Role for Physical Fitness in the Association between Age and Cognitive Function in Older Adults: A Mediation Analysis of the SABE Colombia Study
Abstract
1. Introduction
2. Methods
2.1. Design, Setting and Participants
2.2. Data Collection
2.2.1. Sociodemographic, Health Disorders History and Lifestyle Data
2.2.2. Anthropometrics Measurement
2.2.3. Physical Fitness Tests
2.2.4. Cognitive Function
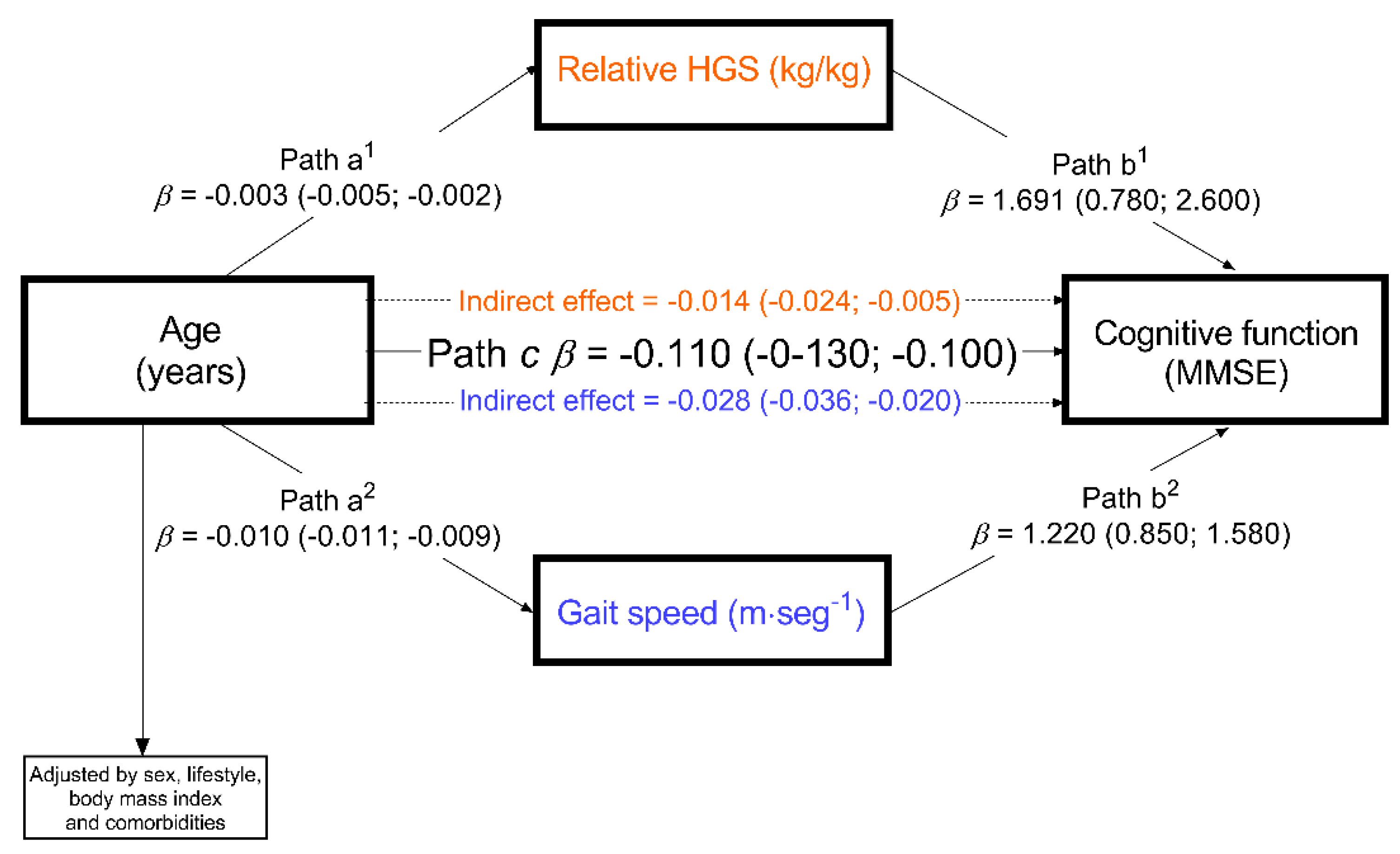
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Population Ageing 2015; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet (Lond. Engl.) 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Sosa, A.L.; Albanese, E.; Stephan, B.C.M.; Dewey, M.; Acosta, D.; Ferri, C.P.; Guerra, M.; Huang, Y.; Jacob, K.S.; Jiménez-Velázquez, I.Z.; et al. Prevalence, distribution, and impact of mild cognitive impairment in Latin America, China, and India: A 10/66 population-based study. PLoS Med. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Díaz Cabezas, R.; Marulanda Mejía, F.; Martínez Arias, M.H. Prevalencia de deterioro cognitivo y demencia en mayores de 65 años en una población urbana colombiana. Acta Neurológ. Colomb. 2013, 29, 141–151. [Google Scholar]
- Daimiel, L.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Schröder, H.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; Lopez-Miranda, J.; et al. Physical fitness and physical activity association with cognitive function and quality of life: Baseline cross-sectional analysis of the PREDIMED-Plus trial. Sci. Rep. 2020, 10, 34. [Google Scholar] [CrossRef]
- Ashby-Mitchell, K.; Jagger, C.; Fouweather, T.; Anstey, K.J. Life expectancy with and without cognitive impairment in seven latin American and Caribbean countries. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Vopat, B.G.; Klinge, S.A.; McClure, P.K.; Fadale, P.D. The Effects of Fitness on the Aging Process. J. Am. Acad. Orthop. Surg. 2014, 22, 576–585. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Senior Fitness Test Manual; Human kinetics: Champaign, IL, USA, 2001; ISBN 1450411185. [Google Scholar]
- Fleg, J.L.; Morrell, C.H.; Bos, A.G.; Brant, L.J.; Talbot, L.A.; Wright, J.G.; Lakatta, E.G. Accelerated Longitudinal Decline of Aerobic Capacity in Healthy Older Adults. Circulation 2005, 112, 674–682. [Google Scholar] [CrossRef]
- Milanović, Z.; Pantelić, S.; Trajković, N.; Sporiš, G.; Kostić, R.; James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 2013, 8, 549–556. [Google Scholar] [CrossRef]
