the title and abstract, 1929 studies were excluded for the following reasons: unrelated title and abstract (1432), animal studies (375), and review studies (122). A total of 78 potentially related articles were screened by full text, while 68 articles were excluded owing to the absence of LCHF or/and HIIT interventions. Overall, 10 studies were included. The flow diagram of study selection is shown in Figure 1.
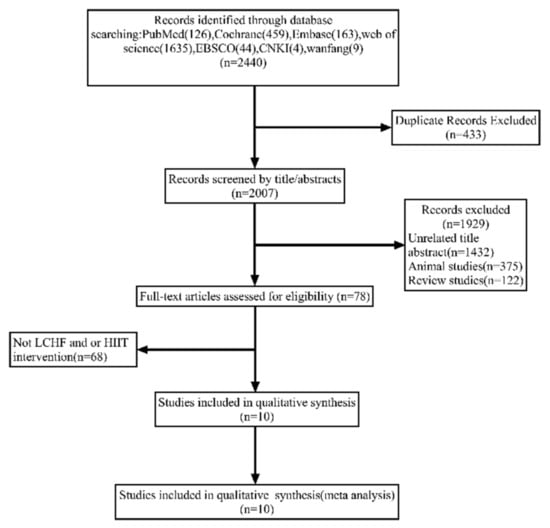
Figure 1.
Flow diagram of the study selection process.
3.2. Characteristics of the Included Studies
The characteristics of the included studies are summarized in Table 1. Among them, seven studies were designed as RCT [39,40,41,42,43,44], and three studies were designed as non-RCT [33,35,46]. The subject’s ages ranged from 18 to 60 yr. Two studies contained only male subjects [48,49], three enrolled only female participants [43,50,51], and five studies included both sexes [41,52,53,54,55]. The participants included obese males and/or females [43,50,51,53,54], endurance-trained males [49], untrained healthy males and/or females [41,48], and metabolic syndrome males and females [52,55]. The intervention duration ranged from 2 weeks to 14 weeks [17,56,57,58].

Table 1.
Characteristics of studies included in the meta-analysis.
3.3. Results from Quality Assessments
Risk of bias (ROB) was used to assess the risk of bias. The ROB of random sequence generation was “low” in seven and “high” in three studies. Regarding allocation concealment, the ROB was “low” in ten studies. For the blinding of the participants and personnel, the ROB was “high” in three, and “unclear” in seven studies. The ROB of blinding of the outcome assessments was “low” in six and “unclear” in four studies. For incomplete outcome data, the ROB was “low” in ten studies. Overall, all studies met the quality level. Therefore, no studies were excluded (Figure 2 and Figure 3).
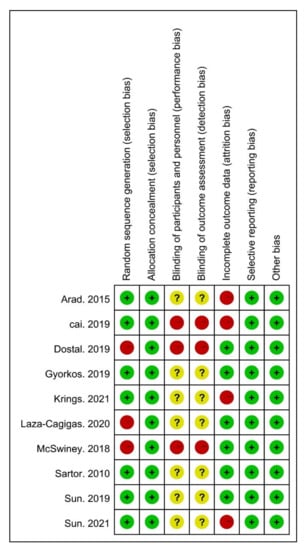
Figure 2.
Risk of bias (ROB) results of quality assessment within included studies. Risk of bias levels: low (green or “+”), unclear (yellow or “?”), and high (red or “–”).
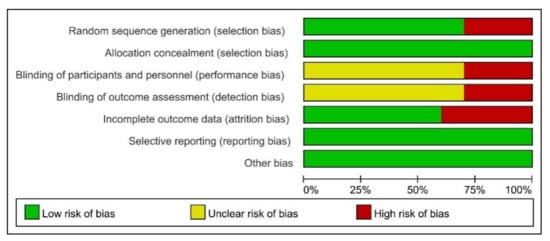
Figure 3.
Risk of bias (ROB) results of quality assessment within included studies. Risk of bias levels: low (green), unclear (yellow), and high (red).
