An Investigation of the Wild Rat Crown Incisor as an Indicator of Lead (Pb) Exposure Using Inductively Couple Plasma Mass Spectrometry (ICP-MS) and Laser Ablation ICP-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals and Exposure
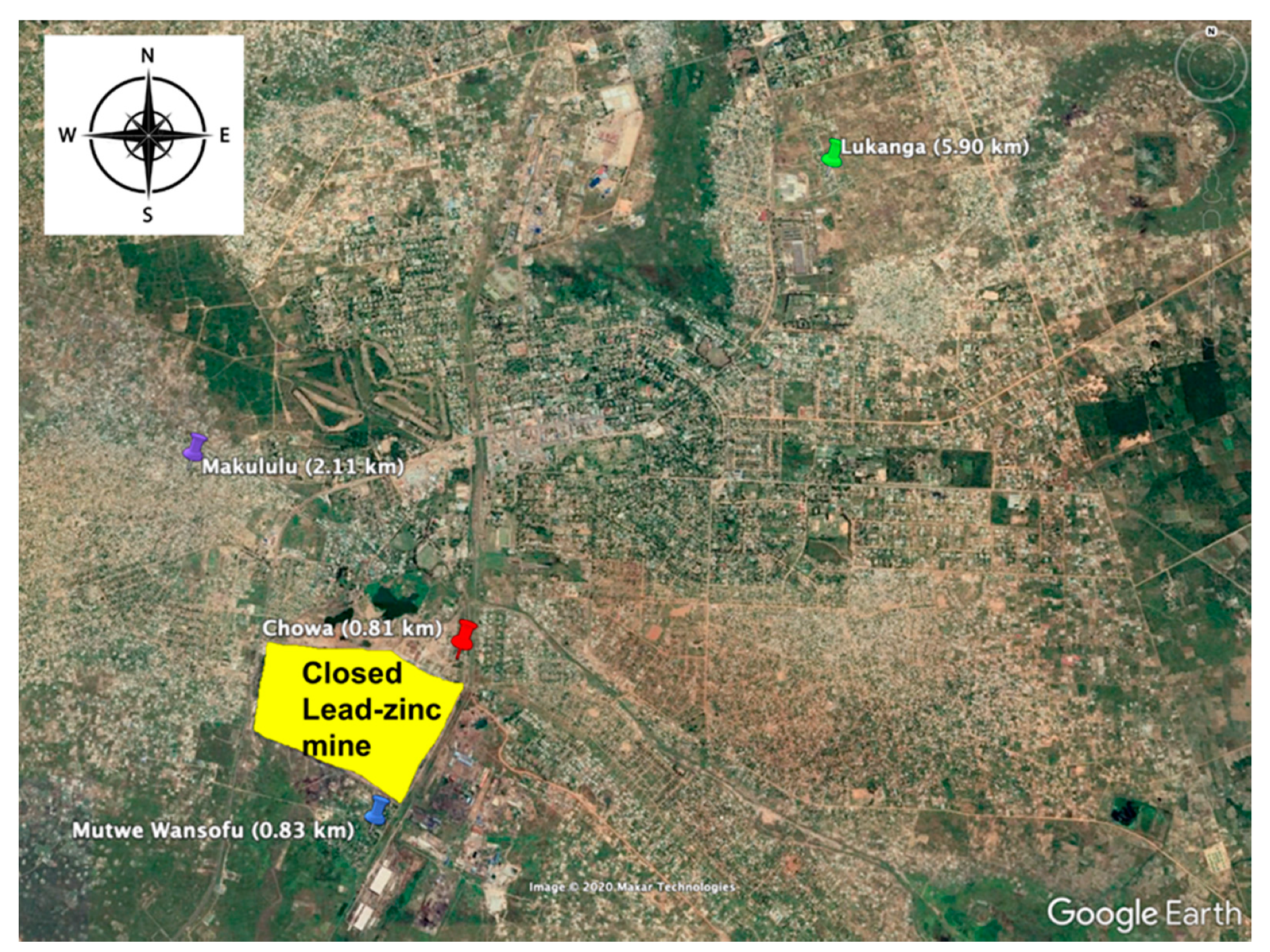
2.2. Wild Rat Sampling and Species Identification
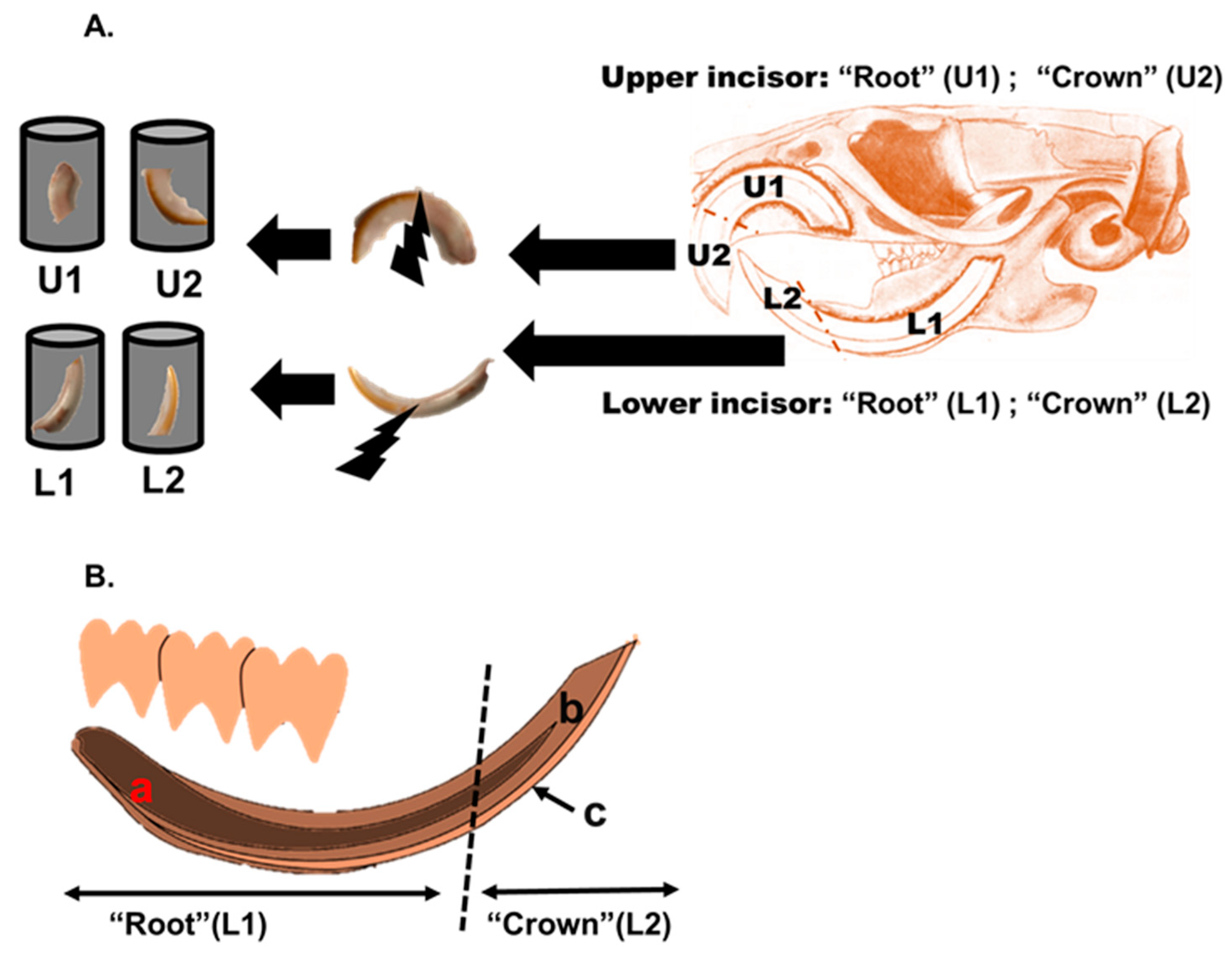
2.3. Digestion and Quantitative Analysis of Lead in Blood and Teeth Subdivisions
2.4. Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) Analysis of Incisor Teeth of Sprague Dawley and R. rattus Rats
2.5. Data Analysis
3. Results
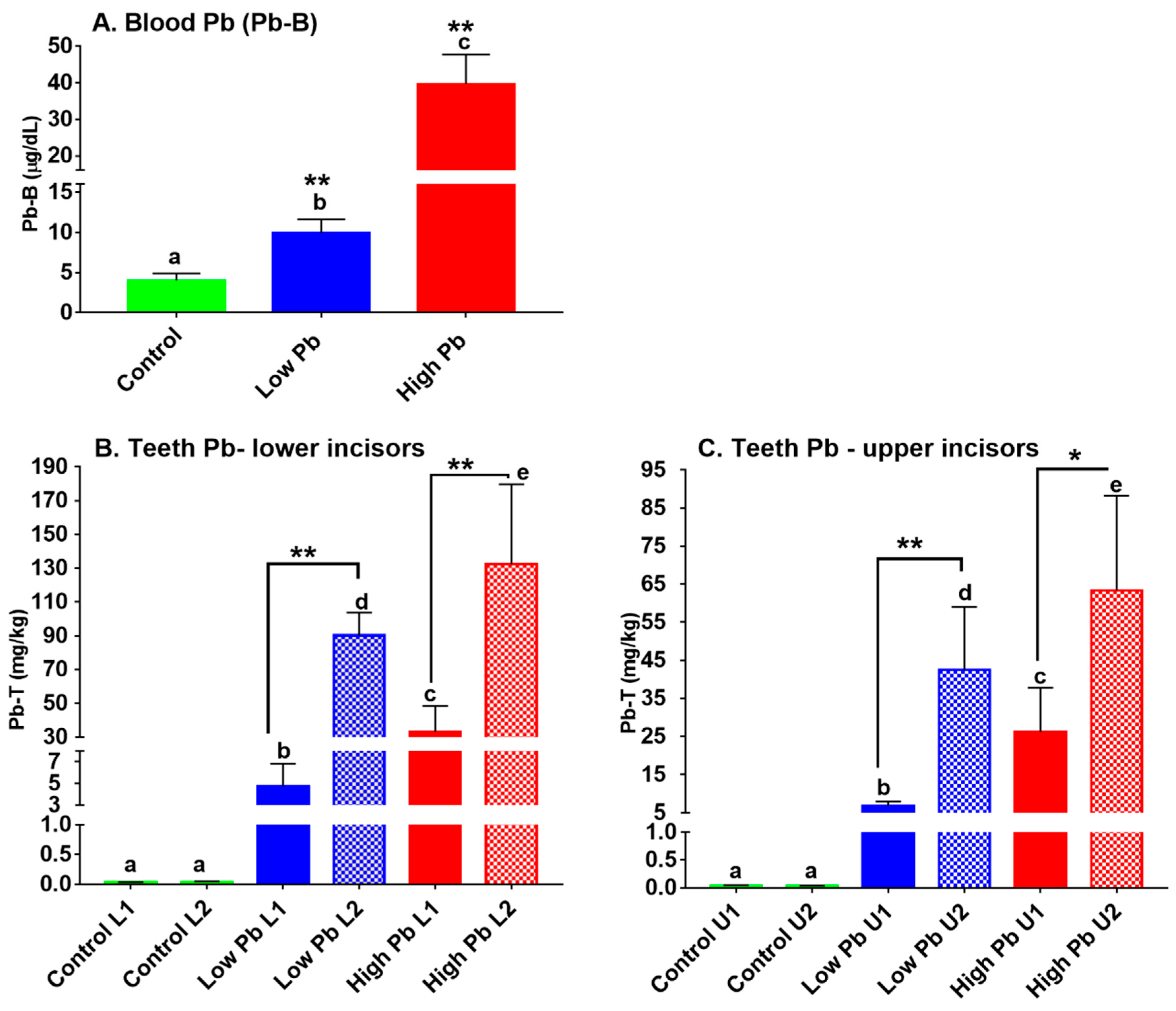
3.1. Lead in Blood (Pb-B) and Incisors Subdivisions of Laboratory-Exposed Sprague Dawley Rats
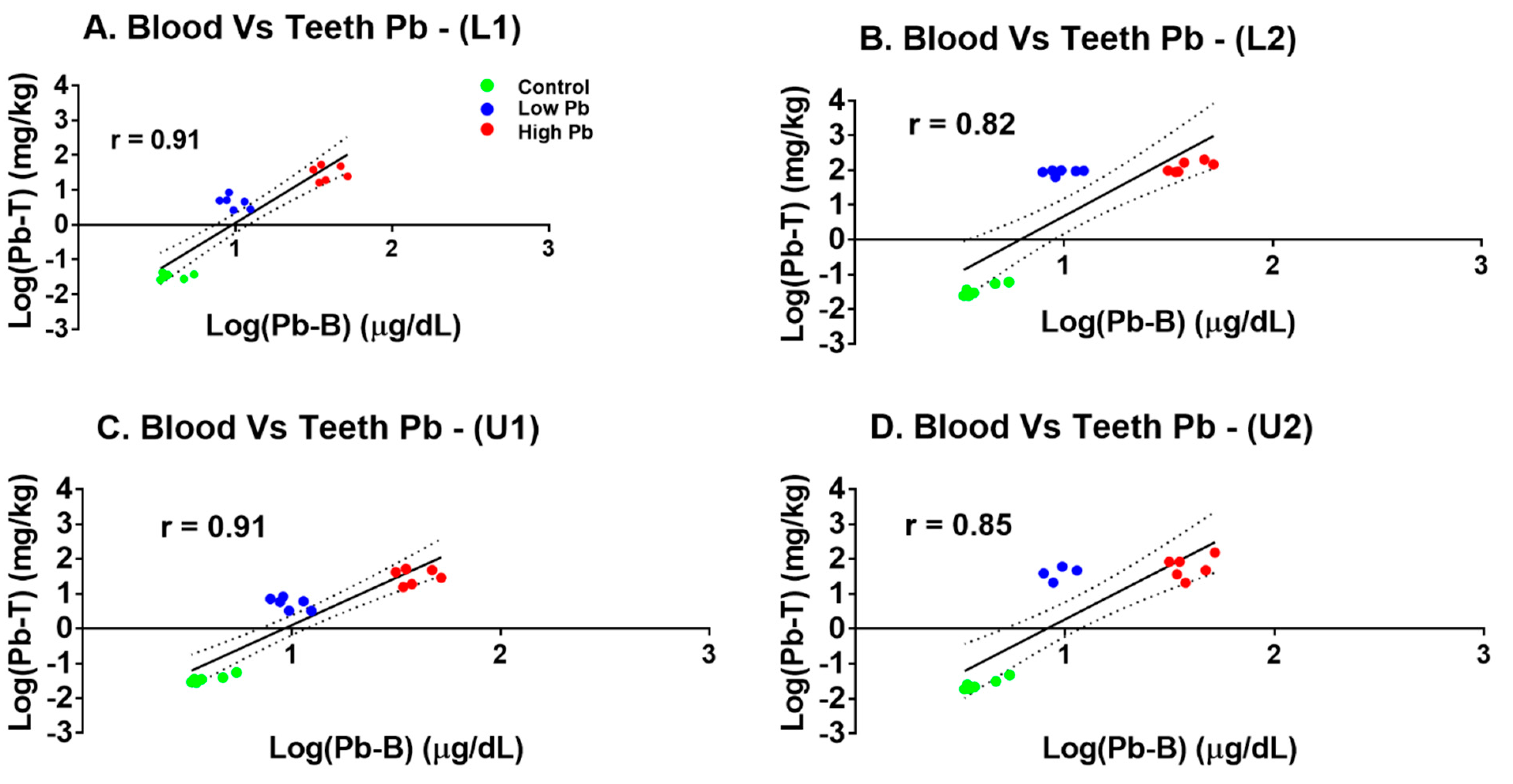
3.2. Relationship between Incisor Teeth Parts Pb and Blood Pb in the Laboratory Exposed Sprague Dawley Rats
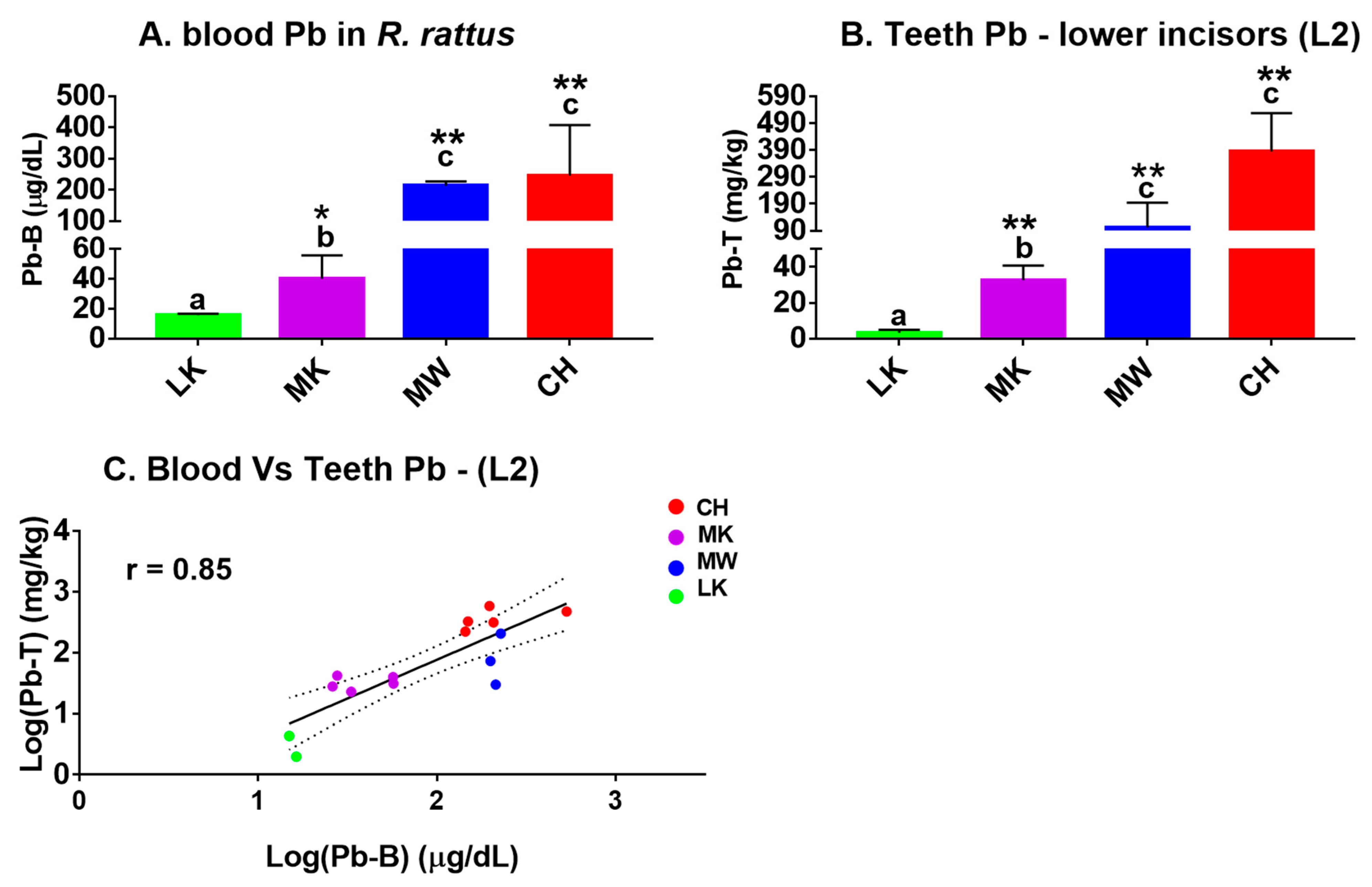
3.3. Lead in Blood and Teeth and Their Relationship in the Lead-Exposed R. rattus Rats
3.4. Lead Distribution in the Incisor Teeth of the Lead-Exposed Laboratory Sprague Dawley and Wild R. rattus Rats Using LA-ICP-MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Wild Rat Species Identification
References
- Galal, M.K.; Elleithy, E.M.; Abdrabou, M.I.; Yasin, N.A.; Shaheen, Y.M. Modulation of caspase-3 gene expression and protective effects of garlic and spirulina against CNS neurotoxicity induced by lead exposure in male rats. NeuroToxicology 2019, 72, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Dapul, H.; Laraque, D. Lead Poisoning in Children. Adv. Pediatr. 