Health Status Is Affected, and Phase I/II Biotransformation Activity Altered in Young Women Using Oral Contraceptives Containing Drospirenone/Ethinyl Estradiol
Abstract
:1. Introduction
2. Material and Methods
2.1. Recruitment of Participants and Sample Collection
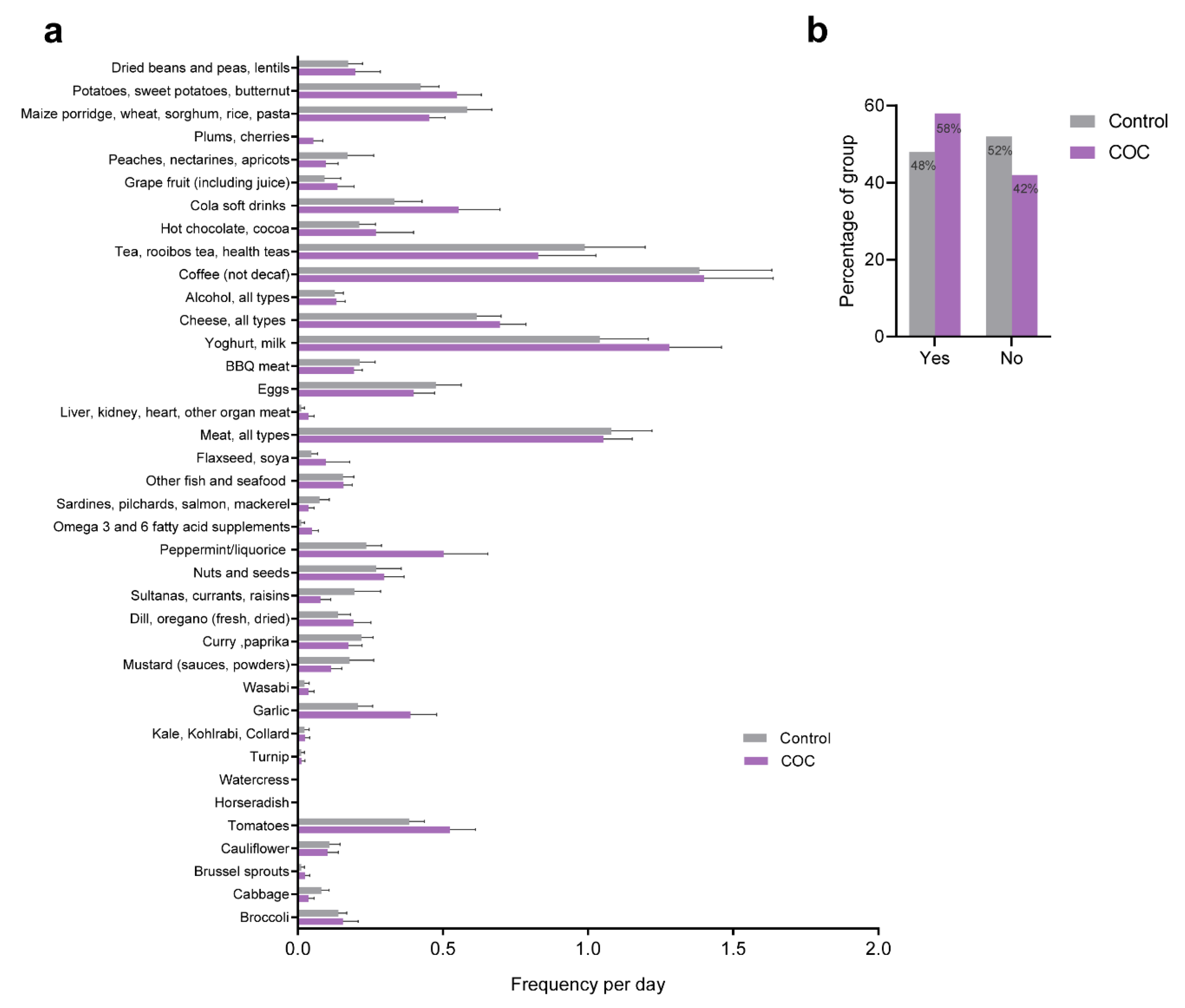
2.2. Dietary Evaluation
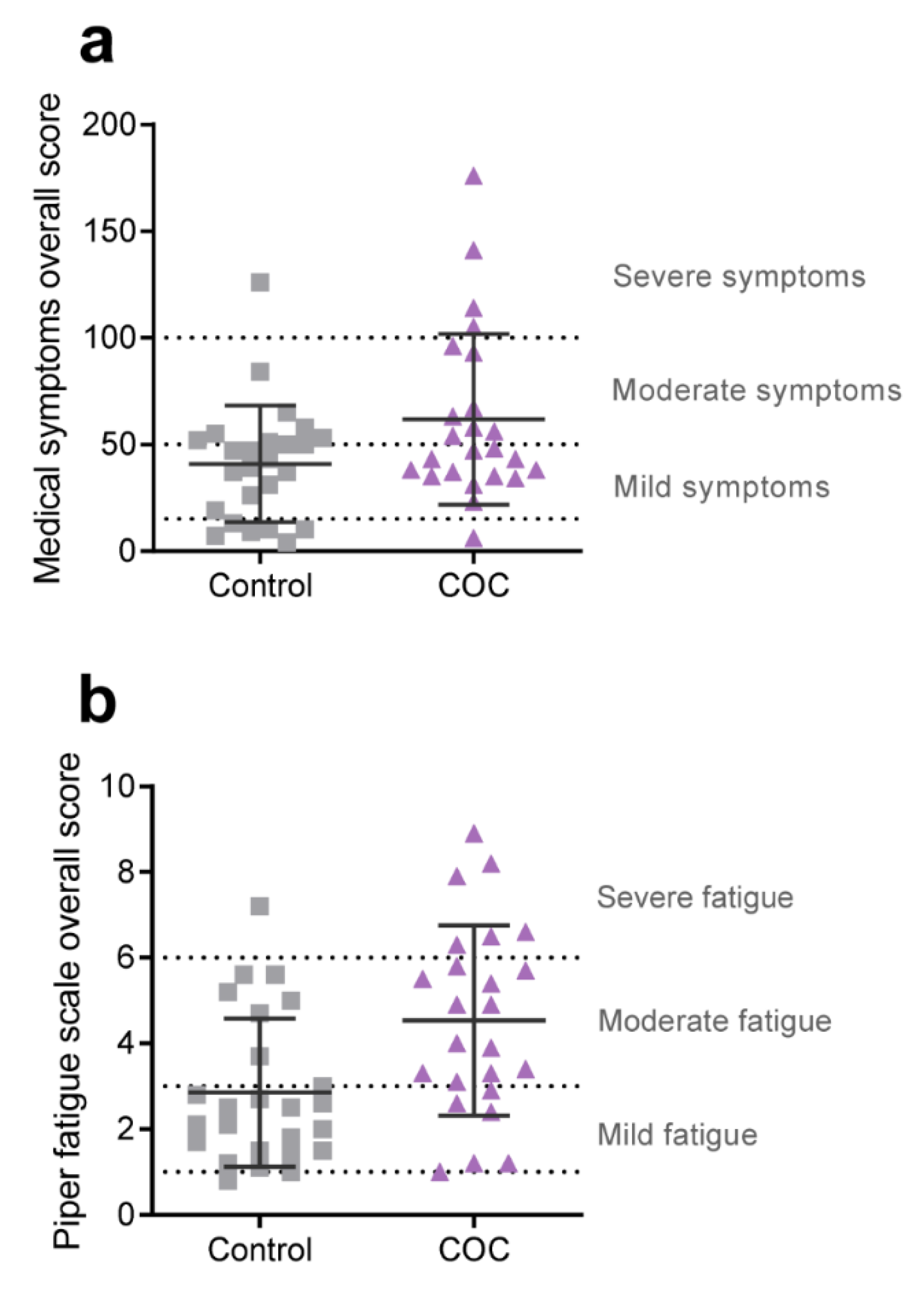
2.3. Medical Symptoms Questionnaire
2.4. Piper Fatigue Scale Assessment
2.5. Biomedical/Physiological Measurements
2.6. Reagents
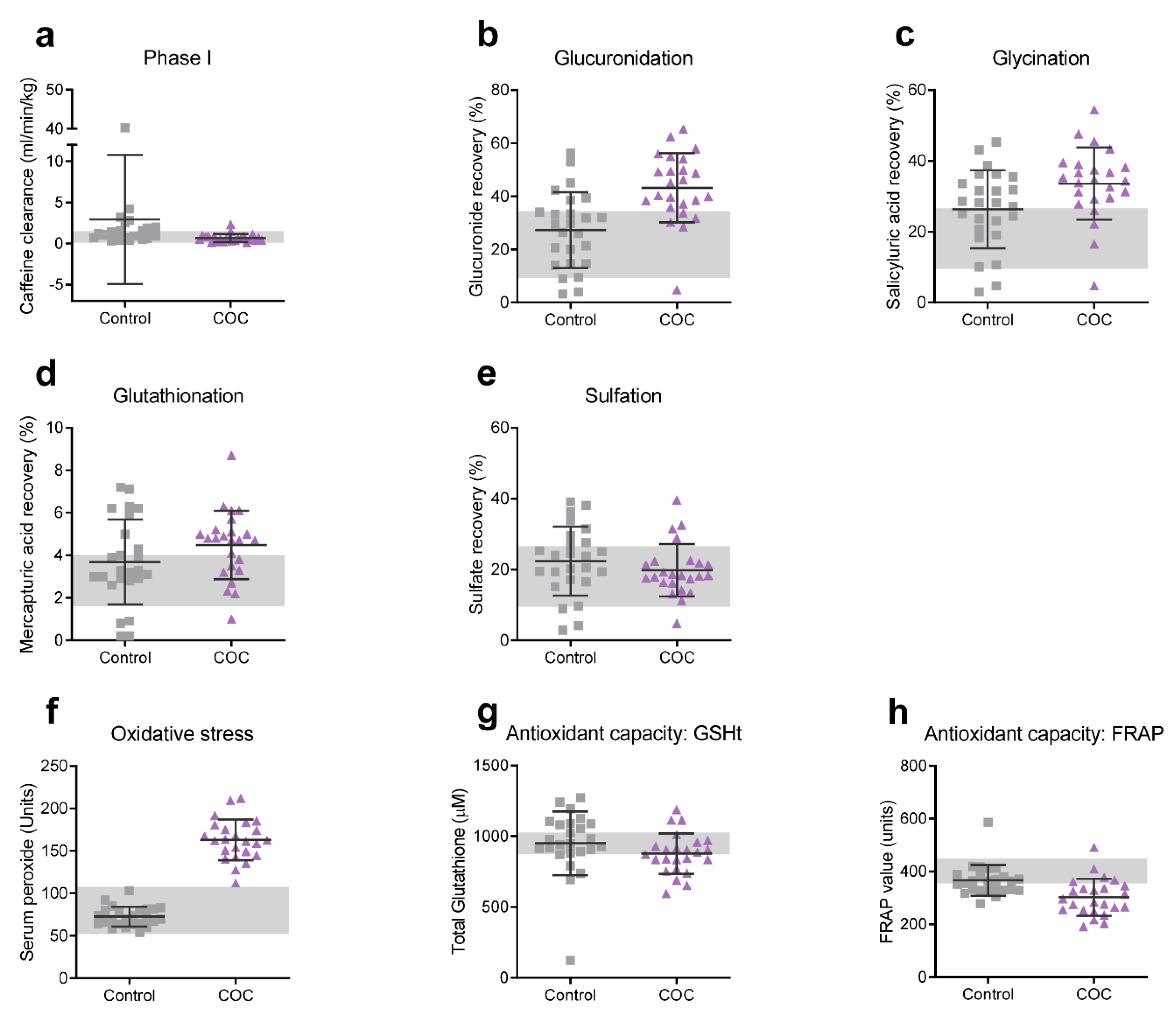
2.7. Biotransformation Efficiency
2.8. Secondary Products from the Acetylsalicylic Acid Challenge
2.9. Additional Biochemical Measurements
2.10. Serum Peroxides
2.11. Total Glutathione
2.12. Ferric Reducing Ability of Plasma (FRAP)
2.13. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Study Population
3.2. Dietary Evaluation
3.3. Health Status and Wellbeing
3.4. Biotransformation Efficiency
3.5. Serum Peroxides and Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Contraceptive Use by Method 2019: Data Booklet; Department of Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Piomboni, P.; Morgante, G. Hormonal contraceptives: Pharmacology tailored to women’s health. Hum. Reprod. Update 2016, 22, 634–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokras, A. Noncontraceptive use of oral combined hormonal contraceptives in polycystic ovary syndrome—Risks versus benefits. Fertil. Steril. 2016, 106, 1572–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, F.; Enatsu, A.; Ota, I.; Toda, T.; Arata, K.; Harada, T. Effects of low dose oral contraceptive pill containing drospirenone/ethinylestradiol in patients with endometrioma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Skrenkova, J.; Hill, M.; Stepan, J.J. Low-dose estrogen combined oral contraceptives may negatively influence physiological bone mineral density acquisition during adolescence. Eur. J. Endocrinol. 