Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome
Abstract
1. Introduction
2. Methods
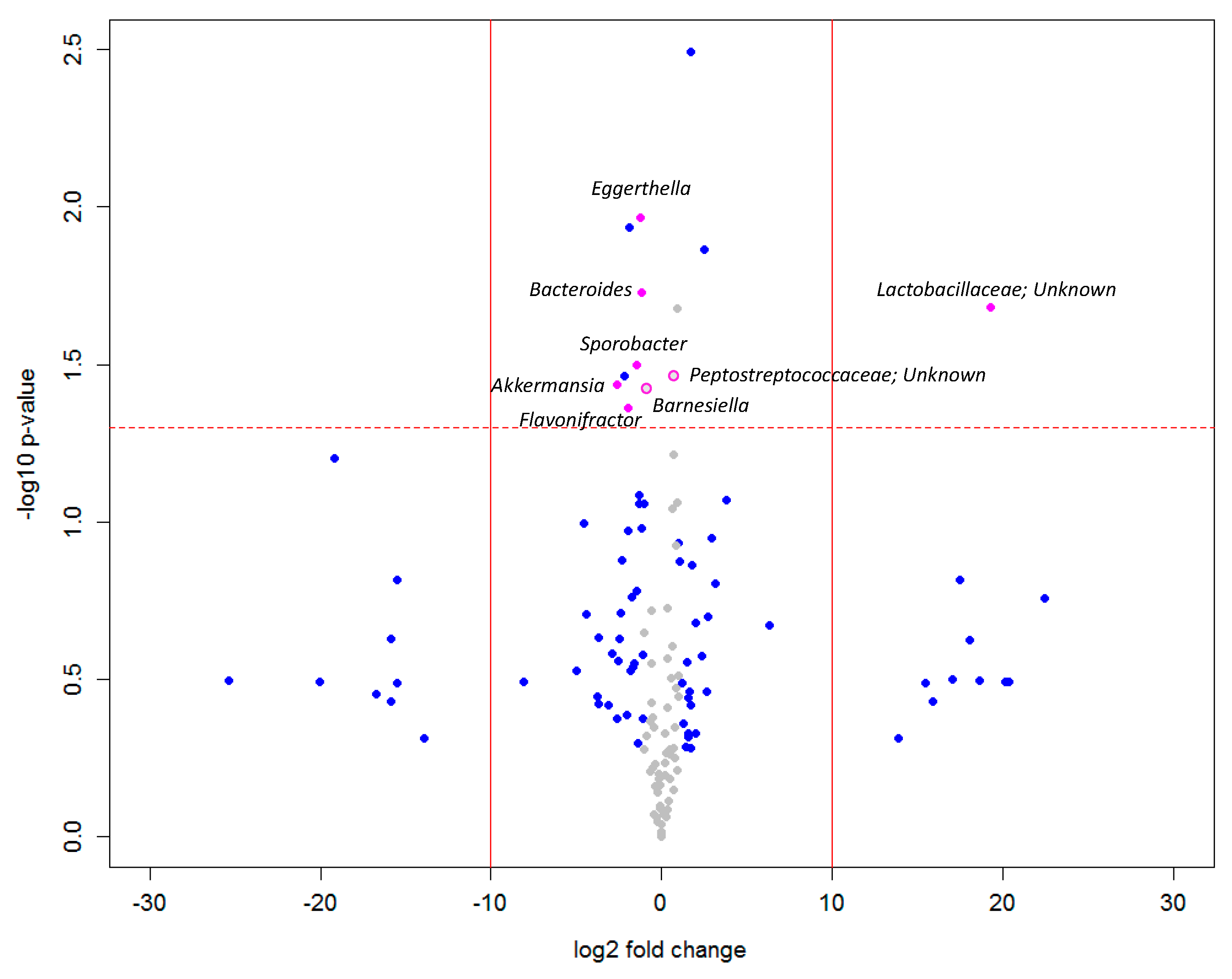
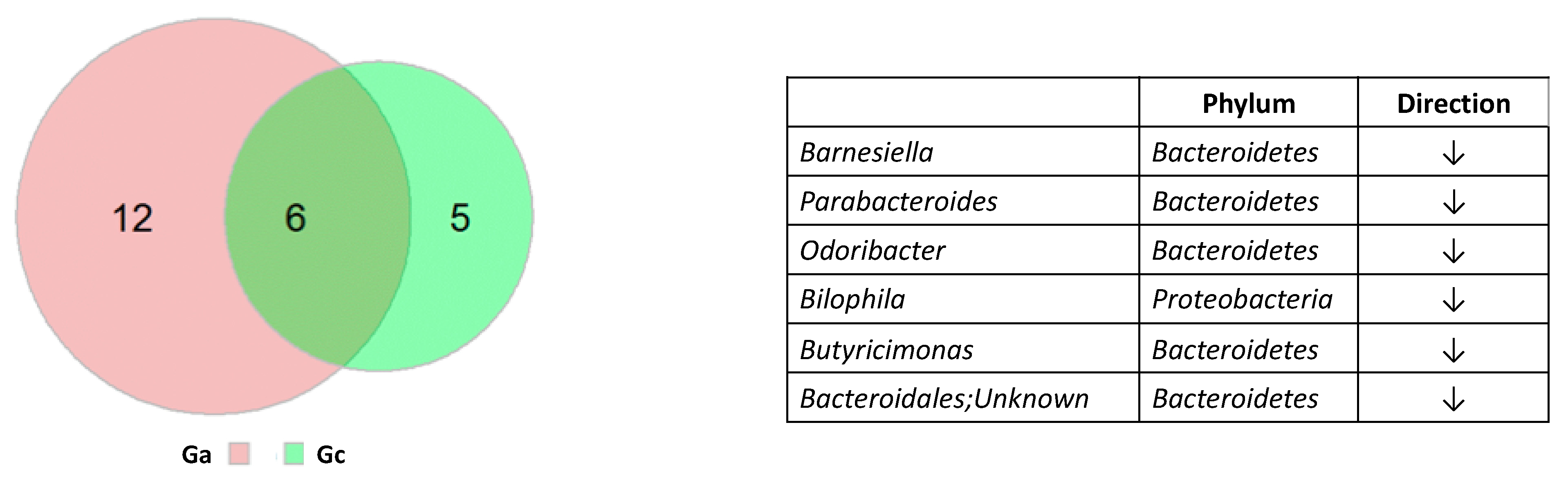
3. Results
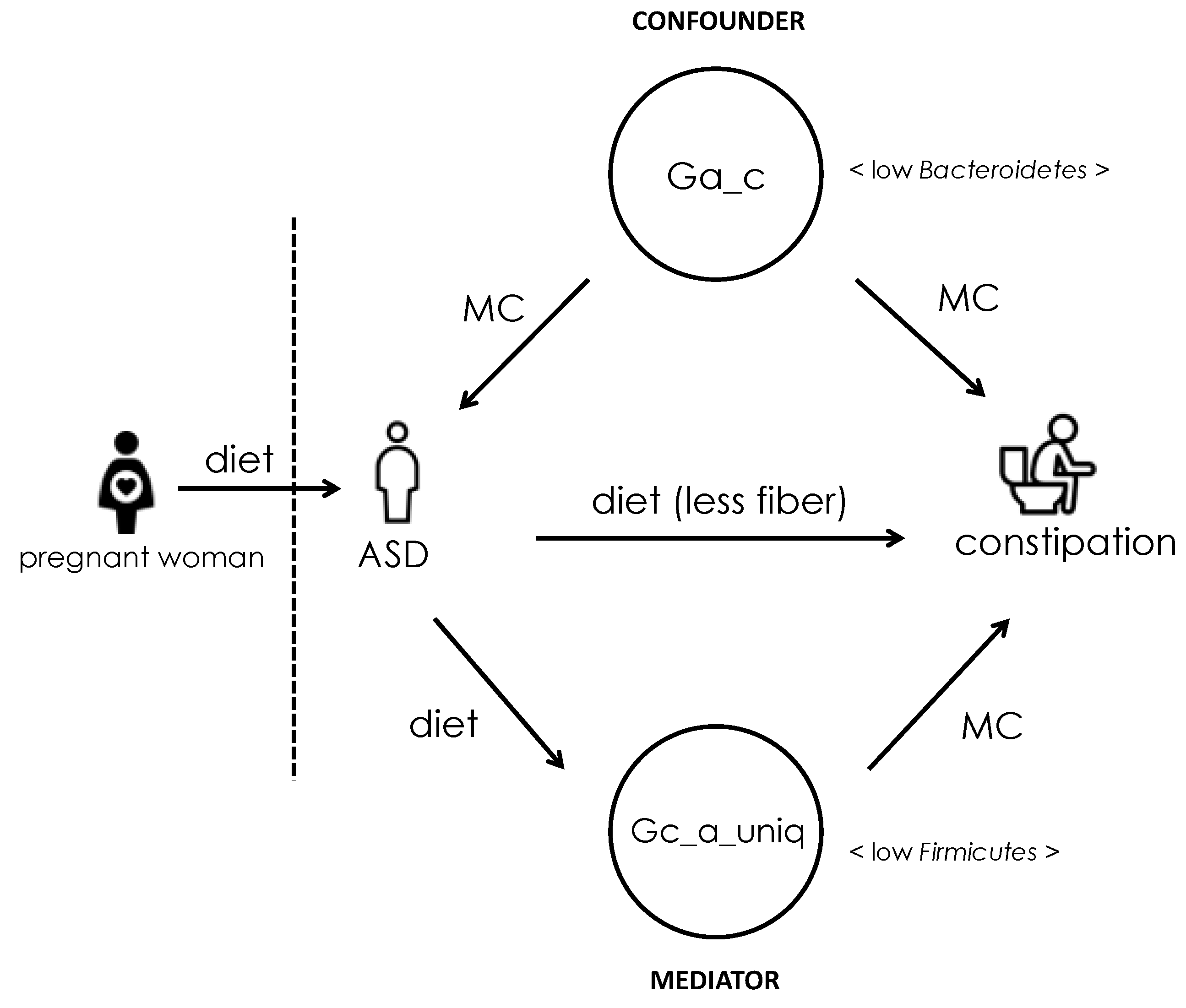
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Methods
Appendix A.1. Population and Study Design
Appendix A.2. Diagnostic Criteria for Autism Spectrum Disorder (ASD) and Constipation
Appendix A.3. Measurement of Microbiome
Appendix A.4. Statistical Analysis
Appendix A.5. Ethics Approval and Consent to Participate
Appendix B. Read Number from the Minimum to the Maximum
| ctrl | AD | |
| 1783 | 6 | |
| 2628 | 14 | |
| 3348 | 49 | |
| 3845 | 78 | |
| 4770 | 1578 | |
| 5165 | 1600 | |
| 5444 | 2176 | |
| 5628 | 3513 | |
| 7553 | 3577 | |
| 8525 | 3730 | |
| 8959 | 4217 | |
| 10,749 | 4266 | |
| 10,956 | 4520 | |
| 11,926 | 4745 | |
| 12,762 | 4856 | |
| 13,607 | 4880 | |
| 13,717 | 5366 | |
| 15,347 | 7307 | |
| 16,331 | 7596 | |
| 17,866 | 8214 | |
| 18,395 | 8934 | |
| 18,858 | 9299 | |
| 18,878 | 11,520 | |
| 18,935 | 11,642 | |
| 19,764 | 12,861 | |
| 19,931 | 13,814 | |
| 21,293 | 15,406 | |
| 21,410 | 15,529 | |
| 21,532 | 18,527 | |
| 21,548 | 19,308 | |
| 21,695 | 19,563 | |
| 23,142 | 21,200 | |
| 27,287 | 21,213 | |
| 29,311 | 22,653 | |
| 34,413 | 22,702 | |
| 37,965 | 27,421 | |
| 41,923 | 30,120 | |
| 42,749 | 33,036 | |
| 42,880 | 48,288 | |
| 46,018 | 72,830 | |
| Average (AD w/o the first 4 samples) | 18,220.9 | 14,666.8611 |
References
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Zablotsky, B.; Black, L.I.; Blumberg, S.J. Estimated Prevalence of Children with Diagnosed Developmental Disabilities in the United States, 2014–2016. NCHS Data Brief 2017, 291, 1–8. [Google Scholar]
- Christensen, D.L.; Braun, K.V.N.; Baio, J.; Bilder, D.; Charles, J.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States. MMWR Surveill. Summ. 2018, 65, 1. [Google Scholar] [PubMed]