- Auyeung, T.W.; Lee, S.W.J.; Leung, J.; Kwok, T.; Woo, J. Age-associated decline of muscle mass, grip strength and gait speed: A 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr. Gerontol. Int. 2014, 14, 76–84. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; Izquierdo, M.; Serra-Rexach, J.A.; Santos-Lozano, A.; Lucia, A. Physical exercise in the oldest old. Compr. Physiol. 2019, 9, 1281–1304. [Google Scholar] [CrossRef]
- Olivares, P.R.; Gusi, N.; Prieto, J.; Hernandez-Mocholi, M.A. Fitness and health-related quality of life dimensions in community-dwelling middle aged and older adults. Health Qual. Life Outcomes 2011, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Firth, J.A.; Large, M.; Rosenbaum, S.; Hallgren, M.; Ward, P.B.; Sarris, J.; Yung, A.R. Grip Strength Is Associated with Cognitive Performance in Schizophrenia and the General Population: A UK Biobank Study of 476559 Participants. Schizophr. Bull. 2018, 44, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Stijntjes, M.; Aartsen, M.J.; Taekema, D.G.; Gussekloo, J.; Huisman, M.; Meskers, C.G.M.; De Craen, A.J.M.; Maier, A.B. Temporal relationship between cognitive and physical performance in middle-aged to oldest old people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; An, Y.; Resnick, S.M.; Studenski, S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: Results from the Baltimore longitudinal study of aging. Age Ageing 2017, 46, 445–451. [Google Scholar] [CrossRef]
- Best, J.R.; Liu-Ambrose, T.; Boudreau, R.M.; Ayonayon, H.N.; Satterfield, S.; Simonsick, E.M.; Studenski, S.; Yaffe, K.; Newman, A.B.; Rosano, C. An evaluation of the longitudinal, bidirectional associations between gait speed and cognition in older women and men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1616–1623. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef]
- Li, Z.; Peng, X.; Xiang, W.; Han, J.; Li, K. The effect of resistance training on cognitive function in the older adults: A systematic review of randomized clinical trials. Aging Clin. Exp. Res. 2018, 30, 1259–1273. [Google Scholar] [CrossRef]
- Foster, P.P.; Rosenblatt, K.P.; Kuljiš, R.O. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Front. Neurol. 2011, 2, 28. [Google Scholar] [CrossRef]
- Alfini, A.J.; Weiss, L.R.; Nielson, K.A.; Verber, M.D.; Smith, J.C. Resting cerebral blood flow after exercise training in mild cognitive impairment. J. Alzheimer’s Dis. 2019, 67, 671–684. [Google Scholar] [CrossRef]