3.4. Publication Bias
Begg’s test confirmed the absence of publication bias for studies assessing the effect of LCHF on BM (p = 0.474), BMI (p = 0.462), FM (p = 1), BFP (p = 0.462), FFM (p = 0.308), and VO2max (p = 0.452). Furthermore, funnel plots exhibited a symmetric distribution, which also proved this point (Supplementary Figure S1–S6).
3.5. Results for Body Components and Maximal Oxygen Uptake
The results of meta-analysis indicate that participants that adhered to the LCHF diet and HIIT showed a significant reduction in BM (WMD = −5.299; 95% CI: −7.223, −3.376, p = 0.000, Figure 4), BMI (WMD = −1.150; 95% CI: −2.225, –0.075, p = 0.036, Figure 5), and BFP (WMD = −2.787; 95% CI: −4.738, −0.835, p = 0.005, Figure 6), and a significant increase in VO2max (WMD = 3.311; 95% CI: 1.705, 4.918, p = 0.000, Figure 7) as compared to the control group, while FM (WMD = −2.221; 95% CI: −4.582, 0.139, p = 0.065, Figure 8) and FFM (WMD = 0.487; 95% CI: −3.512, 4.469, p = 0.814, Figure 9) remained unchanged. No significant heterogeneity was detected in all indicators.
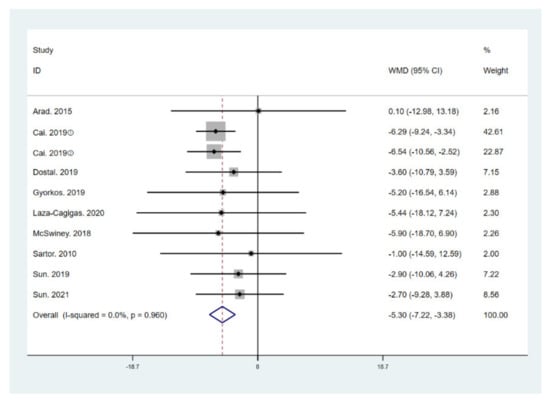
Figure 4.
Forest plot for the effect of a low-carbohydrate high-fat (LCHF) diet combined with HIIT on body mass (BM). For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies.
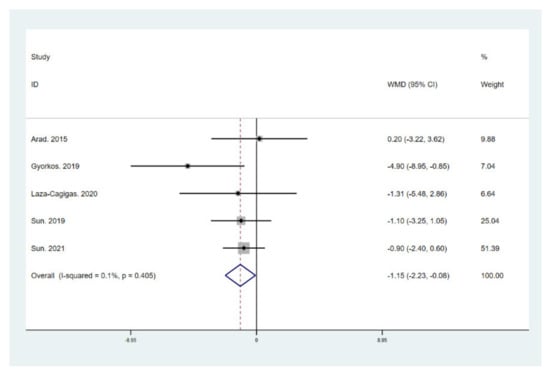
Figure 5.
Forest plot for the effect of a low-carbohydrate high-fat (LCHF) diet combined with HIIT on body mass index (BMI). For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies.
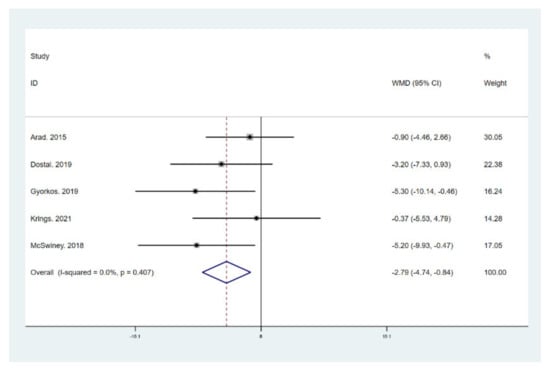
Figure 6.
Forest plot for the effect of a low-carbohydrate high-fat (LCHF) diet combined with HIIT on body fat percentage (BFP). For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies.