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal Dysfunction in Autism: Parental Report, Clinical Evaluation, and Associated Factors. Autism Res. 2012, 5, 101–108. [Google Scholar] [CrossRef]
- Wang, L.W.; Tancredi, D.J.; Thomas, D.W. The Prevalence of Gastrointestinal Problems in Children Across the United States with Autism Spectrum Disorders from Families with Multiple Affected Members. J. Dev. Behav. Pediatr. 2011, 32, 351–360. [Google Scholar] [CrossRef]
- Buie, T.; Fuchs, G.J.; Furuta, G.T.; Kooros, K.; Levy, J.; Lewis, J.D.; Wershil, B.K.; Winter, H. Recommendations for Evaluation and Treatment of Common Gastrointestinal Problems in Children with ASDs. Pediatrics 2010, 125, S19–S29. [Google Scholar] [CrossRef]
- Coury, D.L.; Ashwood, P.; Fasano, A.; Fuchs, G.; Geraghty, M.; Kaul, A.; Mawe, G.; Patterson, P.; Jones, N.E. Gastrointestinal Conditions in Children with Autism Spectrum Disorder: Developing a Research Agenda. Pediatrics 2012, 130, S160–S168. [Google Scholar] [CrossRef]
- Holingue, C.; Newill, C.; Lee, L.-C.; Pasricha, P.J.; Fallin, M.D. Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef]
- Werth, B.L.; Williams, K.A.; Fisher, M.J.; Pont, L.G. Defining constipation to estimate its prevalence in the community: Results from a national survey. BMC Gastroenterol. 2019, 19, 75. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Y.; Wu, B.-L. Genetic architecture, epigenetic influence and environment exposure in the pathogenesis of Autism. Sci. China Life Sci. 2015, 58, 958–967. [Google Scholar] [CrossRef]
- Karahmadi, M.; Karimi, P.; Kamali, E.; Mousavi, S.M. Environmental factors influencing the risk of autism. J. Res. Med. Sci. 2017, 22, 27. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, X.; Li, J.; Li, F. Association between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liang, J.; Dai, M.; Wang, J.; Luo, J.; Zhang, Z.; Jing, J. Altered Gut Microbiota in Chinese Children with Autism Spectrum Disorders. Front. Cell. Infect. Microbiol. 