Hybrid Decision Support to Monitor Atrial Fibrillation for Stroke Prevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Need Definition
2.2. Requirements Analysis
2.3. Specification Refinement
- (i)
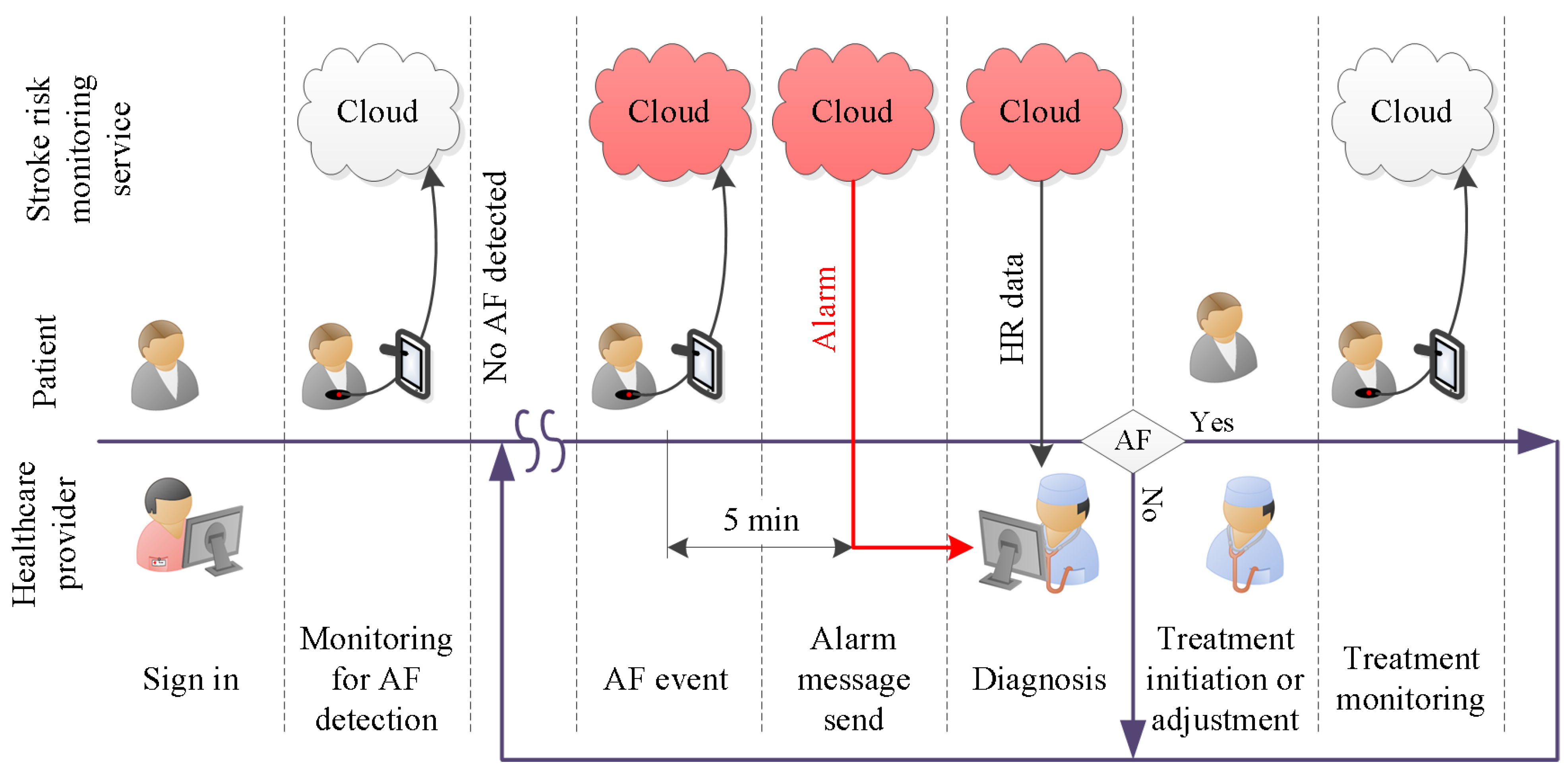
- Smart device activation:The smart device activation service enables a patient’s device to activate and establish an account with the healthcare provider. At the start of the service subscription, the healthcare provider registers the patient with the database on a cloud server. The unique account contains patient information. The necessary fields are: patient ID, assigned physician, service start date, service end date. The registration will provide the cloud server login key. This login key is used for both user authentication and data acquisition setup.
- (ii)
- Cloud server storage:The patient’s HR data and the DL classification results are stored in the cloud server. This service allows the authorized users to retrieve the data anytime and anywhere.
- (iii)
- Real-time HR monitoring service:The patient wears a breast strap with an embedded HR sensor. The sensor picks up the HR signals. These real-time data are displayed on patient smart devices. The patient co-creates value by providing and integrating the data into the AF detection service.
- (iv)
- Automated AF detection and alarm service:The DL algorithm analyses patient real-time HR data and classifies the data as AF or non-AF. Once an AF sequence is detected, the system will send an alarm message to the assigned physician. The DL algorithm creates the core value for the system.
- (v)
- Physician diagnosis support service:The physician support service incorporates algorithm support in the form of DL results and diagnosis support tools. It helps the physician to verify the DL results and to reach a diagnosis. The value of this diagnosis is twofold. First and foremost, it helps to initiate treatment, which might improve the outcomes for the patient. A secondary use for an established diagnosis arises when we consider improving the DL algorithm. To be specific, a diagnosis becomes the ground truth, which can be used to continuously retrain the DL model. That continued retraining has the potential to improve the detection quality of the algorithm.