- Barnes, J.N.; Taylor, J.L.; Kluck, B.N.; Johnson, C.P.; Joyner, M.J. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J. Appl. Physiol. 2013, 114, 1383–1387. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Corchuelo, J.; Curcio, C.-L.; Calzada, M.-T.; Mendez, F. SABE Colombia: Survey on Health, Well-Being, and Aging in Colombia—Study Design and Protocol. Curr. Gerontol. Geriatr. Res. 2016, 2016, 7910205. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; García-Hermoso, A.; Cano, C.A.; Izquierdo, M. Reference values for handgrip strength and their association with intrinsic capacity domains among older adults. J. Cachexia Sarcopenia Muscle 2019, 10, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Albala, C.; Lebrao, M.L.; Léon Díaz, E.M.; Ham-Chande, R.; Hennis, A.J.; Palloni, A.; Peláez, M.; Pratts, O. The health, well-being, and aging (“SABE”) survey: Methodology applied and profile of the study population. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2005, 17, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Publications: New York, NY, USA, 2018; ISBN 1462534651. [Google Scholar]
- Peel, N.M.; Alapatt, L.J.; Jones, L.V.; Hubbard, R.E. The Association Between Gait Speed and Cognitive Status in Community-Dwelling Older People: A Systematic Review and Meta-analysis. J. Gerontol. Ser. A 2018. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jerskey, B.A.; Cote, D.M.; Walsh, E.G.; Hassenstab, J.J.; Ladino, M.E.; Clark, U.S.; Labbe, D.R.; Gunstad, J.J.; Poppas, A.; et al. Cerebrovascular perfusion among older adults is moderated by strength training and gender. Neurosci. Lett. 2014, 560, 26–30. [Google Scholar] [CrossRef]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—A systematic review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sport. Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Jiménez Maldonado, A.; Rentería, I.; García-Suárez, P.C.; Moncada-Jiménez, J.; Freire-Royes, L.F. The Impact of High-Intensity Interval Training on Brain Derived Neurotrophic Factor in Brain: A mini-review. Front. Neurosci. 2018, 12, 839. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, S.K.; Hong, S.B.; Kim, H.J. The effects of strength exercise on hippocampus volume and functional fitness of older women. Exp. Gerontol. 2017, 97, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Ito, K.; Shimokata, H.; Washimi, Y.; Endo, H.; Kato, T. A Randomized Controlled Trial of Multicomponent Exercise in Older Adults with Mild Cognitive Impairment. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Grau, J.W.; Huie, J.R.; Lee, K.H.; Hoy, K.C.; Huang, Y.-J.; Turtle, J.D.; Strain, M.M.; Baumbauer, K.M.; Miranda, R.M.; Hook, M.A.; et al. Metaplasticity and behavior: How training and inflammation affect plastic potential within the spinal cord and recovery after injury. Front. Neural Circuits 2014, 8, 100. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Grande, G.; Triolo, F.; Nuara, A.; Welmer, A.K.; Fratiglioni, L.; Vetrano, D.L. Measuring gait speed to better identify prodromal dementia. Exp. Gerontol. 2019, 124, 110625. [Google Scholar] [CrossRef]