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wan, J.; Rong, H.; He, F.; Wang, H.; Zhou, J.; Cai, C.; Wang, Y.; Xu, R.; Yin, Z.; et al. Alterations in Gut Glutamate Metabolism Associated with Changes in Gut Microbiota Composition in Children with Autism Spectrum Disorder. mSystems 2019, 4, e00321-18. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Coretti, L.; Paparo, L.; Riccio, M.P.; Amato, F.; Cuomo, M.; Natale, A.; Borrelli, L.; Corrado, G.; De Caro, C.; Comegna, M.; et al. Gut Microbiota Features in Young Children with Autism Spectrum Disorders. Front. Microbiol. 2018, 9, 3146. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- I Kushak, R.; Buie, T.; Murray, K.F.; Newburg, D.S.; Chen, C.; Nestoridi, E.; Winter, H.S. Evaluation of Intestinal Function in Children with Autism and Gastrointestinal Symptoms. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 687–691. [Google Scholar] [CrossRef]
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism spectrum disorders and intestinal microbiota. Gut Microbes 2015, 6, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Pulikkan, J.; Maji, A.; Dhakan, D.B.; Saxena, R.; Mohan, B.; Anto, M.M.; Agarwal, N.; Grace, T.; Sharma, V.K. Gut Microbial Dysbiosis in Indian Children with Autism Spectrum Disorders. Microb. Ecol. 2018, 76, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Microbiome Disturbances and Autism Spectrum Disorders. Drug Metab. Dispos. 2015, 43, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Dhamayanti, M.; Noviandhari, A.; Supriadi, S.; Judistiani, R.T.; Setiabudiawan, B. Association of maternal vitamin D deficiency and infants’ neurodevelopmental status: A cohort study on vitamin D and its impact during pregnancy and childhood in Indonesia. J. Paediatr. Child Health 2019, 56, 16–21. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.