2012, 166, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Hickson, S.S.; Miles, K.L.; McDonnell, B.J.; Yasmin; Cockcroft, J.R.; Wilkinson, I.B.; McEniery, C.M. Use of the oral contraceptive pill is associated with increased large artery stiffness in young women: The ENIGMA study. J. Hypertens. 2011, 29, 1155–1159. [Google Scholar] [CrossRef]
- Grandi, G.; Napolitano, A.; Cagnacci, A. Metabolic impact of combined hormonal contraceptives containing estradiol. Expert Opin. Drug Metab. Toxicol. 2016, 12, 779–787. [Google Scholar] [CrossRef]
- Borges, L.E.; Andrade, R.P.; Aldrighi, J.M.; Guazelli, C.; Yazlle, M.E.H.; Isaia, C.F.; Petracco, A.; Peixoto, F.C.; Camargos, A.F. Effect of a combination of ethinylestradiol 30 μg and drospirenone 3 mg on tolerance, cycle control, general well-being and fluid-related symptoms in women with premenstrual disorders requesting contraception. Contraception 2006, 74, 446–450. [Google Scholar] [CrossRef]
- Sulak, P.J. Ovulation suppression of premenstrual symptoms using oral contraceptives. Am. J. Manag. Care 2005, 11, S492–S497. [Google Scholar] [PubMed]
- Loder, E.W.; Buse, D.C.; Golub, J.R. Headache as a side effect of combination estrogen-progestin oral contraceptives: A systematic review. Am. J. Obstet. Gynecol. 2005, 193, 636–649. [Google Scholar] [CrossRef]
- Gierisch, J.M.; Coeytaux, R.R.; Urrutia, R.P.; Havrilesky, L.J.; Moorman, P.G.; Lowery, W.J.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sanders, G.D.; et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1931–1943. [Google Scholar] [CrossRef] [Green Version]