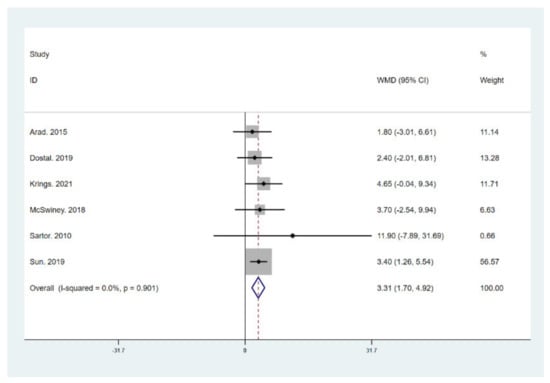
Figure 7.
Forest plot for the effect of a low-carbohydrate high-fat (LCHF) diet combined with HIIT on maximal oxygen uptake (VO2 max). For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies.
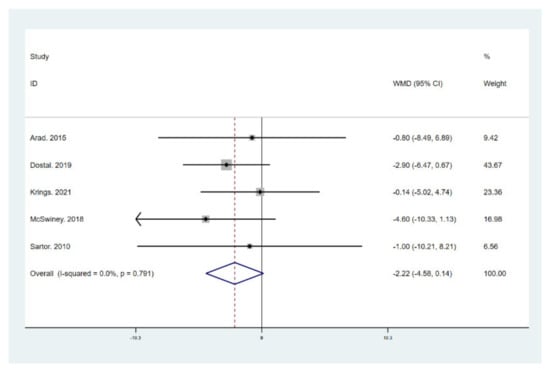
Figure 8.
Forest plot for the effect of a low-carbohydrate high-fat (LCHF) diet combined with HIIT on fat mass (FM). For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies.
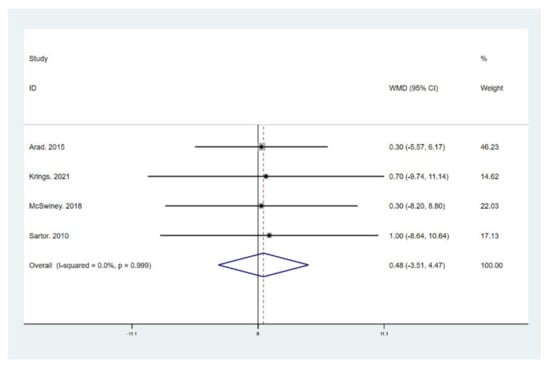
Figure 9.
Forest plot for the effect of a low-carbohydrate high-fat (LCHF) diet combined with HIIT on fat free mass (FFM). For each study, squares represent the mean difference in intervention effects, with horizontal lines intersecting them as the lower and upper limits of the 95% CI. The size of each square represents the relative weight of the studies conducted in the meta-analysis. The diamond represents the results of the meta-analysis combining the individual studies.
4. Discussion
This meta-analysis evaluated the impact of LCHF on human body composition with the addition of HIIT. The results of the ten studies included for analysis show that LCHF significantly decreased human BM, BMI, and BFP by the combination with HIIT, while FM and FFM remained unchanged. Moreover, VO2max was augmented following the intervention.
Recently, attention has been paid to LCHF on account of the reported influences on body composition. Extensive research has demonstrated the remarkable effect of LCHF on weight loss and fat loss [9,10,11] [9 (RCT), 10 (RCT), 11 (RCT)]. Similar to the previous studies, our results support the evidence that individuals that adhere to LCHF diet exhibit a larger decline in BM, FM, and BFP than those assigned to a non-LCHF diet group. The underlying mechanisms of LCHF-induced weight and fat reduction may relate to a limited appetite or the changing metabolic modulators. Some investigations indicated that the LCHF diet elicits physiological ketosis, which is characterized by the increment in ketone bodies, such as β-hydroxybutyrate, acetoacetate, and acetone [56,59]. The ketosis effect is assumed to suppress appetite [57] and thus decrease total calorie intake, as multiple kinds of research find the LCHF diet group consumes fewer calories in ad libitum patterns [17,19,20,60]. More importantly, this view is confirmed by the fact that the LCHF diet group only resulted in more BM and FM loss in ad libitum studies [17,19,20,60] but not in isocaloric studies [18,58,61]. However, on the other hand, Sun et al. performed a four-week isocaloric LCHF dietary pattern and still detected a reduction in BM and BMI in overweight/obese Chinese females [43], which suggests this weight-reducing effect may be generated by other possible reasons. Lipogenesis reduction and lipolysis escalation may be the plausible mechanisms of weight and fat loss for the isocaloric LCHF diet, as proven by the decreased insulin [62]. Another possible reason may involve the extra need for gluconeogenesis for energy production, which is a process consuming extra energy [63,64]. Nevertheless, dietary intervention alone may bring a negative impact on muscle mass. Therefore, the combination with an exercise intervention, such as HIIT and RT, has received lots of attention. A recent meta-analysis indicated that the ketogenic diet decreased FFM, and this negative effect could not be ameliorated by combining it with RT [36]. However, surprisingly, our result reveals that LCHF combined with HIIT has no significant effect on FFM, which implies that HIIT prevents greater muscle mass loss during LCHF than RT.