- Angevaren, M.; Aufdemkampe, G.; Verhaar, H.J.J.; Aleman, A.; Vanhees, L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2008, CD005381. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Crockett, R.A.; Liu-Ambrose, T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging 2019, 79, 119–130. [Google Scholar] [CrossRef]
- McGrath, R.P.; Kraemer, W.J.; Snih, S.A.; Peterson, M.D. Handgrip Strength and Health in Aging Adults. Sports Med. 2018, 48, 1993–2000. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef] [PubMed]
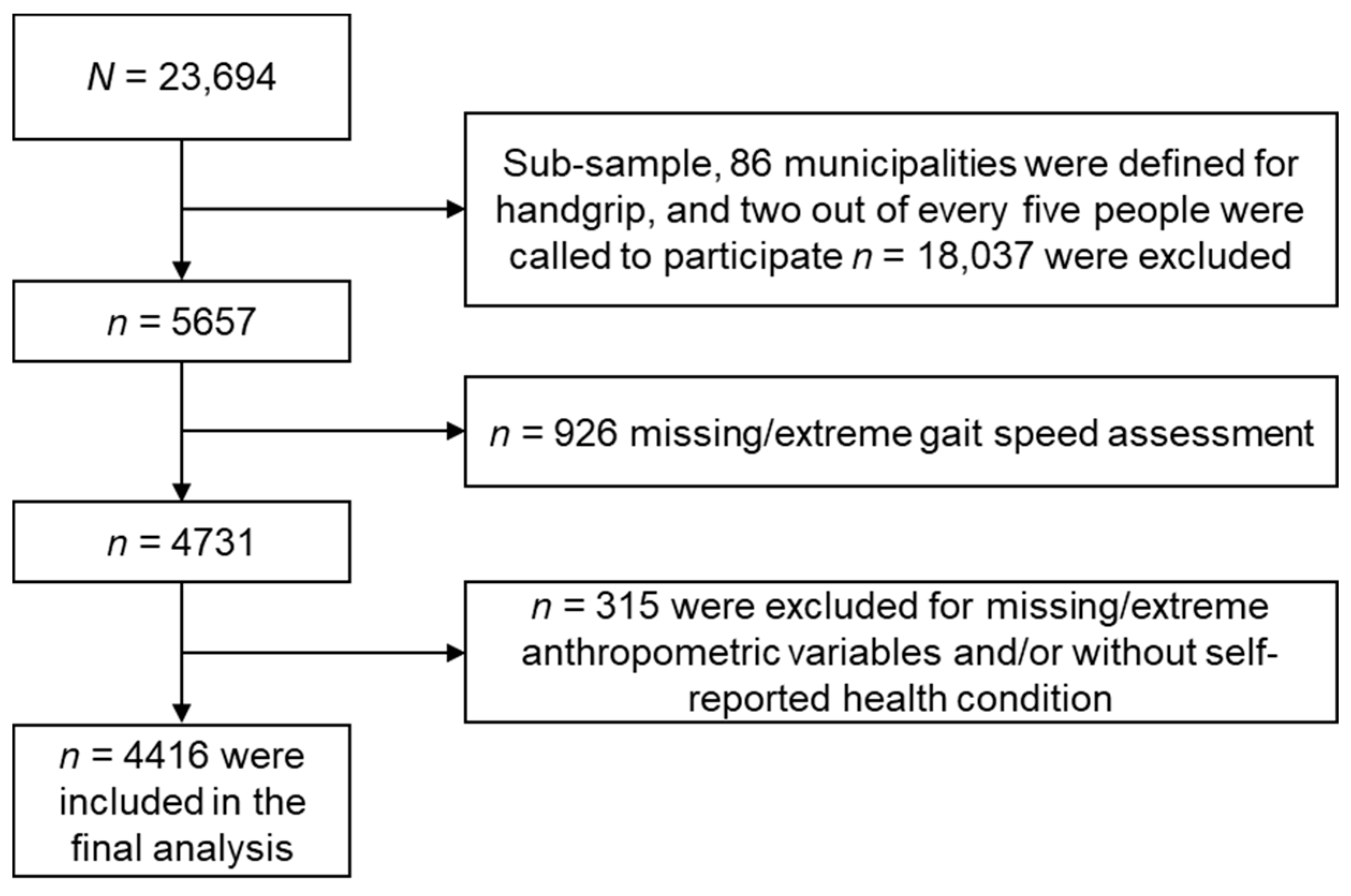
| Sociodemographic Characteristics | Overall | No Cognitive Impairment n = 3906 (88.5%) | Cognitive Impairment n = 510 (11.5%) | p-Value |
|---|---|---|---|---|
| Sex (female), n (%) | 2531 (57.3) | 2224 (56.9) | 307 (60.2) | 0.001 |
| Sex (male), n (%) | 1885 (42.7) | 1682 (43.1) | 203 (39.8) | |
| Age group, n (%) | ||||
| 60–69 | 2512 (56.9) | 2366 (60.6) | 146 (28.6) | 0.001 |
| 70–79 | 1431 (32.4) | 1234 (31.6) | 197 (38.6) | |
| 80+ | 473 (10.7) | 306 (7.8) | 167 (32.7) | |
| Nutritional status, n (%) | ||||
| Underweight | 83 (1.9) | 73 (1.9) | 10 (2.0) | 0.001 |
| Normal weight | 1344 (30.4) | 1141 (29.2) | 203 (39.8) | |
| Overweight | 1809 (41.0) | 1628 (41.7) | 181 (35.5) | |
| Obese | 1180 (26.7) | 1064 (27.2) | 116 (22.7) | |
| Socioeconomic status, n (%) | ||||
| Level I–II (low) | 3371 (76.3) | 2934 (75.3) | 428 (83.9) | 0.001 |
| Level III–IV (medium) | 1007 (22.8) | 926 (23.7) | 81 (15.9) | |
| Level V–VI (high) | 38 (0.8) | 37 (0.9) | 1 (0.2) | ― |
| Ethnic group, n (%) | ||||
| Indigenous | 267 (6.0) | 267 (6.8) | 0 (0.0) | ― |
| Black | 369 (8.4) | 369 (9.4) | 0 (0.0) | ― |
| White | 1234 (27.9) | 1234 (31.6) | 0 (0.0) | ― |
| Others | 2036 (46.1) | 2036 (52.1) | 0 (0.0) | ― |
| Non-ethnic | 510 (11.5) | 0 (0.0) | 510 (11.5) | ― |
| Living area, n (%) | ||||
| Urban | 3406 (77.1) | 3060 (78.3) | 346 (67.8) | 0.001 |
| Rural | 1010 (22.9) | 846 (21.7) | 164 (32.2) | |
| Lifestyle outcomes, n (%) | ||||
| Alcohol | 594 (13.5) | 559 (14.3) | 35 (6.9) | 0.001 |
| Smoking | 487 (11.0) | 422 (10.8) | 65 (12.8) | |
| Non-physically active | 3555 (80.6) | 3098 (79.3) | 457 (89.8) | |
| Comorbid chronic diseases, n (%) | ||||
| HBP | 2374 (53.9) | 2077 (53.3) | 297 (58.3) | 0.001 |