- Nappi, R.E.; Lete, I.; Lee, L.K.; Flores, N.M.; Micheletti, M.-C.; Tang, B. Real-world experience of women using extended-cycle vs monthly-cycle combined oral contraception in the United States: The National Health and Wellness Survey. BMC Women’s Health 2018, 18, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, D. Experiences with Yasmin: The acceptability of a novel oral contraceptive and its effect on well-being. Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2002, 7 (Suppl 3), 35–41. [Google Scholar]
- Adeyanju, O.; Soetan, O.A.; Olatunji, L.A. Drospirenone-containing contraceptive exerts positive effects on cardiac uric acid and PAI-1 but not GSK-3: Improved safety profiles in contraception? Pathophysiology 2019, 26, 227–231. [Google Scholar] [CrossRef]
- Witjes, H.; Creinin, M.D.; Sundström-Poromaa, I.; Martin Nguyen, A.; Korver, T. Comparative analysis of the effects of nomegestrol acetate/17 β-estradiol and drospirenone/ethinylestradiol on premenstrual and menstrual symptoms and dysmenorrhea. Eur. J. Contracept. Reprod. Health Care 2015, 20, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruzzetti, F.; Lello, S.; Lazzarini, V.; Fratta, S.; Orru, M.; Sorge, R.; Minerba, L.; Ricci, C.; Genazzani, A.R.; Melis, G.B.; et al. The oral contraceptive containing 30 microg of ethinylestradiol plus 3 mg of drospirenone is able to antagonize the increase of extracellular water occurring in healthy young women during the luteal phase of the menstrual cycle: An observational study. Contraception 2007, 75, 199–203. [Google Scholar] [CrossRef]
- Sabatini, R.; Orsini, G.; Cagiano, R.; Loverro, G. Noncontraceptive benefits of two combined oral contraceptives with antiandrogenic properties among adolescents. Contraception 2007, 76, 342–347. [Google Scholar] [CrossRef]
- Li, J.; Ren, J.; Sun, W. A comparative systematic review of Yasmin (drospirenone pill) versus standard treatment options for symptoms of polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 13–21. [Google Scholar] [CrossRef]
- Oelkers, W. Drospirenone, a progestogen with antimineralocorticoid properties: A short review. Mol. Cell. Endocrinol. 2004, 217, 255–261. [Google Scholar] [CrossRef]