Except for the weight-reducing effect, the LCHF diet may also become a possible dietary intervention to enhance sports performance. The LCHF diet has been demonstrated to cause a decline in total carbohydrate oxidation and an increase in fat oxidation and lipolysis during prolonged exercise [63,65], which would in turn improve exercise performance. However, there is much debate on its effect on VO2max, as some studies found a reduction in the VO2max of the LCHF diet group [42], whereas others did not find this [16,43]. A systematic review proposed that the duration of consuming LCHF may be an essential factor affecting physical performance. Long-term intervention demands metabolic adaptations to minimize adverse effects [37]. Our meta-analysis indicated that LCHF combined with HIIT for 2–14 weeks has a positive effect on VO2max, which suggests that the negative effect at the initiation of the diet can be reversed by the addition of HIIT.
The scope of this study was limited by the following terms. First, since all included studies lasted less than 14 weeks, we are unable to explain the long-term effect of LCHF combined with HIIT on body composition and VO2max. Second, the included studies employed different estimates of VO2max (i.e., VO2max or VO2peak), which may lead to deviation. Third, dietary intervention and modes of HIIT were performed in different patterns (i.e., ad libitum or isocaloric, cycling or unarmed training). Fourth, the populations involved were of different types (i.e., overweight/obese people, healthy untrained individuals, or elite athletes).
5. Conclusions
LCHF combined with HIIT reduces body weight and fat mass while maintaining lean body mass and enhancing aerobic capacity. However, considering the adverse effects of LCHF, whether its combination with HIIT can be used in a range of patients and its long-term safety remains unknown. Further large, long-term, well-designed randomized trials on this topic are needed to assess the long-term safety, efficacy, and adherence to the combination of LCHF and HIIT. Further studies are also necessary to explore the impact of other types of exercise (i.e., moderate-intensity interval training, aerobic training, etc.) on body composition and physical performance during LCHF.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph182010740/s1, Figure S1: Funnel plot of studies referring to BM, Figure S2: Funnel plot of studies referring to BMI, Figure S3: Funnel plot of studies referring to FM, Figure S4: Funnel plot of studies referring to BFP, Figure S5: Funnel plot of studies referring to FFM, Figure S6: Funnel plot of studies referring to VO2max.