- (vi)
- Feedback and intervention service:Once the physician has reached a diagnosis, the feedback service can be used to communicate the result to the patient. Social media, email and personal phone calls can be used to provide feedback. Timely appropriate intervention can be carried out to boost the outcomes for patients. Another use for the feedback service is the dissemination of patient compliance messages. For example, through data analytics, it is possible to establish if there is a signal interruption. A compliance message over the feedback channel might help to re-establish the data flow.
3. Results
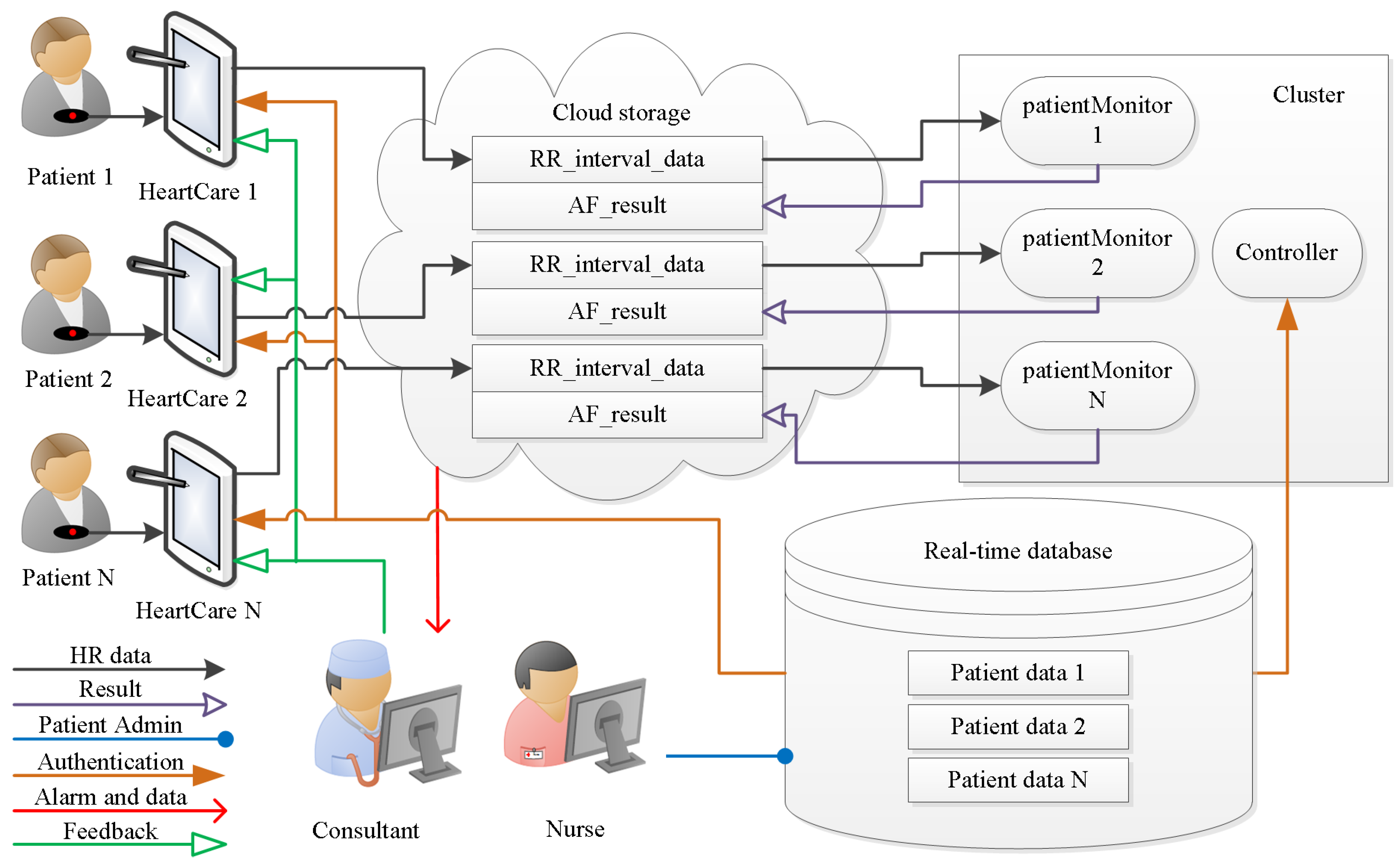
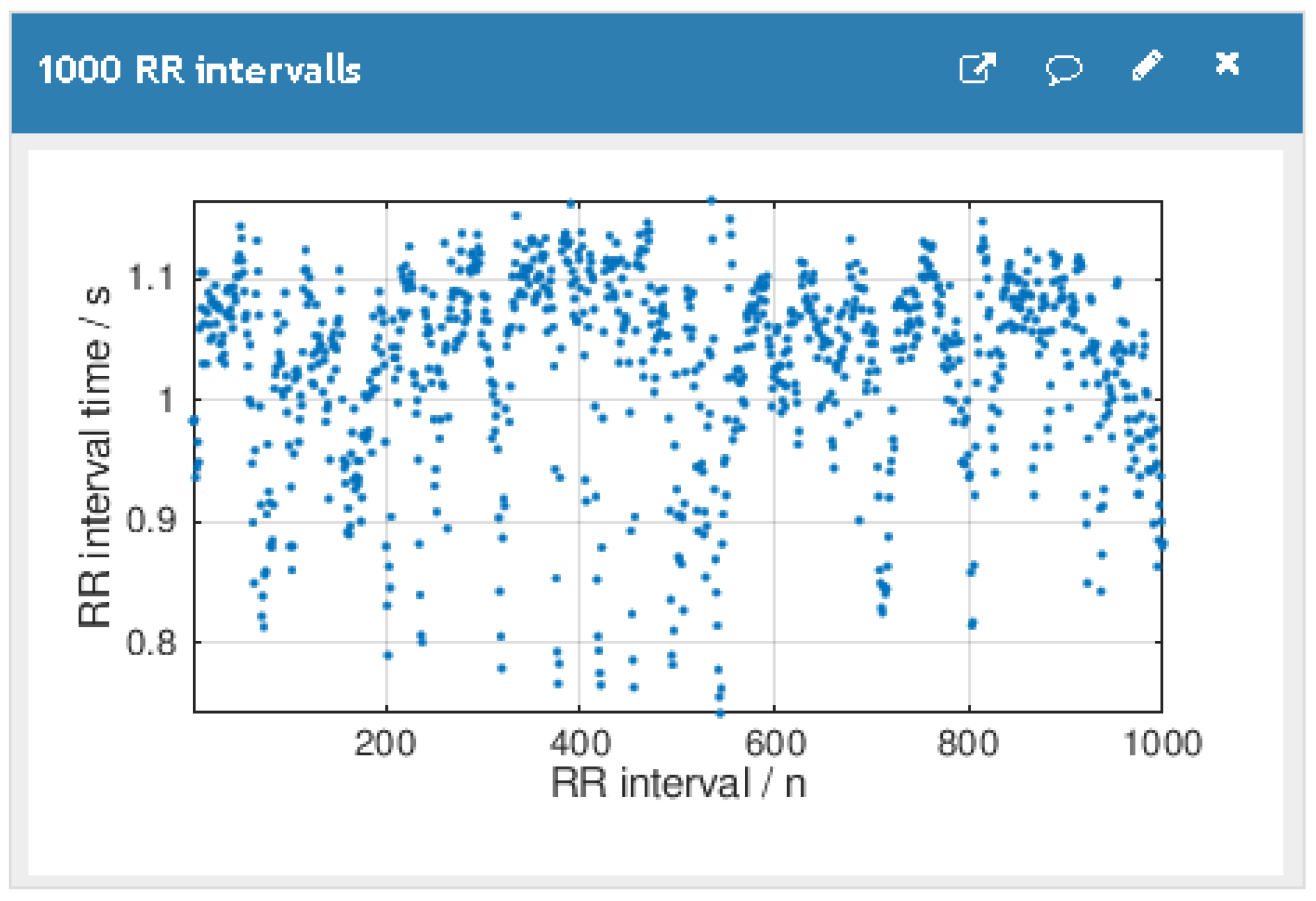
3.1. Real-Time Database
3.2. HeartCare Mobile App
3.3. Cloud Storage
3.4. Patient HR Data Processing in the Cluster
3.5. Physician Support
3.6. Feedback and Intervention
4. Discussion
4.1. Limitations
- (i)
- An alarm message is sent when a dangerous situation arises. Initially, what constitutes a dangerous condition could follow Holter monitoring protocols. For example, an AF event is detected when the estimated AF probability is above 0.5 for at least 30 s [58]. However, it is not known if such an approach is sensitive and indeed specific enough to capture the stroke risk for patients.
- (ii)
- Obtaining necessary regulatory approvals (not just the U.K. and EU) especially as regulatory requirements are increasing significantly with the transition to the much more demanding Medical Device Regulations; this can be a long and iterative process.
- (iii)
- Negotiating and executing mutually beneficial and sustainable agreements with appropriate commercial partners.
- (iv)
- Speed to market; alternative less sophisticated solutions are already available, and new solutions are in development.
4.2. Future Work
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Accuracy |
| AF | Atrial Fibrillation |
| AFDB | Atrial Fibrillation Database |