- Lete, I.; Chabbert-Buffet, N.; Jamin, C.; Lello, S.; Lobo, P.; Nappi, R.E.; Pintiaux, A. Haemostatic and metabolic impact of estradiol pills and drospirenone-containing ethinylestradiol pills vs. levonorgestrel-containing ethinylestradiol pills: A literature review. Eur. J. Contracept. Reprod. Health Care 2015, 20, 329–343. [Google Scholar] [CrossRef]
- De Groote, D.; d’Hauterive, S.P.; Pintiaux, A.; Balteau, B.; Gerday, C.; Claesen, J.; Foidart, J.-M. Effects of oral contraception with ethinylestradiol and drospirenone on oxidative stress in women 18–35 years old. Contraception 2009, 80, 187–193. [Google Scholar] [CrossRef]
- Dinger, J.; Möhner, S.; Heinemann, K. Cardiovascular risks associated with the use of drospirenone-containing combined oral contraceptives. Contraception 2016, 93, 378–385. [Google Scholar] [CrossRef]
- Dinger, J.; Bardenheuer, K.; Heinemann, K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: Final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception 2014, 89, 253–263. [Google Scholar] [CrossRef]
- Dinger, J.; Assmann, A.; Möhner, S.; Minh, T.D. Risk of venous thromboembolism and the use of dienogest- and drospirenone-containing oral contraceptives: Results from a German case-control study. J. Fam. Plan. Reprod. Health Care 2010, 36, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Stegeman, B.H.; De Bastos, M.; Rosendaal, F.R.; Vlieg, A.V.H.; Helmerhorst, F.M.; Stijnen, T.; Dekkers, O.M. Different combined oral contraceptives and the risk of venous thrombosis: Systematic review and network meta-analysis. BMJ 2013, 347, f5298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer-plc. Yasmin film-coated tablets 0.03mg/3mg—Summary of Product Characteristics (SmPC). 2019. Available online: https://www.medicines.org.uk/emc/product/1607/smpc (accessed on 3 April 2020).