Author Contributions
All authors contributed to this research article as follows: Conceptualization and project administration, J.L. and R.W.; methodology and statistical analysis, J.H. and B.L.; writing—original draft preparation, Z.W. and J.H.; writing—review and editing, Z.W. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (grant no. 31471133) and Fundamental Research Funds for the Central Universities (grant no. 2020025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the included studies are in Table 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burke, L.M. Re-Examining High-Fat Diets for Sports Performance: Did We Call the ‘Nail in the Coffin’ Too Soon? Sports Med. 2015, 45, S33–S49. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, S.A. Re: National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. J. Urol. 2011, 186, 1982–1983. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedecke, J.H.; Christie, C.; Wilson, G.; Dennis, S.C.; Noakes, T.D.; Hopkins, W.G.; Lambert, E.V. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism 1999, 48, 1509–1517. [Google Scholar] [CrossRef]
- Adam-Perrot, A.; Clifton, P.; Brouns, F. Low-carbohydrate diets: Nutritional and physiological aspects. Obes. Rev. 2006, 7, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.; Volek, J.S.; Phinney, S.D. Low-carbohydrate diets for athletes: What evidence? Br. J. Sports Med. 2014, 48, 1077–1078. [Google Scholar] [CrossRef] [Green Version]
- Volek, J.S.; Noakes, T.; Phinney, S.D. Rethinking fat as a fuel for endurance exercise. Eur. J. Sport Sci. 2015, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S., Jr.; Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef]
- Moreno, B.; Crujeiras, A.B.; Bellido, D.; Sajoux, I.; Casanueva, F.F. Obesity treatment by very low-calorie-ketogenic diet at two years: Reduction in visceral fat and on the burden of disease. Endocrine 2016, 54, 681–690. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [Green Version]
- Brouns, F. Overweight and diabetes prevention: Is a low-carbohydrate-high-fat diet recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea Meira, I.; Romão, T.T.; Pires do Prado, H.J.; Krüger, L.T.; Pires, M.E.P.; da Conceição, P.O. Ketogenic Diet and Epilepsy: What We Know So Far. Front. Neurosci. 2019, 13, 5. [Google Scholar] [CrossRef] [Green Version]
- Włodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsharairi, N.A. The Role of Short-Chain Fatty Acids in the Interplay between a Very Low-Calorie Ketogenic Diet and the Infant Gut Microbiota and Its Therapeutic Implications for Reducing Asthma. Int. J. Mol. Sci. 2020, 21, 9580. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Maunder, E.D.; Dulson, D.K. Effect of a Ketogenic Diet on Submaximal Exercise Capacity and Efficiency in Runners. Med. Sci. Sports Exerc. 2019, 51, 2135–2146. [Google Scholar] [CrossRef]
- Vargas-Molina, S.; Petro, J.L.; Romance, R.; Kreider, R.B.; Schoenfeld, B.J.; Bonilla, D.A.; Benitez-Porres, J. Effects of a ketogenic diet on body composition and strength in trained women. J. Int. Soc. Sports Nutr. 2020, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Vargas, S.; Romance, R.; Petro, J.L.; Bonilla, D.A.; Galancho, I.; Espinar, S.; Kreider, R.B.; Benitez-Porres, J. Efficacy of ketogenic diet on body composition during resistance training in trained men: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 31. [Google Scholar] [CrossRef] [Green Version]
- LaFountain, R.A.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.T.; Beeler, M.K.; Buga, A.; Sapper, T.N.; Short, J.A.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, e538–e547. [Google Scholar] [CrossRef]
- Greene, D.A.; Varley, B.J.; Hartwig, T.B.; Chapman, P.; Rigney, M. A Low-Carbohydrate Ketogenic Diet Reduces Body Mass Without Compromising Performance in Powerlifting and Olympic Weightlifting Athletes. J. Strength Cond. Res. 2018, 32, 3373–3382. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; Konig, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, R.; Dugré, N.; Allan, G.M.; Lindblad, A.J. Ketogenic diet for weight loss. Can. Fam. Physician Med. Fam. Can. 2018, 64, 906. [Google Scholar]