| AFL | Atrial Flutter |
| AI | Artificial Intelligence |
| CPU | Central Process Unit |
| DB | Database |
| DL | Deep Learning |
| ECG | Electrocardiogram |
| GUI | Graphical User Interface |
| HR | Heart Rate |
| HRVAS | Heart Rate Variability Analysis Software |
| IoT | Internet of Things |
| LSTM | Long Short-Term Memory |
| NSR | Normal Sinus Rhythm |
| RNN | Recurrent Neural Network |
| SEN | Sensitivity |
| SPE | Specificity |
References
- Callow, A.D. Cardiovascular disease 2005—The global picture. Vasc. Pharmacol. 2006, 45, 302–307. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E., III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Daniel, K.; Wolfe, C.D.; Busch, M.A.; McKevitt, C. What are the social consequences of stroke for working-aged adults? A systematic review. Stroke 2009, 40, e431–e440. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.; Murad, S.; Eliahoo, J.; Majeed, A. Stroke incidence and risk factors in a population-based prospective cohort study. Health Stat. Q. 2001, 12, 18–26. [Google Scholar]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–255. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Jamil-Copley, S.; Kanagaratnam, P. Stroke in atrial fibrillation—Hope on the horizon? J. R. Soc. Interface 2010, 7, S765–S769. [Google Scholar] [CrossRef]
- Fitzmaurice, D.A.; Hobbs, F.R.; Jowett, S.; Mant, J.; Murray, E.T.; Holder, R.; Raftery, J.; Bryan, S.; Davies, M.; Lip, G.Y.; et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: Cluster randomised controlled trial. BMJ 2007, 335, 383. [Google Scholar] [CrossRef]
- Cadilhac, D.A. The economics of atrial fibrillation: A time for review and prioritization. Int. J. Stroke 2012, 7, 477–479. [Google Scholar] [CrossRef]
- Public Health England. Atrial Fibrillation Prevalence Estimates in England: Application of Recent Population Estimates of AF in Sweden; Technical Report; National Health Service: London, UK, 2017.