-B. Intestinal microbiota and chronic constipation. Springerplus 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Parekh, T.; Lipkin, W.I. Application of Novel PCR-Based Methods for Detection, Quantitation, and Phylogenetic Characterization of Sutterella Species in Intestinal Biopsy Samples from Children with Autism and Gastrointestinal Disturbances. mBio 2012, 3, 211–261. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T. High carbohydrate diets and Alzheimer’s disease. Med. Hypotheses 2004, 62, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, W.; Boeing, H.; Robra, B.-P.; Seidler, A.; Vieregge, P.; Nischan, P.; Joerg, J.; Oertel, W.; Schneider, E.; Ulm, G. Diet and Parkinson’s disease II: A possible role for the past intake of specific nutrients: Results from a self-administered food-frequency questionnaire in a case-control study. Neurology 1996, 47, 644–650. [Google Scholar] [CrossRef]
- Abbott, R.D.; Ross, G.W.; White, L.R.; Sanderson, W.T.; Burchfiel, C.M.; Kashon, M.; Sharp, D.S.; Masaki, K.H.; Curb, J.D.; Petrovitch, H. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: Recent findings from the Honolulu-Asia Aging Study. J. Neurol. 2003, 250, iii30–iii39. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wang, H. The Association between Depression and Gastroesophageal Reflux based on Phylogenetic Analysis of miRNA Biomarkers. In Current Medicinal Chemistry; Bentham Science Publishers: Sharjah, Emirate, 2020; Volume 27. [Google Scholar]
- Nor, N.K.; Ghozali, A.H.; Ismail, J. Prevalence of Overweight and Obesity Among Children and Adolescents With Autism Spectrum Disorder and Associated Risk Factors. Front. Pediatr. 2019, 7, 38. [Google Scholar] [CrossRef]
- Ley, R.E. Obesity and the human microbiome. Curr. Opin. Gastroenterol. 2010, 26, 5–11. [Google Scholar] [CrossRef]