- Kosinski, C.; Jornayvaz, F.R. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517. [Google Scholar] [CrossRef]
- Jelleyman, C.; Yates, T.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef] [Green Version]
- Consitt, L.A.; Dudley, C.; Saxena, G. Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults. Nutrients 2019, 11, 2636. [Google Scholar] [CrossRef] [Green Version]
- Hallsworth, K.; Thoma, C.; Hollingsworth, K.G.; Cassidy, S.; Anstee, Q.M.; Day, C.P.; Trenell, M.I. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Sci. 2015, 129, 1097–1105. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, P.; Kuljiratitikal, K.; Aksornchanya, O.; Chaiyasoot, K.; Bandidniyamanon, W.; Charatcharoenwitthaya, N. Moderate-Intensity Aerobic vs. Resistance Exercise and Dietary Modification in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. Clin. Transl. Gastroenterol. 2021, 12, e00316. [Google Scholar] [CrossRef] [PubMed]
- Dun, Y.; Smith, J.R.; Liu, S.; Olson, T.P. High-Intensity Interval Training in Cardiac Rehabilitation. Clin. Geriatr. Med. 2019, 35, 469–487. [Google Scholar] [CrossRef]
- Williams, M.A.; Stewart, K.J. Impact of strength and resistance training on cardiovascular disease risk factors and outcomes in older adults. Clin. Geriatr. Med. 2009, 25, 703–714. [Google Scholar] [CrossRef]
- Chin, E.C.; Yu, A.P.; Lai, C.W.; Fong, D.Y.; Chan, D.K.; Wong, S.H.; Sun, F.; Ngai, H.H.; Yung, P.S.H.; Siu, P.M. Low-Frequency HIIT Improves Body Composition and Aerobic Capacity in Overweight Men. Med. Sci. Sports Exerc. 2020, 52, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Pinto, R.S.; Bottaro, M.; Izquierdo, M. Strength and endurance training prescription in healthy and frail elderly. Aging Dis. 2014, 5, 183–195. [Google Scholar]
- Moro, T.; Marcolin, G.; Bianco, A.; Bolzetta, F.; Berton, L.; Sergi, G.; Paoli, A. Effects of 6 Weeks of Traditional Resistance Training or High Intensity Interval Resistance Training on Body Composition, Aerobic Power and Strength in Healthy Young Subjects: A Randomized Parallel Trial. Int. J. Environ. Res. Public Health 2020, 17, 4093. [Google Scholar] [CrossRef]
- Fisher, G.; Brown, A.W.; Bohan Brown, M.M.; Alcorn, A.; Noles, C.; Winwood, L.; Resuehr, H.; George, B.; Jeansonne, M.M.; Allison, D.B. High Intensity Interval—vs. Moderate Intensity—Training for Improving Cardiometabolic Health in Overweight or Obese Males: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0138853. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.K.; Dorff, T.B.; Todd Schroeder, E.; Lane, C.J.; Gross, M.E.; Dieli-Conwright, C.M. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: A pilot randomized controlled trial. BMC Cancer 2018, 18, 368. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, H.; Arabzadeh, E. Metabolic cross-talk between skeletal muscle and adipose tissue in high-intensity interval training vs. moderate-intensity continuous training by regulation of PGC-1α. Eat. Weight. Disord. 2020, 25, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Asbaghi, O.; Tinsley, G.M.; Kooti, W.; Abbasnezhad, A.; Afrisham, R.; Wong, A. Effects of resistance training combined with a ketogenic diet on body composition: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef]
- Murphy, N.E.; Carrigan, C.T.; Margolis, L.M. High-Fat Ketogenic Diets and Physical Performance: A Systematic Review. Adv. Nutr. 2021, 12, 223–233. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar]
- Impey, S.G.; Hearris, M.A.; Hammond, K.M.; Bartlett, J.D.; Louis, J.; Close, G.L.; Morton, J.P. Fuel for the Work Required: A Theoretical Framework for Carbohydrate Periodization and the Glycogen Threshold Hypothesis. Sports Med. 2018, 48, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- Knuiman, P.; Hopman, M.T.; Mensink, M. Glycogen availability and skeletal muscle adaptations with endurance and resistance exercise. Nutr. Metab. 2015, 12, 59. [Google Scholar] [CrossRef] [Green Version]
