Responses to Low- and High-Intensity Exercise in Adolescents with Type 1 Diabetes in Relation to Their Level of VO2 Max
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Group
2.2. Experimental Protocol
2.3. Physiological Analyses
2.4. The Control of Blood Glucose Concentrations
2.5. Biochemical Analyses
2.6. Statistical Analyses
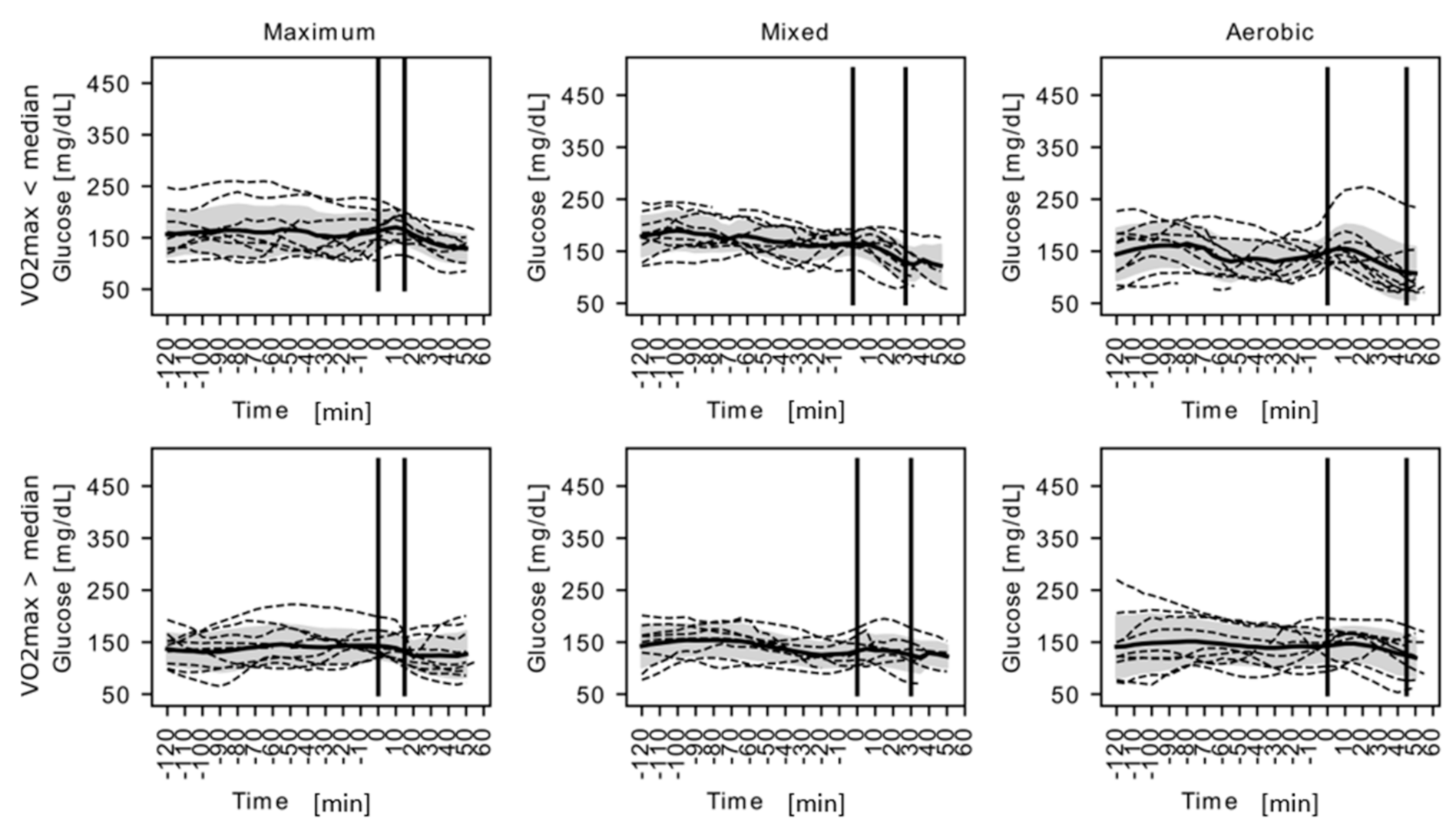
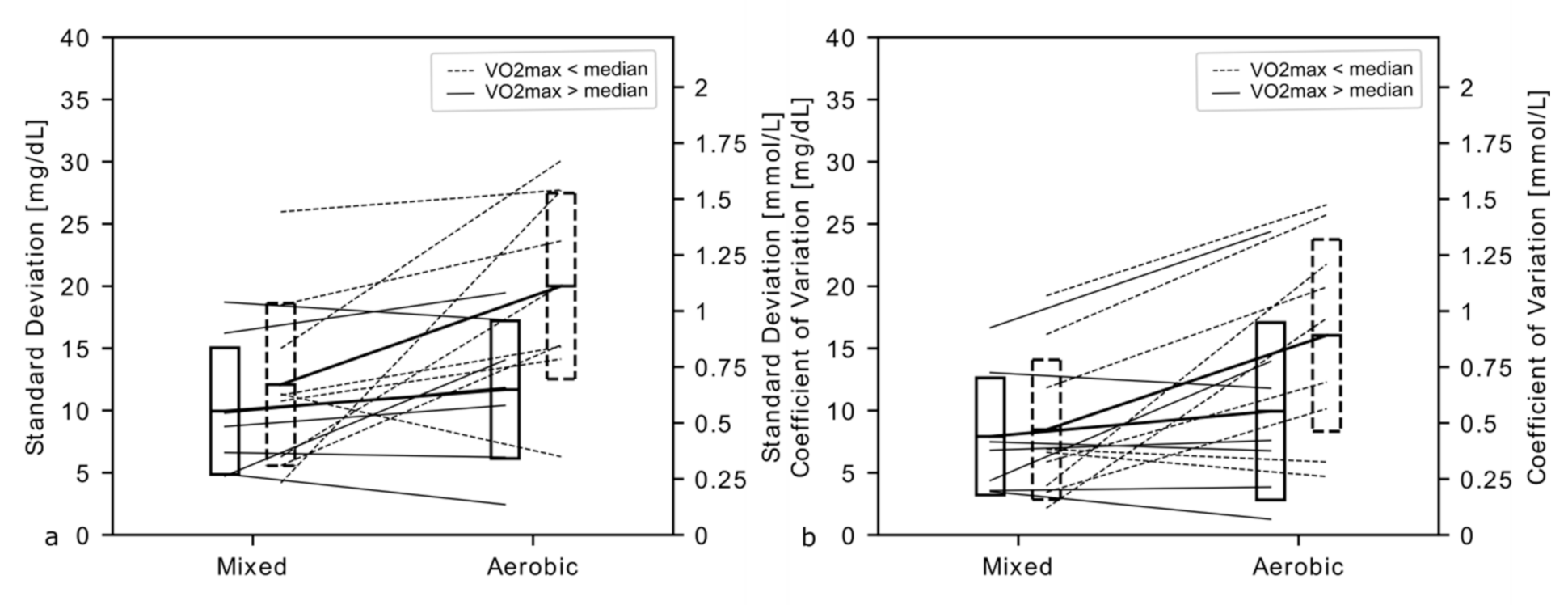
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krause, M.S.; Bittencourt, P.I., Jr. Type 1 diabetes: Can exercise impair the autoimmune event? The L-arginine/glutamine coupling hypothesis. Cell. Biochem. Funct. 2008, 26, 406–433. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.N.; Anderson, L.; Morton, J.P.; Wagenmakers, A.J.M.; Riddell, M.C. Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance? Nutrients 2019, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, V.; Gesuita, R.; Skrami, E.; Rabbone, I.; Bonfanti, R.; Arnaldi, C.; D’Annunzio, G.; Frongia, A.; Lombardo, F.; Piccinno, E.; et al. Optimal predictive low glucose management settings during physical exercise in adolescents with type 1 diabetes. Pediatr. Diabetes 2019, 20, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chetty, T.; Shetty, V.; Fournier, P.A.; Adolfsson, P.; Jones, T.W.; Davis, E.A. Exercise Management for Young People With Type 1 Diabetes: A Structured Approach to the Exercise Consultation. Front. Endocrinol. (Lausanne) 2019, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; O’Donnell, H.K.; Stanek, K.; Youngkin, E.; Gomer, T.; Driscoll, K.A. Suicide Risk Assessment in Youth and Young Adults With Type 1 Diabetes. Diabetes Care 2020, 43, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19, 7–19. [Google Scholar] [CrossRef]
- Clinical recommendations for the management of patients with diabetes 2020. Statement of the Polish Diabetological Society. Diabetol. Pract. 2020, 6, 105–106.