2014, 61, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Bose-O’Reilly, S.; Yabe, J.; Makumba, J.; Schutzmeier, P.; Ericson, B.; Caravanos, J. Lead intoxicated children in Kabwe, Zambia. Environ. Res. 2018, 165, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Yabe, J.; Nakayama, S.; Ikenaka, Y.; Yohannes, Y.B.; Bortey-Sam, N.; Oroszlany, B.; Muzandu, K.; Choongo, K.; Kabalo, A.N.; Ntapisha, J.; et al. Lead poisoning in children from townships in the vicinity of a lead–zinc mine in Kabwe, Zambia. Chemosphere 2015, 119, 941–947. [Google Scholar] [CrossRef]
- Yabe, J.; Nakayama, S.M.; Nakata, H.; Toyomaki, H.; Yohannes, Y.B.; Muzandu, K.; Kataba, A.; Zyambo, G.; Hiwatari, M.; Narita, D.; et al. Current trends of blood lead levels, distribution patterns and exposure variations among household members in Kabwe, Zambia. Chemosphere 2020, 243, 125412. [Google Scholar] [CrossRef]
- Dooyema, C.A.; Neri, A.; Lo, Y.; Durant, J.; Dargan, P.I.; Swarthout, T.; Biya, O.; Gidado, S.O.; Haladu, S.; Sani-Gwarzo, N.; et al. Outbreak of Fatal Childhood Lead Poisoning Related to Artisanal Gold Mining in Northwestern Nigeria, 2010. Environ. Health Perspect. 2012, 120, 601–607. [Google Scholar] [CrossRef]
- Haeflinger, P.; Mathieu-Nolf, M.; Lociciro, S.; Ndiaye, C.; Coly, M.; Diouf, A.; Faye, L.A.; Sow, A.; Tempowski, J.; Pronczuk, J.; et al. Mass Lead Intoxication from Informal Used Lead-Acid Battery Recycling in Darkar, Senegal. Environ. Health Perspect. 2009, 117. [Google Scholar] [CrossRef]
- Bellinger, D.C. Childhood Lead Exposure and Adult Outcomes. JAMA 2017, 317, 1219–1220. [Google Scholar] [CrossRef]
- Chang, S.-H.; Cheng, B.-H.; Lee, S.-L.; Chuang, H.-Y.; Yang, C.-Y.; Sung, F.-C.; Wu, T.-N. Low blood lead concentration in association with infertility in women. Environ. Res. 2006, 101, 380–386. [Google Scholar] [CrossRef]
- Barbosa, F.; Tanus-Santos, J.E.; Gerlach, R.F.; Parsons, P.J. A Critical Review of Biomarkers Used for Monitoring Human Exposure to Lead: Advantages, Limitations, and Future Needs. Environ. Health Perspect. 2005, 113, 1669–1674. [Google Scholar] [CrossRef]