- Kearley, K.; Selwood, M.; Van den Bruel, A.; Thompson, M.; Mant, D.; Hobbs, F.R.; Fitzmaurice, D.; Heneghan, C. Triage tests for identifying atrial fibrillation in primary care: A diagnostic accuracy study comparing single-lead ECG and modified BP monitors. BMJ Open 2014, 4, e004565. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.H.; Kerr, C.R.; Connolly, S.J.; Klein, G.; Boone, J.A.; Green, M.; Sheldon, R.; Talajic, M.; Dorian, P.; Newman, D. New-onset atrial fibrillation: Sex differences in presentation, treatment, and outcome. Circulation 2001, 103, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Faust, O.; Shenfield, A.; Kareem, M.; San, T.R.; Fujita, H.; Acharya, U.R. Automated detection of atrial fibrillation using long short-term memory network with RR interval signals. Comput. Biol. Med. 2018, 102, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Faust, O.; Ciaccio, E.J.; Koh, J.E.W.; Oh, S.L.; San Tan, R.; Garan, H. Application of nonlinear methods to discriminate fractionated electrograms in paroxysmal versus persistent atrial fibrillation. Comput. Methods Programs Biomed. 2019, 175, 163–178. [Google Scholar] [CrossRef]
- Faust, O.; Hagiwara, Y.; Hong, T.J.; Lih, O.S.; Acharya, U.R. Deep learning for healthcare applications based on physiological signals: A review. Comput. Methods Programs Biomed. 2018, 161, 1–13. [Google Scholar] [CrossRef]
- Rajaganeshan, R.; Ludlam, C.; Francis, D.; Parasramka, S.; Sutton, R. Accuracy in ECG lead placement among technicians, nurses, general physicians and cardiologists. Int. J. Clin. Pract. 2008, 62, 65–70. [Google Scholar] [CrossRef]
- Acharya, U.R.; Ghista, D.N.; KuanYi, Z.; Min, L.C.; Ng, E.; Sree, S.V.; Faust, O.; Weidong, L.; Alvin, A. Integrated index for cardiac arrythmias diagnosis using entropies as features of heart rate variability signal. In Proceedings of the 2011 1st Middle East Conference on Biomedical Engineering, Sharjah, UAE, 22–25 February 2011; pp. 371–374. [Google Scholar]
- Nguyen, T.T.; Yuldashev, Z.; Sadykova, E. A remote cardiac rhythm monitoring system for detecting episodes of atrial fibrillation. Biomed. Eng. 2017, 51, 189–194. [Google Scholar] [CrossRef]
- Asgari, S.; Mehrnia, A.; Moussavi, M. Automatic detection of atrial fibrillation using stationary wavelet transform and support vector machine. Comput. Biol. Med. 2015, 60, 132–142. [Google Scholar] [CrossRef]
- Koivisto, T.; Pänkäälä, M.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Saraste, A.; Airaksinen, J. Automatic detection of atrial fibrillation using MEMS accelerometer. In Proceedings of the Computing in Cardiology Conference (CinC), Nice, France, 6–9 September 2015; pp. 829–832. [Google Scholar]
- Harju, J.; Tarniceriu, A.; Parak, J.; Vehkaoja, A.; Yli-Hankala, A.; Korhonen, I. Monitoring of heart rate and inter-beat intervals with wrist plethysmography in patients with atrial fibrillation. Physiol. Meas. 2018, 39, 065007. [Google Scholar] [CrossRef]
- Larburu, N.; Lopetegi, T.; Romero, I. Comparative study of algorithms for atrial fibrillation detection. In Proceedings of the Computing in Cardiology, Hangzhou, China, 18–21 September 2011; pp. 265–268. [Google Scholar]
- Erl, T. SOA Principles of Service Design (Paperback); Prentice Hall Press: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
- Faust, O.; Lei, N.; Chew, E.; Ciaccio, E.J.; Acharya, U.R. A Smart Service Platform for Cost Efficient Cardiac Health Monitoring. Int. J. Environ. Res. Public Health 2020, 17, 6313. [Google Scholar] [CrossRef]