- Adolfsson, P.; Riddell, M.C.; Taplin, C.E.; Davis, E.A.; Fournier, P.A.; Annan, F.; Scaramuzza, A.E.; Hasnani, D.; Hofer, S.E. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19, 11–14. [Google Scholar] [CrossRef]
- Adolfsson, P.; Nilsson, S.; Albertsson-Wikland, K.; Lindblad, B. Hormonal response during physical exercise of different intensities in adolescents with type 1 diabetes and healthy controls. Pediatr. Diabetes 2012, 13, 587–596. [Google Scholar] [CrossRef]
- Gallen, I.W.; Redgrave, A.; Redgrave, S. Olympic diabetes. Clin. Med. (London) 2003, 3, 333–337. [Google Scholar] [CrossRef]
- Francescato, M.P.; Carrato, S. Management of exercise-induced glycemic imbalances in type 1 diabetes. Curr. Diabetes Rev. 2011, 7, 253–263. [Google Scholar] [CrossRef]
- Chimen, T.; Kennedy, A.; Nirantharakumar, K.; Pang, T.T.; Andrews, R.; Narendran, P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012, 55, 542–551. [Google Scholar] [CrossRef]
- Jendle, J.H.; Riddell, M.C. Editorial: Physical Activity and Type 1 Diabetes. Front. Endocrinol. (Lausanne) 2019, 10, 860. [Google Scholar] [CrossRef]
- Kodama, S.; Tanaka, S.; Heianza, Y.; Fujihara, K.; Horikawa, C.; Shimano, H.; Saito, K.; Yamada, N.; Ohashi, Y.; Sone, H. Association between physical activity and risk of all-cause mortality and cardiovascular disease in patients with diabetes: A meta-analysis. Diabetes Care 2013, 36, 471–479. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Hu, Y.; Zong, G.; Li, S.; Rimm, E.E.; Hu, F.B.; Manson, J.E.; Rexrode, K.M.; Shin, H.J.; et al. Influence of Lifestyle on Incident Cardiovascular Disease and Mortality in Patients with Diabetes Mellitus. J. Am. Coll. Cardiol. 2018, 71, 2867–2876. [Google Scholar] [CrossRef]
- Najafipour, F.; Mobasseri, M.; Yavari, A.; Nadrian, H.; Aliasgarzedeh, A.; Abbasi, N.M.; Niafar, M.; Gharamaleki, J.H.; Sadra, V. Effect of regular exercise training on changes in HbA1c, BMI and VO2max among patients with type 2 diabetes mellitus: An 8-year trial. BMJ Open Diabetes Res. Care 2017, 5, e000414. [Google Scholar] [CrossRef]
- Nöthlings, U.; Ford, E.S.; Kröger, J.; Boeing, H. Lifestyle factors and mortality among adults with diabetes: Findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam study. J. Diabetes 2010, 2, 112–117. [Google Scholar]
- Cuenca-Garcia, M.; Jago, R.; Shield, J.P.H.; Burren, C.P. How does physical activity and fitness influence glycemic control in young people with type 1 diabetes? Diabet. Med. 2012, 29, e369–e376. [Google Scholar] [CrossRef]
- Yardley, J.E.; Sigal, R.J. Exercise strategies for hypoglycemia prevention in individuals with type 1 diabetes. Diabetes Spectr. 2015, 28, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, A.C.; de Lourdes Lima, M.; Nunes, F.; Cambuí, Z.; Barbosa, C.; Andrade, A.; Viana, A.; Martins, M.; Abrantes, V.; Aragão, C.; et al. The effect of resistance versus aerobic training on metabolic control in patients with type-1 diabetes mellitus. Diabetes Res. Clin. Pract. 