- De Figueiredo, F.A.T.; Ramos, J.; Kawakita, E.R.H.; Bilal, A.S.; De Sousa, F.B.; Swaim, W.D.; Issa, J.P.M.; Gerlach, R.F. Lead line in rodents: An old sign of lead intoxication turned into a new method for environmental surveillance. Environ. Sci. Pollut. Res. 2016, 23, 21475–21484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Specht, A.J.; Liu, Y.; Finney, L.; Maxey, E.; Vogt, S.; Zheng, W.; Weisskopf, M.; Nie, L.H. Microdistribution of lead in human teeth using microbeam synchrotron radiation X-ray fluorescence (μ-SRXRF). X-ray Spectrom. 2016, 46, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E. Low Level Environmental Lead Exposure—A Continuing Challenge. Clin. Biochem. Rev. 2008, 29, 63–70. [Google Scholar] [PubMed]
- Steenhout, A.; Pourtois, M. Lead accumulation in teeth as a function of age with different exposures. Occup. Environ. Med. 1981, 38, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Winneke, G.; Hrdina, K.-G.; Brockhaus, A. Neuropsychological studies in children with elevated tooth-lead concentrations. Int. Arch. Occup. Environ. Health 1982, 51, 169–183. [Google Scholar] [CrossRef]
- Arora, M.; Hare, D.J. Tooth lead levels as an estimate of lead body burden in rats following pre- and neonatal exposure. RSC Adv. 2015, 5, 67308–67314. [Google Scholar] [CrossRef]
- Evans, R.D.; Richner, P.; Outridge, P.M. Micro-spatial variations of heavy metals in the teeth of walrus as determined by laser ablation ICP-MS: The potential for reconstructing a history of metal exposure. Arch. Environ. Contam. Toxicol. 1995, 28, 55–60. [Google Scholar] [CrossRef]