- Cauci, S.; Buligan, C.; Marangone, M.; Francescato, M.P. Oxidative stress in female athletes using combined oral contraceptives. Sports Med. Open 2016, 2, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Cui, D.; Wang, B.; Han, Y.H.; Balimane, P.; Yang, Z.; Sinz, M.; Rodrigues, A.D. Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: A new look at an old drug. Clin. Pharmacokinet. 2007, 46, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Liska, D.; Lyon, M.; Jones, D.S. Detoxification and biotransformational imbalances. Explore 2006, 2, 122–140. [Google Scholar] [CrossRef]
- Erasmus, E.; Steffens, F.E.; Van Reenen, M.; Vorster, B.C.; Reinecke, C.J. Biotransformation profiles from a cohort of chronic fatigue women in response to a hepatic detoxification challenge. PLoS ONE 2019, 14, e0216298. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Quinn, S. Textbook of Functional Medicine, 3rd ed.; The Institute for Functional Medicine: Gig Harbor, WA, USA, 2010. [Google Scholar]
- Piper, B.F.; Dibble, S.L.; Dodd, M.J.; Weiss, M.C.; Slaughter, R.E.; Paul, S.M. The revised piper fatigue scale: Psychometric evaluation in women with breast cancer. Oncol. Nurs. Forum 1998, 25, 677–684. [Google Scholar]
- Biaggi, R.R.; Vollman, M.W.; Nies, M.A.; Brener, C.E.; Flakoll, P.J.; Levenhagen, D.K.; Sun, M.; Karabulut, Z.; Chen, K.Y. Comparison of air-displacement plethysmography with hydrostatic weighing and bioelectrical impedance analysis for the assessment of body composition in healthy adults. Am. J. Clin. Nutr. 1999, 69, 898–903. [Google Scholar] [CrossRef] [Green Version]
- Talbert, E.; Flynn, M.G.; Bell, J.W.; Carrillo, A.; Dill, M.D.; Christensen, C.N.; Thompson, C.M. Comparison of body composition measurements using a new caliper, two established calipers, hydrostatic weighing, and bodpod. Int. J. Exerc. Sci. 2009, 2, 19–27. [Google Scholar] [PubMed]
- Verde, V.; Fogliano, V.; Ritieni, A.; Maiani, G.; Morisco, F.; Caporaso, N. Use of N,N-dimethyl-p-phenylenediamine to evaluate the oxidative status of human plasma. Free. Radic. Res. 2002, 36, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groppe, D. MATLAB Central File Exchange. 2021. Available online: https://www.mathworks.com/matlabcentral/fileexchange/27418-fdr_bh (accessed on 11 June 2021).
- Hyman, M. MSQ—Medical Symptom/Toxicity Questionnaire. Available online: http://drhyman.com/downloads/MSQ_Fillable.pdf (accessed on 19 March 2021).
- Ibrahim, S. Medical Symptoms Questionnaire. Available online: https://thevitalityclinic.co.uk/videos/medical-symptoms-questionnaire/ (accessed on 19 March 2021).