- Dostal, T.; Plews, D.J.; Hofmann, P.; Laursen, P.B.; Cipryan, L. Effects of a 12-Week Very-Low Carbohydrate High-Fat Diet on Maximal Aerobic Capacity, High-Intensity Intermittent Exercise, and Cardiac Autonomic Regulation: Non-randomized Parallel-Group Study. Front. Physiol. 2019, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Glowka, N.; Ziobrowska, A.; Podgorski, T. Is a Four-Week Ketogenic Diet an Effective Nutritional Strategy in CrossFit-Trained Female and Male Athletes? Nutrients 2021, 13, 864. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Kong, Z.W.; Shi, Q.D.; Hu, M.Z.; Zhang, H.F.; Zhang, D.; Nie, J.L. Non-Energy-Restricted Low-Carbohydrate Diet Combined with Exercise Intervention Improved Cardiometabolic Health in Overweight Chinese Females. Nutrients 2019, 11, 3051. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Fu, J.; Sun, S.; Zhao, G.; Cheng, W.; Dou, C.; Quan, M. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PLoS ONE 2019, 14, e0210644. [Google Scholar] [CrossRef] [PubMed]
- Vella, C.A.; Taylor, K.; Drummer, D. High-intensity interval and moderate-intensity continuous training elicit similar enjoyment and adherence levels in overweight and obese adults. Eur. J. Sport Sci. 2017, 17, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Edmunds, R.M.; Clark, A.; King, L.; Gallant, R.A.; Namm, S.; Fischer, A.; Wood, K.M. High-Intensity Interval Training Increases Cardiac Output and V O2max. Med. Sci. Sports Exerc. 2017, 49, 265–273. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Krings, B.M.; Waldman, H.S.; Shepherd, B.D.; McAllister, M.J.; Fountain, B.J.; Lamberth, J.G.; Smith, J.W. The metabolic and performance effects of carbohydrate timing in resistance trained males undergoing a carbohydrate restricted diet. Appl. Physiol. Nutr. Metab. 2021, 46, 626–636. [Google Scholar] [CrossRef]
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; Lafountain, R.A.; Volek, J.S.; Doyle, L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metab. Clin. Exp. 2018, 81, 25–34. [Google Scholar] [CrossRef]
- Arad, A.D.; DiMenna, F.J.; Thomas, N.; Tamis-Holland, J.; Weil, R.; Geliebter, A.; Albu, J.B. High-intensity interval training without weight loss improves exercise but not basal or insulin-induced metabolism in overweight/obese African American women. J. Appl. Physiol. 2015, 119, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Kong, Z.; Shi, Q.; Zhang, H.; Lei, O.K.; Nie, J. Carbohydrate Restriction with or without Exercise Training Improves Blood Pressure and Insulin Sensitivity in Overweight Women. Healthcare 2021, 9, 637. [Google Scholar] [CrossRef]
- Gyorkos, A.; Baker, M.H.; Miutz, L.N.; Lown, D.A.; Jones, M.A.; Houghton-Rahrig, L.D. Carbohydrate-restricted Diet and High-intensity Interval Training Exercise Improve Cardio-metabolic and Inflammatory Profiles in Metabolic Syndrome: A Randomized Crossover Trial. Cureus 2019, 11, e5596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, F.; de Morree, H.M.; Matschke, V.; Marcora, S.M.; Milousis, A.; Thom, J.M.; Kubis, H.P. High-intensity exercise and carbohydrate-reduced energy-restricted diet in obese individuals. Eur. J. Appl. Physiol. 2010, 110, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Chen, M. Epidemiological experiments on weight-loss using by ketogenic diet. J. Taishan Med. Coll. 2019, 40, 672–675. [Google Scholar]
- Laza-Cagigas, R.; Chan, S.; Sumner, D.; Rampal, T. Effects and feasibility of a prehabilitation programme incorporating a low-carbohydrate, high-fat dietary approach in patients with type 2 diabetes: A retrospective study. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Paoli, A.; Cenci, L.; Pompei, P.; Sahin, N.; Bianco, A.; Neri, M.; Caprio, M.; Moro, T. Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders. Nutrients 2021, 13, 374. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [Green Version]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in CrossFit Trainees: A Pilot Study. Sports 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.M.; Volek, J.S.; D’Agostino, D.P. Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Men. J. Strength Cond. Res. 2020, 34, 3463–3474. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Veldhorst, M.A.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am. J. Clin. Nutr. 2009, 90, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Fine, E.J.; Feinman, R.D. Thermodynamics of weight loss diets. Nutr. Metab. 2004, 1, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).