- Cox, A.; Keenan, F.; Cooke, M.; Appleton, J. Trace element profiling of dental tissues using laser ablation-inductively coupled plasma-mass spectrometry. Anal. Bioanal. Chem. 1996, 354, 254–258. [Google Scholar] [CrossRef]
- Uryu, T.; Yoshinaga, J.; Yanagisawa, Y.; Endo, M.; Takahashi, J. Analysis of Lead in Tooth Enamel by Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Anal. Sci. 2003, 19, 1413–1416. [Google Scholar] [CrossRef][Green Version]
- Yohannes, Y.B.; Ikenaka, Y.; Ito, G.; Nakayama, S.M.M.; Mizukawa, H.; Wepener, V.; Smit, N.J.; Van Vuren, J.H.J.; Ishizuka, M. Assessment of DDT contamination in house rat as a possible bioindicator in DDT-sprayed areas from Ethiopia and South Africa. Environ. Sci. Pollut. Res. 2017, 24, 23763–23770. [Google Scholar] [CrossRef]
- Ardizzone, M.; Vizio, C.; Bozzetta, E.; Pezzolato, M.; Meistro, S.; Dondo, A.; Giorgi, I.; Seghesio, A.; Mirabelli, D.; Capella, S.; et al. The wild rat as sentinel animal in the environmental risk assessment of asbestos pollution: A pilot study. Sci. Total Environ. 2014, 479, 31–38. [Google Scholar] [CrossRef]
- Martiniakova, M.; Omelka, R.; Jančová, A.; Stawarz, R.; Formicki, G. Concentrations of Selected Heavy Metals in Bones and Femoral Bone Structure of Bank (Myodes glareolus) and Common (Microtus arvalis) Voles from Different Polluted Biotopes in Slovakia. Arch. Environ. Contam. Toxicol. 2011, 60, 524–532. [Google Scholar] [CrossRef]
- Togao, M.; Nakayama, S.; Ikenaka, Y.; Mizukawa, H.; Makino, Y.; Kubota, A.; Matsukawa, T.; Yokoyama, K.; Hirata, T.; Ishizuka, M. Bioimaging of Pb and STIM1 in mice liver, kidney and brain using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and immunohistochemistry. Chemosphere 2020, 238, 124581. [Google Scholar] [CrossRef]