- Stickdorn, M.; Hormess, M.E.; Lawrence, A.; Schneider, J. This Is Service Design Doing: Applying Service Design Thinking in the Real World; O’Reilly Media, Inc.: Newton, MA, USA, 2018. [Google Scholar]
- Ali, A.N.; Abdelhafiz, A. Clinical and economic implications of AF related stroke. J. Atr. Fibrillation 2016, 8, 1279. [Google Scholar] [PubMed]
- Romero, J.R.; Wolf, P.A. Epidemiology of stroke: Legacy of the Framingham Heart Study. Glob. Heart 2013, 8, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Paszkiel, S. Using BCI in IoT Implementation. In Analysis and Classification of EEG Signals for Brain—Computer Interfaces; Springer: Berlin/Heidelberg, Germany, 2020; pp. 111–128. [Google Scholar]
- Paszkiel, S. Using Neural Networks for Classification of the Changes in the EEG Signal Based on Facial Expressions. In Analysis and Classification of EEG Signals for Brain–Computer Interfaces; Springer: Berlin/Heidelberg, Germany, 2020; pp. 41–69. [Google Scholar]
- Faust, O.; Ciaccio, E.J.; Acharya, U.R. A Review of Atrial Fibrillation Detection Methods as a Service. Int. J. Environ. Res. Public Health 2020, 17, 3093. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S. ThingSpeak based sensing and monitoring system for IoT with Matlab Analysis. Int. J. New Technol. Res. 2016, 2, 19–23. [Google Scholar]
- Faust, O.; Yu, W.; Acharya, U.R. The role of real-time in biomedical science: A meta-analysis on computational complexity, delay and speedup. Comput. Biol. Med. 2015, 58, 73–84. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Moody, G.B.; Mark, R.G. A new method for detecting atrial fibrillation using R-R intervals. Comput. Cardiol. 1983, 10, 227–230. [Google Scholar]
- Yadav, S.; Shukla, S. Analysis of k-fold cross-validation over hold-out validation on colossal datasets for quality classification. In Proceedings of the 2016 IEEE 6th International Conference on Advanced Computing (IACC), Bhimavaram, India, 27–28 February 2016; pp. 78–83. [Google Scholar]
- Faust, O.; Kareem, M.; Shenfield, A.; Ali, A.; Acharya, U.R. Validating the robustness of an internet of things based atrial fibrillation detection system. Pattern Recognit. Lett. 2020, 133, 55–61. [Google Scholar] [CrossRef]
- Lih, O.S.; Jahmunah, V.; San, T.R.; Ciaccio, E.J.; Yamakawa, T.; Tanabe, M.; Kobayashi, M.; Faust, O.; Acharya, U.R. Comprehensive electrocardiographic diagnosis based on deep learning. Artif. Intell. Med. 2020, 103, 101789. [Google Scholar] [CrossRef]
- Ramshur, J.T. Design, Evaluation, and Applicaion of Heart Rate Variability Analysis Software (HRVAS). Master’s Thesis, The University of Memphis, Memphis, TN, USA, 2010. [Google Scholar]
- Faust, O. Improving the safety of atrial fibrillation monitoring systems through human verification. Biomed. Signal Process. Control. 2014, 13, 295–305. [Google Scholar] [CrossRef]
- Faust, O.; Ciaccio, E.J.; Majid, A.; Acharya, U.R. Improving the safety of atrial fibrillation monitoring systems through human verification. Saf. Sci. 2019, 118, 881–886. [Google Scholar] [CrossRef]
- Kareem, M.; Faust, O. Establishing the safety of a smart heart health monitoring service through validation. In Proceedings of the 2019 IEEE International Conference on Big Data (Big Data), Los Angeles, CA, USA, 9–12 December 2019; pp. 6089–6091. [Google Scholar]