| High cholesterol | 2159 (49.1) | 1930 (49.6) | 229 (45.5) | |
| Diabetes | 715 (16.2) | 635 (16.3) | 80 (15.7) | |
| Cancer (any type) | 210 (4.8) | 195 (5.0) | 15 (2.9) | |
| COPD | 443 (10.0) | 375 (9.6) | 68 (13.4) | |
| CVD | 600 (13.6) | 520 (13.3) | 80 (15.7) | |
| Stroke | 167 (3.8) | 138 (3.5) | 29 (5.7) | |
| Arthritis | 1192 (27.1) | 1075 (27.6) | 117 (23.1) | |
| Osteoporosis | 499 (11.4) | 446 (11.5) | 53 (10.4) |
| Variables | No Cognitive Impairment | Cognitive Impairment | Model 1 p-Value | Model 2 p-Value | Model 3 p-Value |
|---|---|---|---|---|---|
| Absolute HGS (kg) | 22.08 | 18.50 | <0.001 | <0.001 | <0.001 |
| Relative HGS (kg/kg) | 0.34 | 0.30 | <0.001 | <0.001 | <0.001 |
| Gait speed (m·s−1) | 0.77 | 0.63 | <0.001 | <0.001 | <0.001 |
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Lower HGS | 1.45 | (1.11; 1.90) | 0.006 | 1.47 | (1.11; 1.93) | 0.006 | 1.55 | (1.16; 2.03) | 0.002 |
| Lower Gait speed | 2.12 | (1.61; 2.79) | 0.007 | 2.06 | (1.55; 2.72) | <0.001 | 2.08 | (1.56; 2.80) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Sousa, M.Á.; del Pozo-Cruz, J.; Olivares, P.R.; Cano-Gutiérrez, C.A.; Izquierdo, M.; Ramírez-Vélez, R. Role for Physical Fitness in the Association between Age and Cognitive Function in Older Adults: A Mediation Analysis of the SABE Colombia Study. Int. J. Environ. Res. Public Health 2021, 18, 751. https://doi.org/10.3390/ijerph18020751
Pérez-Sousa MÁ, del Pozo-Cruz J, Olivares PR, Cano-Gutiérrez CA, Izquierdo M, Ramírez-Vélez R. Role for Physical Fitness in the Association between Age and Cognitive Function in Older Adults: A Mediation Analysis of the SABE Colombia Study. International Journal of Environmental Research and Public Health. 2021; 18(2):751. https://doi.org/10.3390/ijerph18020751
Chicago/Turabian StylePérez-Sousa, Miguel Ángel, Jesús del Pozo-Cruz, Pedro R. Olivares, Carlos A. Cano-Gutiérrez, Mikel Izquierdo, and Robinson Ramírez-Vélez. 2021. "Role for Physical Fitness in the Association between Age and Cognitive Function in Older Adults: A Mediation Analysis of the SABE Colombia Study" International Journal of Environmental Research and Public Health 18, no. 2: 751. https://doi.org/10.3390/ijerph18020751
APA StylePérez-Sousa, M. Á., del Pozo-Cruz, J., Olivares, P. R., Cano-Gutiérrez, C. A., Izquierdo, M., & Ramírez-Vélez, R. (2021). Role for Physical Fitness in the Association between Age and Cognitive Function in Older Adults: A Mediation Analysis of the SABE Colombia Study. International Journal of Environmental Research and Public Health, 18(2), 751. https://doi.org/10.3390/ijerph18020751