- Ikenaka, Y.; Nakayama, S.M.M.; Muzandu, K.; Choongo, K.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Heavy Metal Contamination of Soil and Sediment in Zambia. Afr. J. Environ. Sci. Technol. 2010, 4, 729–739. [Google Scholar]
- Nakayama, S.; Ikenaka, Y.; Hamada, K.; Muzandu, K.; Choongo, K.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Metal and metalloid contamination in roadside soil and wild rats around a Pb–Zn mine in Kabwe, Zambia. Environ. Pollut. 2011, 159, 175–181. [Google Scholar] [CrossRef]
- Robins, J.H.; Hingston, M.; Matisoo-Smith, E.; Ross, H.A. Identifying Rattus species using mitochondrial DNA. Mol. Ecol. Notes 2007, 7, 717–729. [Google Scholar] [CrossRef]
- Nakata, H.; Nakayama, S.; Yabe, J.; Liazambi, A.; Mizukawa, H.; Darwish, W.S.; Ikenaka, Y.; Ishizuka, M. Reliability of stable Pb isotopes to identify Pb sources and verifying biological fractionation of Pb isotopes in goats and chickens. Environ. Pollut. 2016, 208, 395–403. [Google Scholar] [CrossRef]
- Nakata, H.; Nakayama, S.M.M.; Oroszlany, B.; Ikenaka, Y.; Mizukawa, H.; Tanaka, K.; Harunari, T.; Tanikawa, T.; Darwish, W.S.; Yohannes, Y.B.; et al. Monitoring Lead (Pb) Pollution and Identifying Pb Pollution Sources in Japan Using Stable Pb Isotope Analysis with Kidneys of Wild Rats. Int. J. Environ. Res. Public Health 2017, 14, 56. [Google Scholar] [CrossRef]
- Ishii, C.; Nakayama, S.M.; Kataba, A.; Ikenaka, Y.; Saito, K.; Watanabe, Y.; Makino, Y.; Matsukawa, T.; Kubota, A.; Yokoyama, K.; et al. Characterization and imaging of lead distribution in bones of lead-exposed birds by ICP-MS and LA-ICP-MS. Chemosphere 2018, 212, 994–1001. [Google Scholar] [CrossRef]
- Mancinelli, E.; Capello, V. Anatomy and Disorders of the Oral Cavity of Rat-like and Squirrel-like Rodents. Veter- Clin. North Am. Exot. Anim. Pr. 2016, 19, 871–900. [Google Scholar] [CrossRef]