- Gibbons, H.; O’Gorman, A.; Brennan, L. Metabolomics as a tool in nutritional research. Curr. Opin. Lipidol. 2015, 26, 30–34. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nat. Cell Biol. 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Xu, Z.; Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 2015, 113, S1–S5. [Google Scholar] [CrossRef]
- Hosseinian, F.; Oomah, B.D.; Campos-Vega, R. Dietary Fibre Functionality in Food and Nutraceuticals: From Plant to Gut; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Martínez, I.; Lattimer, J.M.; Hubach, K.L.; A Case, J.; Yang, J.; Weber, C.G.; A Louk, J.; Rose, D.J.; Kyureghian, G.; A Peterson, D.; et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2012, 7, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and metabolic disease: Revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aslam, Z.; Kausar, F.; Irshad, H.; Anwer, P. Maternal malnutrition and its kick on child growth: An alarming trim for Pakistan. J. Food Nutr. Popul. Health 2017, 1, 24. [Google Scholar]
- Agrawal, S.; Rao, S.C.; Bulsara, M.K.; Patole, S.K. Prevalence of Autism Spectrum Disorder in Preterm Infants: A Meta-analysis. Pediatrics 2018, 142, e20180134. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.-C.; Lee, C.-H.; Wang, H. Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome. Int. J. Environ. Res. Public Health 2021, 18, 667. https://doi.org/10.3390/ijerph18020667
Fu S-C, Lee C-H, Wang H. Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome. International Journal of Environmental Research and Public Health. 2021; 18(2):667. https://doi.org/10.3390/ijerph18020667
Chicago/Turabian StyleFu, Shih-Chen, Chung-Han Lee, and Hsiuying Wang. 2021. "Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome" International Journal of Environmental Research and Public Health 18, no. 2: 667. https://doi.org/10.3390/ijerph18020667
APA StyleFu, S.-C., Lee, C.-H., & Wang, H. (2021). Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome. International Journal of Environmental Research and Public Health, 18(2), 667. https://doi.org/10.3390/ijerph18020667