2006, 72, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Wittenberg, A.; Castle, J.R.; El Youssef, J.; Winters-Stone, K.; Gillingham, M.; Jacobs, P.G. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults with Type 1 Diabetes. Can. J. Diabetes 2019, 43, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Laursen, P.B.; Shing, C.M.; Peake, J.M.; Coombes, J.S.; Jenkins, D.G. Interval training program optimization in highly trained endurance cyclists. Med. Sci. Sports Exerc. 2002, 34, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, J.H.; Costill, D.L.; Kenney, W.L. Physiology of Sport and Exercise. Hum. Kinetics. 2008, 192–201. [Google Scholar] [CrossRef]
- Kjaer, M.; Farrell, P.A.; Christensen, N.J.; Galbo, H. Increased epinephrine response and inaccurate glucoregulation in exercising athletes. J. Appl. Physiol. 1986, 61, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.E.; Hattersley, A.; Donaghue, K.C. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr. Diabetes 2009, 10, 3–12. [Google Scholar] [CrossRef]
- Kowalski, K.C.; Crocker, P.R.E.; Donen, R.M.D. The Physical Activity Questionnaire for Older Children (PAQ-C) and Adolescents (PAQ-A) Manual; College of Kinesiology, University of Saskatchewan: Saskatoon, SK, Canada, 2004; pp. 1–37. [Google Scholar]
- Jastrzębski, Z.; Rompa, P.; Szutowicz, M.; Radzimiński, Ł. Effects of applied training loads on the aerobic capacity of young soccer players during a soccer season. J. Strength Cond. Res. 2013, 27, 916–923. [Google Scholar] [CrossRef]
- Myśliwec, A.; Skalska, M.; Knechtle, B.; Nikolaidis, P.T.; Rosemann, T.; Szmigiero-Kawko, M.; Lejk, A.; Jastrzębska, J.; Radzimiński, Ł.; Wakuluk, D.; et al. Acute Responses to Low and High Intensity Exercise in Type 1 Diabetic Adolescents in Relation to Their Level of Serum 25(OH)D. Nutrients 2020, 12, 454. [Google Scholar]
- Pagacz, K.; Stawiski, K.; Szadkowska, A.; Mlynarski, W.; Fendler, W. GlyCulator2: An update on a web application for calculation of glycemic variability indices. Acta Diabetol. 2018, 55, 877–880. [Google Scholar] [CrossRef]
- Komatsu, W.R.; Gabbay, M.A.; Castro, M.L.; Saraiva, G.L.; Chacra, A.R.; de Barros Neto, T.L.; Dib, S.A. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr. Diabetes 2015, 6, 145–149. [Google Scholar] [CrossRef]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuousglucose monitoring (CGM) and intermittently scanned CGM(isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and ofthe International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Pediatr. Diabetes 2020, 21, 1375–1393. [Google Scholar] [PubMed]
- Biagi, L.; Bertachi, A.; Quirós, C.; Giménez, M.; Conget, I.; Bondia, J.; Vehí, J. Accuracy of Continuous Glucose Monitoring before, during, and after Aerobic and Anaerobic Exercise in Patients with Type 1 Diabetes Mellitus. Biosensors 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Groat, D.; Chan, O.; Hung, M.; Sharma, A.; Varner, M.W.; Gouripeddi, R.; Facelli, J.C.; Fisher, S.J. Alarm Settings of Continuous Glucose Monitoring Systems and Associations to Glucose Outcomes in Type 1 Diabetes. J. Endocr. Soc. 2020, 4, bvz005. [Google Scholar] [CrossRef] [PubMed]