- Park, M.K.; Min, S.-Y.; Song, J.S.; Lee, J.-H.; Jung, H.-S.; Kim, S.-O. Estimated Time of Biomineralization in Developing Rat Incisors. J. Korean Acad. Pedtatric Dent. 2017, 44, 138–146. [Google Scholar] [CrossRef]
- Suzuki, T.; Sakata, S.; Makino, Y.; Obayashi, H.; Ohara, S.; Hattori, K.; Hirata, T. iQuant2: Software for Rapid and Quantitative Imaging Using Laser Ablation-ICP Mass Spectrometry. Mass Spectrom. 2018, 7, A0065. [Google Scholar] [CrossRef]
- Rabinowitz, M. Relating tooth and blood lead levels in children. Bull. Environ. Contam. Toxicol. 1995, 55, 853–857. [Google Scholar] [CrossRef]
- Bellis, D.J.; Hetter, K.M.; Jones, J.; Amarasiriwardena, D.; Parsons, P.J. Lead in teeth from lead-dosed goats: Microdistribution and relationship to the cumulative lead dose. Environ. Res. 2008, 106, 34–41. [Google Scholar] [CrossRef][Green Version]
- Grobler, S.; Theunissen, F.S.; Kotze, T. The relation between lead concentrations in human dental tissues and in blood. Arch. Oral Biol. 2000, 45, 607–609. [Google Scholar] [CrossRef]
- Teeth as Indicators of Environmental Pollution with Lead. J. Environ. Anal. Toxicol. 2012, 2, 1–5. [CrossRef]
- Barton, H.J. Advantages of the Use of Deciduous Teeth, Hair, and Blood Analysis for Lead and Cadmium Bio-Monitoring in Children. A Study of 6-Year-Old Children from Krakow (Poland). Biol. Trace Element Res. 2010, 143, 637–658. [Google Scholar] [CrossRef]
- Johnston, J.E.; Franklin, M.; Roh, H.; Austin, C.; Arora, M. Lead and Arsenic in Shed Deciduous Teeth of Children Living Near a Lead-Acid Battery Smelter. Environ. Sci. Technol. 2019, 53, 6000–6006. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, G.R.C.; Saraiva, M.; Barbosa, F.; Krug, F.J.; Cury, J.A.; Sousa, M.D.L.R.D.; Buzalaf, M.A.R.; Gerlach, R.F. Lead contents in the surface enamel of deciduous teeth sampled in vivo from children in uncontaminated and in lead-contaminated areas. Environ. Res. 2007, 104, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Toyomaki, H.; Yabe, J.; Nakayama, S.M.; Yohannes, Y.B.; Muzandu, K.; Liazambi, A.; Ikenaka, Y.; Kuritani, T.; Nakagawa, M.; Ishizuka, M. Factors associated with lead (Pb) exposure on dogs around a Pb mining area, Kabwe, Zambia. Chemosphere 2020, 247, 125884. [Google Scholar] [CrossRef] [PubMed]
- Yabe, J.; Nakayama, S.; Ikenaka, Y.; Muzandu, K.; Choongo, K.; Mainda, G.; Kabeta, M.; Ishizuka, M.; Umemura, T. Metal distribution in tissues of free-range chickens near a lead-zinc mine in Kabwe, Zambia. Environ. Toxicol. Chem. 2012, 32, 189–192. [Google Scholar] [CrossRef]
- Hegde, S.; Sridhar, M.; Bolar, D.R.; Bhaskar, S.A.; Sanghavi, M.B. Relating tooth- and blood-lead levels in children residing near a zinc-lead smelter in India. Int. J. Paediatr. Dent. 2010, 20, 186–192. [Google Scholar] [CrossRef]
- Hare, D.J.; Austin, C.; Doble, P.; Arora, M. Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. J. Dent. 2011, 39, 397–403. [Google Scholar] [CrossRef]
- Olympio, K.; Huila, M.F.; Cardoso, C.D.A.B.; Ferreira, A.P.S.D.S.; Ortiz, A.G.; Toma, H.E.; Da Silva, R.H.A.; Luz, M.S.; Cardoso, M.R.A.; Kelmer, G.A.R.; et al. Can in vivo surface dental enamelmicrobiopsies be used to measure remote lead exposure? Environ. Sci. Pollut. Res. 2017, 25, 9322–9329. [Google Scholar] [CrossRef] [PubMed]
- Cleymaet, R.; Collys, K.; Retief, D.H.; Michotte, Y.; Slop, D.; Taghon, E.; Maex, W.; Coomans, D. Relation between lead in surface tooth enamel, blood, and saliva from children residing in the vicinity of a non-ferrous metal plant in Belgium. Occup. Environ. Med. 1991, 48, 702–709. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataba, A.; Nakayama, S.M.M.; Nakata, H.; Toyomaki, H.; Yohannes, Y.B.; Yabe, J.; Muzandu, K.; Zyambo, G.; Kubota, A.; Matsukawa, T.; et al. An Investigation of the Wild Rat Crown Incisor as an Indicator of Lead (Pb) Exposure Using Inductively Couple Plasma Mass Spectrometry (ICP-MS) and Laser Ablation ICP-MS. Int. J. Environ. Res. Public Health 2021, 18, 767. https://doi.org/10.3390/ijerph18020767
Kataba A, Nakayama SMM, Nakata H, Toyomaki H, Yohannes YB, Yabe J, Muzandu K, Zyambo G, Kubota A, Matsukawa T, et al. An Investigation of the Wild Rat Crown Incisor as an Indicator of Lead (Pb) Exposure Using Inductively Couple Plasma Mass Spectrometry (ICP-MS) and Laser Ablation ICP-MS. International Journal of Environmental Research and Public Health. 2021; 18(2):767. https://doi.org/10.3390/ijerph18020767
Chicago/Turabian StyleKataba, Andrew, Shouta M. M. Nakayama, Hokuto Nakata, Haruya Toyomaki, Yared B. Yohannes, John Yabe, Kaampwe Muzandu, Golden Zyambo, Ayano Kubota, Takehisa Matsukawa, and et al. 2021. "An Investigation of the Wild Rat Crown Incisor as an Indicator of Lead (Pb) Exposure Using Inductively Couple Plasma Mass Spectrometry (ICP-MS) and Laser Ablation ICP-MS" International Journal of Environmental Research and Public Health 18, no. 2: 767. https://doi.org/10.3390/ijerph18020767
APA StyleKataba, A., Nakayama, S. M. M., Nakata, H., Toyomaki, H., Yohannes, Y. B., Yabe, J., Muzandu, K., Zyambo, G., Kubota, A., Matsukawa, T., Yokoyama, K., Ikenaka, Y., & Ishizuka, M. (2021). An Investigation of the Wild Rat Crown Incisor as an Indicator of Lead (Pb) Exposure Using Inductively Couple Plasma Mass Spectrometry (ICP-MS) and Laser Ablation ICP-MS. International Journal of Environmental Research and Public Health, 18(2), 767. https://doi.org/10.3390/ijerph18020767