- Piper, B.F.; Dibble, S.L.; Dodd, M.J.; Weiss, M.C.; Slaughter, R.E.; Paul, S.M. Scoring Piper Fatigue Scale (PFS); University at Buffalo: Buffalo, NY, USA; The State University of New York: Albany, NY, USA, 1998. [Google Scholar]
- Shimada, T.; Yamazaki, H.; Mimura, M.; Inui, Y.; Guengerich, F.P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994, 270, 414–423. [Google Scholar]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Balogh, A.; Klinger, G.; Henschel, L.; Börner, A.; Vollanth, R.; Kuhnz, W. Influence of ethinylestradiol-containing combination oral contraceptives with gestodene or levonorgestrel on caffeine elimination. Eur. J. Clin. Pharmacol. 1995, 48, 161–166. [Google Scholar] [CrossRef]
- Granfors, M.T.; Backman, J.T.; Laitila, J.; Neuvonen, P.J. Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2. Clin. Pharmacol. Ther. 2005, 78, 400–411. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, C., II; Tuveson, D.A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruzzetti, F.; Lazzarini, V.; Ricci, C.; Quirici, B.; Gambacciani, M.; Paoletti, A.M.; Genazzani, A.R. Effect of an oral contraceptive containing 30 microg ethinylestradiol plus 3 mg drospirenone on body composition of young women affected by premenstrual syndrome with symptoms of water retention. Contraception 2007, 76, 190–194. [Google Scholar] [CrossRef]
- Giribela, C.R.G.; Consolim-Colombo, F.M.; Nisenbaum, M.G.; Moraes, T.L.D.; Giribela, A.H.G.; Baracat, E.C.; Melo, N.R.D. Effects of a combined oral contraceptive containing 20 mcg of ethinylestradiol and 3 mg of drospirenone on the blood pressure, renin-angiotensin-aldosterone system, insulin resistance, and androgenic profile of healthy young women. Gynecol. Endocrinol. 2015, 31, 912–915. [Google Scholar] [CrossRef]
- Nisenbaum, M.G.; de Melo, N.R.; Giribela, C.R.G.; de Morais, T.L.; Guerra, G.M.; de Angelis, K.; Mostarda, C.; Baracat, E.C.; Consolim-Colombo, F.M. Effects of a contraceptive containing drospirenone and ethinyl estradiol on blood pressure and autonomic tone: A prospective controlled clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 62–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apter, D.; Borsos, A.; Baumgärtner, W.; Melis, G.B.; Vexiau-Robert, D.; Colligs-Hakert, A.; Palmer, M.; Kelly, S. Effect of an oral contraceptive containing drospirenone and ethinylestradiol on general well-being and fluid-related symptoms. Eur. J. Contracept. Reprod. Health Care 2003, 8, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Davies, E.; Fearns, S.; McKinnon, C.; Carter, R.; Gerlinger, C.; Smithers, A. Effects of oral contraceptives containing ethinylestradiol with either drospirenone or levonorgestrel on various parameters associated with well-being in healthy women. Clin. Drug Investig. 2010, 30, 325–336. [Google Scholar] [CrossRef]
- Borenstein, J.; Yu, H.T.; Wade, S.; Chiou, C.F.; Rapkin, A. Effect of an oral contraceptive containing ethinyl estradiol and drospirenone on premenstrual symptomatology and health-related quality of life. J. Reprod. Med. 2003, 48, 79–85. [Google Scholar] [PubMed]
- Foidart, J.M. Added benefits of drospirenone for compliance. Climacteric 2005, 8, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, J.; Paoletti, A.M. Added benefits and user satisfaction with a low-dose oral contraceptive containing drospirenone: Results of three multicentre trials. Clin. Drug Investig. 2009, 29, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauci, S.; Xodo, S.; Buligan, C.; Colaninno, C.; Barbina, M.; Barbina, G.; Francescato, M. Oxidative stress is increased in combined oral contraceptives users and is positively associated with high-sensitivity c-reactive protein. Molecules 2021, 26, 1070. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Brix, T.H.; Kyvik, K.O.; Brøsen, K. The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics 2002, 12, 473–478. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.-I.