- Puhr, S.; Derdzinski, M.; Welsh, J.B.; Parker, A.S.; Walker, T.; Price, D.A. Real-World Hypoglycemia Avoidance with a Continuous Glucose Monitoring System’s Predictive Low Glucose Alert. Diabetes Technol. Ther. 2019, 21, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Puhr, S.; Derdzinski, M.; Parker, A.S.; Welsh, J.B.; Price, D.A. Real-World Hypoglycemia Avoidance with a Predictive Low Glucose Alert Does Not Depend on Frequent Screen Views. J. Diabetes Sci. Technol. 2020, 14, 83–86. [Google Scholar] [CrossRef]
- Hermanns, N.; Heinemann, L.; Freckmann, G.; Waldenmaier, D.; Ehrmann, D. Impact of CGM on the Management of Hypoglycemia Problems: Overview and Secondary Analysis 0f the HypoDE Study. J. Diabetes Sci. Technol. 2019, 13, 636–644. [Google Scholar] [CrossRef]
- Ceriello, A.; Monnier, L.; Owens, D. Glycaemic variability in diabetes: Clinical and therapeutic implications. Lancet Diabetes Endocrionol. 2019, 7, 221–230. [Google Scholar] [CrossRef]
- Lachin, J.M.; Bebu, I.; Bergenstal, R.M.; Pop-Busui, R.; Service, F.J.; Zinman, B.; Nathan, D.M.; DCCT/EDIC Research Group. Association of Glycemic Variability in Type 1 Diabetes with Progression of Microvascular Outcomes in the Diabetes Control and Complications Trial. Diabetes Care 2017, 40, 777–783. [Google Scholar] [CrossRef]
- Vesco, A.T.; Jedraszko, A.M.; Garza, K.P.; Weissberg-Benchell, J. Continuous Glucose Monitoring Associated with Less Diabetes-Specific Emotional Distress and Lower A1c Among Adolescents with Type 1 Diabetes. J. Diabetes Sci. Technol. 2018, 12, 792–799. [Google Scholar] [CrossRef]
| Characteristics | All (n = 20) | LFG (n = 10) | HFG (n = 10) | p-Value |
|---|---|---|---|---|
| Age (years) | 14.3 ± 1.6 | 14.0 ± 1.7 | 14.7 ± 1.5 | 0.4076 |
| Diabetes duration (years) | 6.7 ± 4.1 | 6.1 ± 3.3 | 7.2 ± 4.9 | 0.5660 |
| CSII (Continuous Subcutaneous Insulin Infusion) duration (years) | 6.0 ± 3.9 | 5.3 ± 3.2 | 6.7 ± 4.5 | 0.4330 |
| HbA1c (%) | 7.3 ± 0.8 | 7.4 ± 0.8 | 7.2 ± 0.9 | 0.6078 |
| DDI (UI/kg) | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.5894 |
| BMI z-score | 0.2 ± 0.7 | 0.5 ± 0.8 | −0.2 ± 0.5 | 0.0401 |
| Body fat (%) | 14.7 ± 6.4 | 19.0 ± 6.2 | 10.5 ± 2.8 | 0.0010 |
| WHR (Inches) | 0.8 ± 0.0 | 0.81 ± 0.04 | 0.78 ± 0.03 | 0.0254 |
| VO2 max (mL/kg/min) | 40.2 ± 5.8 | 36.2 ± 5.3 | 44.3 ± 2.5 | N/A |
| TSH (uU/mL) | 1.4 ± 0.8 | 1.3 ± 0.5 | 1.6 ± 1.0 | 0.7912 # |
| FT4 (pmol/L) | 11.9 ± 1.1 | 12.0 ± 1.1 | 11.9 ± 1.1 | 0.8342 |
| Alt (U/L) | 19.3 ± 7.6 | 18.7 ± 4.3 | 19.8 ± 10.0 | 0.7905 # |
| Ast (U/L) | 15.0 ± 4.2 | 15.2 ± 4.7 | 14.7 ± 3.8 | 0.7975 |
| TC (mg/dL) | 160.0 ± 25.5 | 171.7 ± 18.9 | 148.3 ± 26.6 | 0.0361 |
| HDL (mg/dL) | 60.4 ± 16.4 | 68.8 ± 14.1 | 52.0 ± 14.4 | 0.0170 |
| LDL (mg/dL) | 86.9 ± 21.0 | 91.3 ± 22.7 | 82.4 ± 19.1 | 0.3562 |
| TG (mg/dL) | 66.5 ± 35.7 | 61.8 ± 20.9 | 71.2 ± 47.0 | 0.9397 # |