- Faust, O.; Kareem, M.; Ali, A.; Ciaccio, E.J.; Acharya, U.R. Automated arrhythmia detection based on RR-intervals. Knowl. Based Syst. 2020. under review. [Google Scholar]
- Ivanovic, M.D.; Atanasoski, V.; Shvilkin, A.; Hadzievski, L.; Maluckov, A. Deep Learning Approach for Highly Specific Atrial Fibrillation and Flutter Detection based on RR Intervals. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1780–1783. [Google Scholar]
- Fujita, H.; Cimr, D. Computer aided detection for fibrillations and flutters using deep convolutional neural network. Inf. Sci. 2019, 486, 231–239. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Lih, O.S.; Hagiwara, Y.; Tan, J.H.; Adam, M. Automated detection of arrhythmias using different intervals of tachycardia ECG segments with convolutional neural network. Inf. Sci. 2017, 405, 81–90. [Google Scholar] [CrossRef]
- Henzel, N.; Wróbel, J.; Horoba, K. Atrial fibrillation episodes detection based on classification of heart rate derived features. In Proceedings of the 2017 MIXDES-24th International Conference Mixed Design of Integrated Circuits and Systems, Bydgoszcz, Poland, 22–24 June 2017; pp. 571–576. [Google Scholar]
- Desai, U.; Martis, R.J.; Acharya, U.R.; Nayak, C.G.; Seshikala, G.; SHETTY K, R. Diagnosis of multiclass tachycardia beats using recurrence quantification analysis and ensemble classifiers. J. Mech. Med. Biol. 2016, 16, 1640005. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Adam, M.; Lih, O.S.; Hong, T.J.; Sudarshan, V.K.; Koh, J.E. Automated characterization of arrhythmias using nonlinear features from tachycardia ECG beats. In Proceedings of the 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Budapest, Hungary, 9–12 October 2016; pp. 533–538. [Google Scholar]
- Hamed, I.; Owis, M.I. Automatic arrhythmia detection using support vector machine based on discrete wavelet transform. J. Med. Imaging Health Inform. 2016, 6, 204–209. [Google Scholar] [CrossRef]
- Xia, Y.; Wulan, N.; Wang, K.; Zhang, H. Detecting atrial fibrillation by deep convolutional neural networks. Comput. Biol. Med. 2018, 93, 84–92. [Google Scholar] [CrossRef]
- Petrėnas, A.; Marozas, V.; Sörnmo, L. Low-complexity detection of atrial fibrillation in continuous long-term monitoring. Comput. Biol. Med. 2015, 65, 184–191. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, H.; Ung, B.; Pickwell-MacPherson, E.; Zhang, Y. Automatic online detection of atrial fibrillation based on symbolic dynamics and Shannon entropy. Biomed. Eng. Online 2014, 13, 18. [Google Scholar] [CrossRef]
- Muthuchudar, A.; Baboo, S.S. A study of the processes involved in ECG signal analysis. Int. J. Sci. Res. Publ. 2013, 3, 1–5. [Google Scholar]
- Yuan, C.; Yan, Y.; Zhou, L.; Bai, J.; Wang, L. Automated atrial fibrillation detection based on deep learning network. In Proceedings of the 2016 IEEE International Conference on Information and Automation (ICIA), Ningbo, China, 31 July–4 August 2016; pp. 1159–1164. [Google Scholar]
- Pudukotai Dinakarrao, S.M.; Jantsch, A. ADDHard: Arrhythmia detection with digital hardware by learning ECG signal. In Proceedings of the 2018 on Great Lakes Symposium on VLSI, Chicago, IL, USA, 23–25 May 2018; pp. 495–498. [Google Scholar]