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep. 2019, 28, 746–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqui, Z.; Mohammad, R.S.; Lokhandwala, M.F.; Banday, A.A. Nrf2 inhibition induces oxidative stress, renal inflammation and hypertension in mice. Clin. Exp. Hypertens. 2021, 43, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.T.; Gutterman, D.D.; Sato, A.; Toyama, K.; Campbell, W.B.; Zeldin, D.C.; Manthati, V.L.; Falck, J.R.; Miura, H. Hydrogen peroxide inhibits cytochrome P450 epoxygenases. Circ. Res. 2008, 102, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; O’Brien, P. Molecular mechanism of 17α-ethinylestradiol cytotoxicity in isolated rat hepatocytes. Can. J. Physiol. Pharmacol. 2014, 92, 21–26. [Google Scholar] [CrossRef]
- Krattenmacher, R. Drospirenone: Pharmacology and pharmacokinetics of a unique progestogen. Contraception 2000, 62, 29–38. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Sangkuhl, K.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet. Genom. 2015, 25, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Rowland, A.; Miners, J.O.; Mackenzie, P. The UDP-glucuronosyltransferases: Their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013, 45, 1121–1132. [Google Scholar] [CrossRef]
- Heurtaux, T.; Benani, A.; Bianchi, A.; Moindrot, A.; Gradinaru, D.; Magdalou, J.; Netter, P.; Minn, A. Redox state alteration modulates astrocyte glucuronidation. Free. Radic. Biol. Med. 2004, 37, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Boverhof, D.R.; Burgoon, L.D.; Williams, K.J.; Zacharewski, T.R. Inhibition of estrogen-mediated uterine gene expression responses by dioxin. Mol. Pharmacol. 2008, 73, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Tee, M.K.; Rogatsky, I.; Tzagarakis-Foster, C.; Cvoro, A.; An, J.; Christy, R.J.; Yamamoto, K.R.; Leitman, D.C. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol. Biol. Cell 2004, 15, 1262–1272. [Google Scholar]
- Swanepoel, A.C.; Bester, J.; Emmerson, O.; Soma, P.; Beukes, D.; Van Reenen, M.; Loots, D.T.; Du Preez, I. Serum metabolome changes in relation to prothrombotic state induced by combined oral contraceptives with drospirenone and ethinylestradiol. OMICS A J. Integr. Biol. 2020, 24, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Knights, K.M.; Sykes, M.J.; Miners, J.O. Amino acid conjugation: Contribution to the metabolism and toxicity of xenobiotic carboxylic acids. Expert Opin. Drug Metab. Toxicol. 2007, 3, 159–168. [Google Scholar] [CrossRef]
| Control | COC | Control vs. COC | ||||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | ES (Cohen’s d) | BH FDR Adjusted p-Value | |
| Weight (kg) | 64.58 | (9.27) | 67.49 | (12.84) | 0.23 | 0.528 |
| Height (m) | 1.68 | (0.06) | 1.69 | (0.08) | 0.16 | 0.528 |
| BMI (kg/m2) | 22.81 | (2.42) | 23.44 | (3.42) | 0.18 | 0.528 |
| Age (years) | 24.96 | (4.71) | 23.38 | (2.89) | 0.34 | 0.377 |
| Systolic BP (mmHg) | 107.52 | (7.10) | 111.54 | (6.45) | 0.57 | 0.178 |
| Diastolic BP (mmHg) | 68.96 | (6.46) | 72.29 | (5.12) | 0.52 | 0.178 |
| Body fat % | 28.79 | (5.54) | 30.41 | (7.72) | 0.21 | 0.528 |
| COC Duration (months) | N/A | 46.0 | (34.8) | |||
| (range) | (3–144) | |||||
| Proportion smokers (<5 cigarettes/day) | 12% | 4% | ||||
| Control | COC | Control vs. COC | ||||
|---|---|---|---|---|---|---|
| Sub-Scale | Mean | (SD) | Mean | (SD) | ES (Cohen’s d) | BH FDR Adjusted p-Value |
| Overall Score | 40.92 | (27.26) | 61.71 | (40.01) | 0.52 | 0.216 |
| Head | 3.28 | (2.46) | 4.29 | (2.94) | 0.34 | 0.380 |
| Ears | 1.24 | (1.96) | 1.83 | (2.10) | 0.28 | 0.455 |
| Eyes | 2.32 | (2.06) | 2.88 | (2.47) | 0.22 | 0.490 |
| Skin | 3.40 | (3.30) | 4.33 | (4.03) | 0.23 | 0.490 |
| Nose | 3.80 | (3.44) | 7.29 | (5.67) | 0.62 | 0.108 |
| Heart | 0.96 | (1.34) | 2.13 | (3.49) | 0.33 | 0.337 |
| Emotions | 4.44 | (3.12) | 6.08 | (4.51) | 0.36 | 0.337 |
| Mind | 5.04 | (4.89) | 5.63 | (4.52) | 0.12 | 0.666 |
| Digestive Track | 4.04 | (3.70) | 7.25 | (7.46) | 0.43 | 0.266 |