| Type of Test | Maximum | Mixed | Aerobic | ANOVA p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroups Regarding to VO2 Max | LFG (n = 10) | HFG (n = 10) | All (n = 20) | LFG (n = 10) | HFG (n = 10) | All (n = 20) | LFG (n = 10) | HFG (n = 10) | All (n = 20) | Type of Test | VO2 Max |
| Carbohydrates consumed before tests (g/kg) | 0.20 ± 0.18 | 0.34 ± 0.33 | 0.27 ± 0.27 | 0.16 ± 0.18 | 0.24 ± 0.19 | 0.20 ± 0.18 | 0.36 ± 0.26 | 0.28 ± 0.31 | 0.32 ± 0.28 | 0.3677 | 0.4410 |
| Starting glycemia (mg/dL) | 159.70 ± 37.84 | 153.60 ± 40.74 | 156.65 ± 38.39 | 165.30 ± 24.78 | 149.30 ± 35.00 | 157.30 ± 30.63 | 140.00 ± 28.78 | 143.80 ± 22.13 | 141.90 ± 25.06 | 0.2663 | 0.4447 |
| Carbohydrates consumed during and after tests (g/kg) | 0.18 ± 0.34 | 0.05 ± 0.12 | 0.12 ± 0.26 # | 0.22 ± 0.26 | 0.13 ± 0.19 | 0.18 ± 0.23 $ | 0.46 ± 0.23 | 0.33 ± 0.29 | 0.40 ± 0.27 # $ | 0.0003 | 0.1896 |
| Last glycemia during tests (mg/dL) | 165.80 ± 32.79 | 145.70 ± 28.83 | 155.75 ± 31.77 # | 136.40 ± 39.81 | 129.80 ± 34.50 | 133.10 ± 36.41 | 118.70 ± 50.20 | 110.70 ± 34.57 | 114.70 ± 42.15 # | 0.0011 | 0.3483 |
| Glycemia at rest (mg/dL) | 158.22 ± 34.66 | 143.00 ± 40.70 | 150.21 ± 37.73 # $ | 120.90 ± 31.73 | 122.50 ± 33.13 | 121.70 ± 31.58 # | 102.00 ± 51.96 | 107.50 ± 35.92 | 104.75 $ ± 43.56 | 0.0021 | 0.9433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myśliwiec, A.; Skalska, M.; Michalak, A.; Chrzanowski, J.; Szmigiero-Kawko, M.; Lejk, A.; Jastrzębska, J.; Radzimiński, Ł.; López-Sánchez, G.F.; Gawrecki, A.; et al. Responses to Low- and High-Intensity Exercise in Adolescents with Type 1 Diabetes in Relation to Their Level of VO2 Max. Int. J. Environ. Res. Public Health 2021, 18, 692. https://doi.org/10.3390/ijerph18020692
Myśliwiec A, Skalska M, Michalak A, Chrzanowski J, Szmigiero-Kawko M, Lejk A, Jastrzębska J, Radzimiński Ł, López-Sánchez GF, Gawrecki A, et al. Responses to Low- and High-Intensity Exercise in Adolescents with Type 1 Diabetes in Relation to Their Level of VO2 Max. International Journal of Environmental Research and Public Health. 2021; 18(2):692. https://doi.org/10.3390/ijerph18020692
Chicago/Turabian StyleMyśliwiec, Artur, Maria Skalska, Arkadiusz Michalak, Jędrzej Chrzanowski, Małgorzata Szmigiero-Kawko, Agnieszka Lejk, Joanna Jastrzębska, Łukasz Radzimiński, Guillermo F. López-Sánchez, Andrzej Gawrecki, and et al. 2021. "Responses to Low- and High-Intensity Exercise in Adolescents with Type 1 Diabetes in Relation to Their Level of VO2 Max" International Journal of Environmental Research and Public Health 18, no. 2: 692. https://doi.org/10.3390/ijerph18020692
APA StyleMyśliwiec, A., Skalska, M., Michalak, A., Chrzanowski, J., Szmigiero-Kawko, M., Lejk, A., Jastrzębska, J., Radzimiński, Ł., López-Sánchez, G. F., Gawrecki, A., & Jastrzębski, Z. (2021). Responses to Low- and High-Intensity Exercise in Adolescents with Type 1 Diabetes in Relation to Their Level of VO2 Max. International Journal of Environmental Research and Public Health, 18(2), 692. https://doi.org/10.3390/ijerph18020692