- Salem, M.; Taheri, S.; Yuan, J. ECG Arrhythmia Classification Using Transfer Learning from 2- Dimensional Deep CNN Features. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Jawad-Ul-Qamar, M.; Chua, W.; Purmah, Y.; Nawaz, M.; Varma, C.; Davis, R.; Maher, A.; Fabritz, L.; Kirchhof, P. Detection of unknown atrial fibrillation by prolonged ECG monitoring in an all-comer patient cohort and association with clinical and Holter variables. Open Heart 2020, 7, e001151. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Catanese, L.; Perera, K.S.; Ntaios, G.; Connolly, S.J. Embolic stroke of undetermined source: A systematic review and clinical update. Stroke 2017, 48, 867–872. [Google Scholar] [CrossRef] [PubMed]
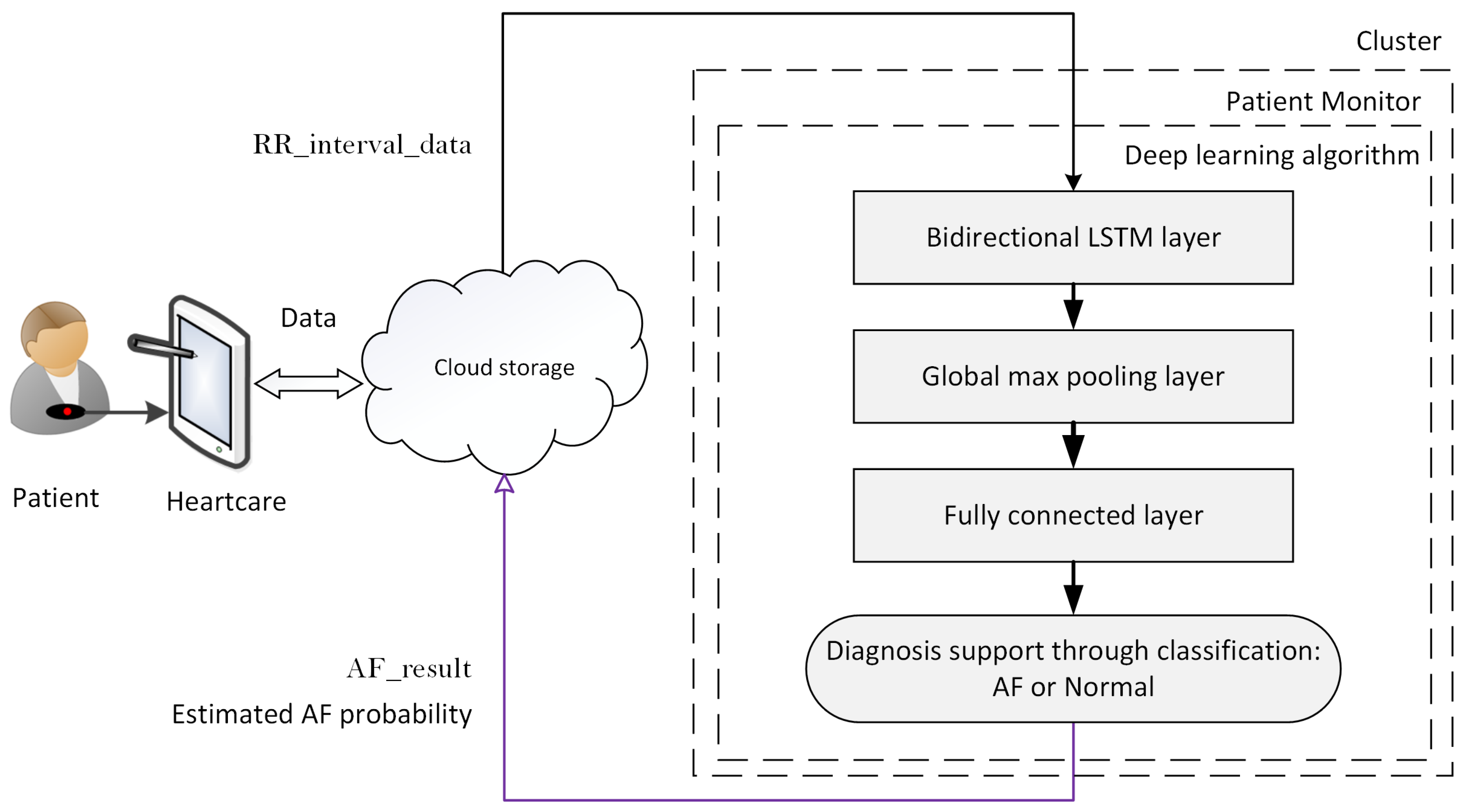
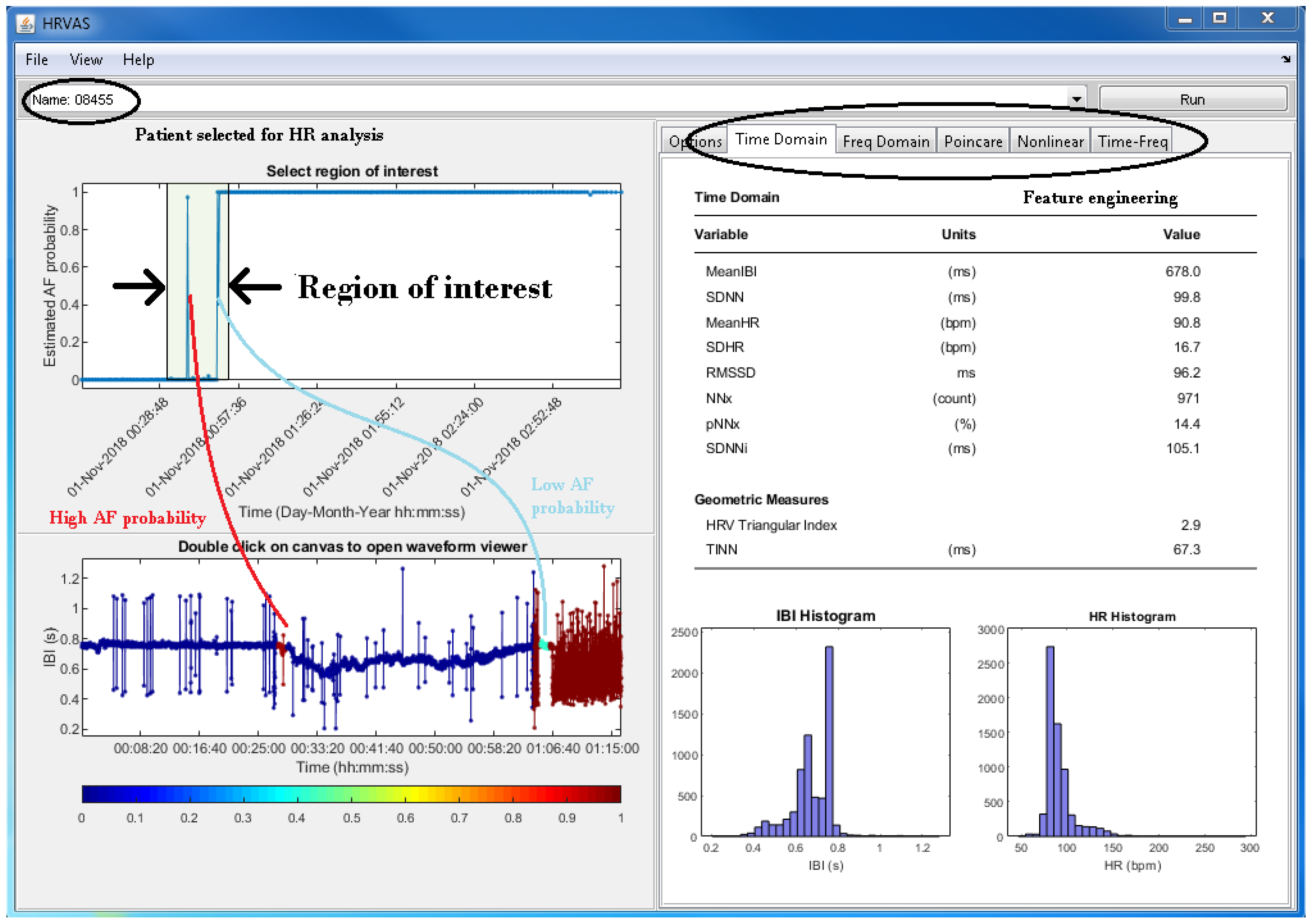
| Stakeholders | Needs and Wants |
|---|---|
| Patients | Reduced stroke risk, less clinical visits, mobility, safety |
| Physicians | Improved clinical outcomes, high quality diagnosis, safety, reduced workload |
| Healthcare providers | High efficiency and quality, improved productivity and outcomes, cost effectiveness |
| Stroke risk monitoring service innovators | Profitability, improved outcome |
| Service | Requirement | Value Proposition |
|---|---|---|
| A | Cost efficient and decision support quality | More infrastructure to help a larger number of patients |
| B | Raise an alarm when AF is detected | Establishing and communicating a suspicion that AF is present in real time |
| C | Present the evidence for raising the alarm | Providing an overview of the estimated AF probability; this can be used to review the DL results that established a suspicion and triggered an alarm message |
| D | Allow selecting a time interval of interest; subsequently, the corresponding HR trace can be analysed | Download the HR trace that corresponds to the selected time interval of interest, and calculate features from that HR trace |
| E | Provide a feedback channel to the patient | Act on the diagnosis by providing appropriate and timely feedback to the patient; act on meta data, such as data stream interruptions, to ensure patient compliance |
| Author Year | Method | Data | Performance | ||||
|---|---|---|---|---|---|---|---|
| Type | DB | Rhythm | ACC | SPE | SEN | ||
| Faust et al. 2020 [43] | Detrending, ResNet | HR | ecgdb | AF Atrial Flutter (AFL) Normal Sinus Rhythm (NSR) | 99.98 | 100.00 | 99.94 |
| Ivanovic et al., 2019 [44] | CNN, LSTM | HR | Hospital | NSR, AF AFL | 88 | 87.09 | |
| Fujita and Cimr, 2019 [45] | CNN with normalization | ECG | afdb, mitdb, vfdb | AF, AFL, VFDB, NSR | 98.45 | 99.87 | 99.27 |
| Faust et al., 2018 [14] | LSTM | HR | afdb | AF NSR | 98.39 | 98.32 | 98.51 |
| Acharya et al., 2017 [46] | CNN with Z-score | ECG | afdb, mitdb, vfdb | AF, AFL, VFIB, NSR | 92.50 | 98.09 | 93.13 |
| Henzel et al., 2017 [47] | Statistical features with generalized linear model | HR | afdb | AF NSR | 93 | 95 | 90 |
| Desai et al., 2016 [48] | RQAwith decision tree, random forest, rotation forest | ECG | afdb, mitdb, vfdb | AF, AFL, VFIB, NSR | 98.37 | ||
| Acharya et al., 2016 [49] | Thirteen nonlinear features with ANOVA with KNN and DT | ECG | afdb, mitdb, vfdb | AF, AFL, VFIB, NSR | 97.78 | 99.76 | 98.82 |
| Hamed and Owis, 2016 [50] | DWT, PCA and SVM | ECG | afdb | AF, AFL, NSR | 98.43 | 96.89 | 98.96 |
| Xia et al., 2018 [51] | STFT/SWTwith CNN | ECG | afdb | AF | 98.63 | 98.79 | 97.87 |
| Petrėnas et al., 2015 [52] | Median filter with threshold | HR | nsrdb, afdb | AF NSR | 98.3 | 97.1 | |
| Zhou et al., 2014 [53] | Median filter and Shannon entropy with threshold | HR | ltafdb, afdb, nsrdb | AF NSR | 96.05 | 95.07 | 96.72 |