| Other | 1.24 | (1.83) | 2.04 | (3.20) | 0.25 | 0.455 |
| Mouth/Throat | 1.28 | (1.95) | 1.71 | (3.06) | 0.14 | 0.601 |
| Lungs | 0.52 | (1.26) | 0.79 | (1.72) | 0.16 | 0.601 |
| Energy/Activity | 3.16 | (2.76) | 4.54 | (3.46) | 0.40 | 0.337 |
| Weight | 3.64 | (3.35) | 7.25 | (4.93) | 0.73 | 0.077 * |
| Joint/Muscle | 2.56 | (2.47) | 3.67 | (3.55) | 0.31 | 0.380 |
| Control | COC | Control vs. COC | ||||
|---|---|---|---|---|---|---|
| Sub-Scale | Mean | (SD) | Mean | (SD) | ES Cohen’s d | BH FDR Adjusted p-Value |
| Overall Score | 2.86 | (1.73) | 4.54 | (2.23) | 0.76 | 0.009 * |
| Behavioral/Severity | 2.19 | (1.53) | 4.22 | (2.60) | 0.78 | 0.005 * |
| Affective Meaning | 2.52 | (2.00) | 4.70 | (2.58) | 0.85 | 0.005 * |
| Sensory | 3.59 | (2.13) | 4.90 | (2.29) | 0.57 | 0.044 * |
| Cognitive/Mood | 3.13 | (1.74) | 4.34 | (2.15) | 0.56 | 0.044 * |
| Control | COC | Control vs. COC | ||||
|---|---|---|---|---|---|---|
| Variable | Mean | (SD) | Mean | (SD) | ES (Cohen’s d) | BH FDR Adjusted p-Value |
| Creatinine | 2.28 | (0.59) | 2.74 | (0.56) | 0.78 | 0.021 * |
| Uric acid | 0.81 | (0.36) | 0.86 | (0.46) | 0.10 | 0.745 |
| Uric acid:Creatinine ratio | 0.16 | (0.11) | 0.10 | (0.05) | 0.57 | 0.036 * |
| Caffeine clearance (Phase I) | 0.91 | (0.69) | 0.47 | (0.26) | 0.63 | 0.020 * |
| APAP-glucuronide | 3.17 | (0.68) | 3.72 | (0.47) | 0.81 | 0.008 * |
| APAP-sulfate | 3.03 | (0.58) | 2.97 | (0.38) | 0.10 | 0.745 |
| APAP-mercapturic acid | 1.43 | (0.54) | 1.66 | (0.33) | 0.42 | 0.145 |
| Salicyluric acid | 3.18 | (0.60) | 3.48 | (0.44) | 0.49 | 0.107 |
| Catechol | 2.39 | (0.76) | 2.18 | (0.65) | 0.29 | 0.412 |
| 2,3-DHBA | 1.41 | (0.64) | 1.26 | (0.57) | 0.23 | 0.525 |
| 2,5-DHBA | 3.85 | (0.85) | 3.97 | (0.98) | 0.12 | 0.745 |
| Carnitine, Total-Free | 0.91 | (0.35) | 0.90 | (0.46) | 0.01 | 0.981 |
| Acyl-Carnitine, Total | 1.10 | (0.44) | 1.28 | (0.44) | 0.41 | 0.253 |
| Acyl-Carnitine:Free Carnitine ratio | 0.94 | (0.53) | 1.16 | (0.65) | 0.35 | 0.287 |
| PhaseI:PhaseII ratio (Sulfation) | 2.07 | (0.92) | 1.42 | (0.73) | 0.70 | 0.023 * |
| PhaseI:PhaseII ratio (Glycination) | 1.94 | (0.93) | 1.06 | (0.65) | 0.94 | 0.003 * |
| PhaseI:PhaseII ratio (Glucuronidation) | 1.96 | (1.04) | 0.94 | (0.64) | 0.98 | 0.002 * |
| Serum Peroxides | 4.29 | (0.15) | 5.09 | (0.15) | 5.26 | <0.001 * |
| FRAP | 5.90 | (0.14) | 5.69 | (0.23) | 0.91 | 0.003 * |
| GSHt | 6.80 | (0.44) | 6.77 | (0.16) | 0.08 | 0.745 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venter, G.; van der Berg, C.L.; van der Westhuizen, F.H.; Erasmus, E. Health Status Is Affected, and Phase I/II Biotransformation Activity Altered in Young Women Using Oral Contraceptives Containing Drospirenone/Ethinyl Estradiol. Int. J. Environ. Res. Public Health 2021, 18, 10607. https://doi.org/10.3390/ijerph182010607
Venter G, van der Berg CL, van der Westhuizen FH, Erasmus E. Health Status Is Affected, and Phase I/II Biotransformation Activity Altered in Young Women Using Oral Contraceptives Containing Drospirenone/Ethinyl Estradiol. International Journal of Environmental Research and Public Health. 2021; 18(20):10607. https://doi.org/10.3390/ijerph182010607
Chicago/Turabian StyleVenter, Gerda, Carien L. van der Berg, Francois H. van der Westhuizen, and Elardus Erasmus. 2021. "Health Status Is Affected, and Phase I/II Biotransformation Activity Altered in Young Women Using Oral Contraceptives Containing Drospirenone/Ethinyl Estradiol" International Journal of Environmental Research and Public Health 18, no. 20: 10607. https://doi.org/10.3390/ijerph182010607
APA StyleVenter, G., van der Berg, C. L., van der Westhuizen, F. H., & Erasmus, E. (2021). Health Status Is Affected, and Phase I/II Biotransformation Activity Altered in Young Women Using Oral Contraceptives Containing Drospirenone/Ethinyl Estradiol. International Journal of Environmental Research and Public Health, 18(20), 10607. https://doi.org/10.3390/ijerph182010607