| Muthuchudar and Baboo, 2013 [54] | UWT NN | ECG | afdb | AF, VFIB, NSR | 96 | ||
| Yuanet al., 2016 [55] | Unsupervised autoencoder NN Softmax regression | ECG | afdb, nsrdb, ltdb, hospital | AF | 98.18 | 98.22 | 98.11 |
| Pudukotai Dinakarrao and Jantsch, 2018 [56] | Daubechies-6 with counters Anomaly detector | ECG | mitdb | AF, VFIB | 99.19 | 98.25 | 78.70 |
| Salem et al., 2018 [57] | Spectrogram with CNN | ECG | afdb, nsrdb, vfdb and edb | AF, AFL VFIB NSR | 97.23 | ||
| Service | Apple Watch and iPhone | KardiaMobile with KardiaPro | Holter Monitor with CardioScan | |
|---|---|---|---|---|
| Performance evaluation | ||||
| Quality | PPV: 95.40% | PPV: 71% (pulse) | 8% AF yield | N/R |
| No. of patients | 82 | N/R | 50 | N/R |
| Dataset | AFDB and LTAFDB | Measurement data | Measurement data | Measurement data |
| System properties | ||||
| Signal | Heart rate | ECG | Finger ECG | ECG |
| Processing | Cloud server | Local | Cloud server | Local |
| Real-time | Yes | Yes | Yes | No |
| Diagnosis | Symbiosis between physician and DL | None | None | Feature support |
| Data storage | Unlimited | None | Snippets | Limited |
| Model update | Retraining the DL model with cloud data | None | None | None |
| Use case scenario | ||||
| Customer | Healthcare provider | Patient | Patient | Healthcare provider |
| Physical equipment | Heart rate sensor and Android phone | Apple Watch and iPhone | KardiaMobile device | Holter monitor |
| Measurement | Patient led | Patient led | Patient led | Expert led |
| Result | Diagnosis DL decision validated by a physician | Suspicion black box decision; follow-up with Holter recording for diagnosis | Suspicion black box decision; no clear follow-up | Diagnosis established by a physician with analysis support |
| Limitations | ||||
| Diagnosis | HR for diagnosis support is a new paradigm | No diagnosis; diagnosis is established through Holter recordings | No diagnosis | Inter- and intra-observer variability; labour intensive |
| Safety | Human and machine | Not critical | Not critical | Human |
| Cost | ||||
| Hardware | £ 300 | £ 1000 | £ 99 and mobile cost | £ 1885.00 |
| Service | £ 30/month | Free | £ 9.99/month | £ 50 for 10 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, N.; Kareem, M.; Moon, S.K.; Ciaccio, E.J.; Acharya, U.R.; Faust, O. Hybrid Decision Support to Monitor Atrial Fibrillation for Stroke Prevention. Int. J. Environ. Res. Public Health 2021, 18, 813. https://doi.org/10.3390/ijerph18020813
Lei N, Kareem M, Moon SK, Ciaccio EJ, Acharya UR, Faust O. Hybrid Decision Support to Monitor Atrial Fibrillation for Stroke Prevention. International Journal of Environmental Research and Public Health. 2021; 18(2):813. https://doi.org/10.3390/ijerph18020813
Chicago/Turabian StyleLei, Ningrong, Murtadha Kareem, Seung Ki Moon, Edward J. Ciaccio, U Rajendra Acharya, and Oliver Faust. 2021. "Hybrid Decision Support to Monitor Atrial Fibrillation for Stroke Prevention" International Journal of Environmental Research and Public Health 18, no. 2: 813. https://doi.org/10.3390/ijerph18020813
APA StyleLei, N., Kareem, M., Moon, S. K., Ciaccio, E. J., Acharya, U. R., & Faust, O. (2021). Hybrid Decision Support to Monitor Atrial Fibrillation for Stroke Prevention. International Journal of Environmental Research and Public Health, 18(2), 813. https://doi.org/10.3390/ijerph18020813







