NoFumo+: A Clinical Trial of an mHealth for Smoking Cessation with Hospitalized Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
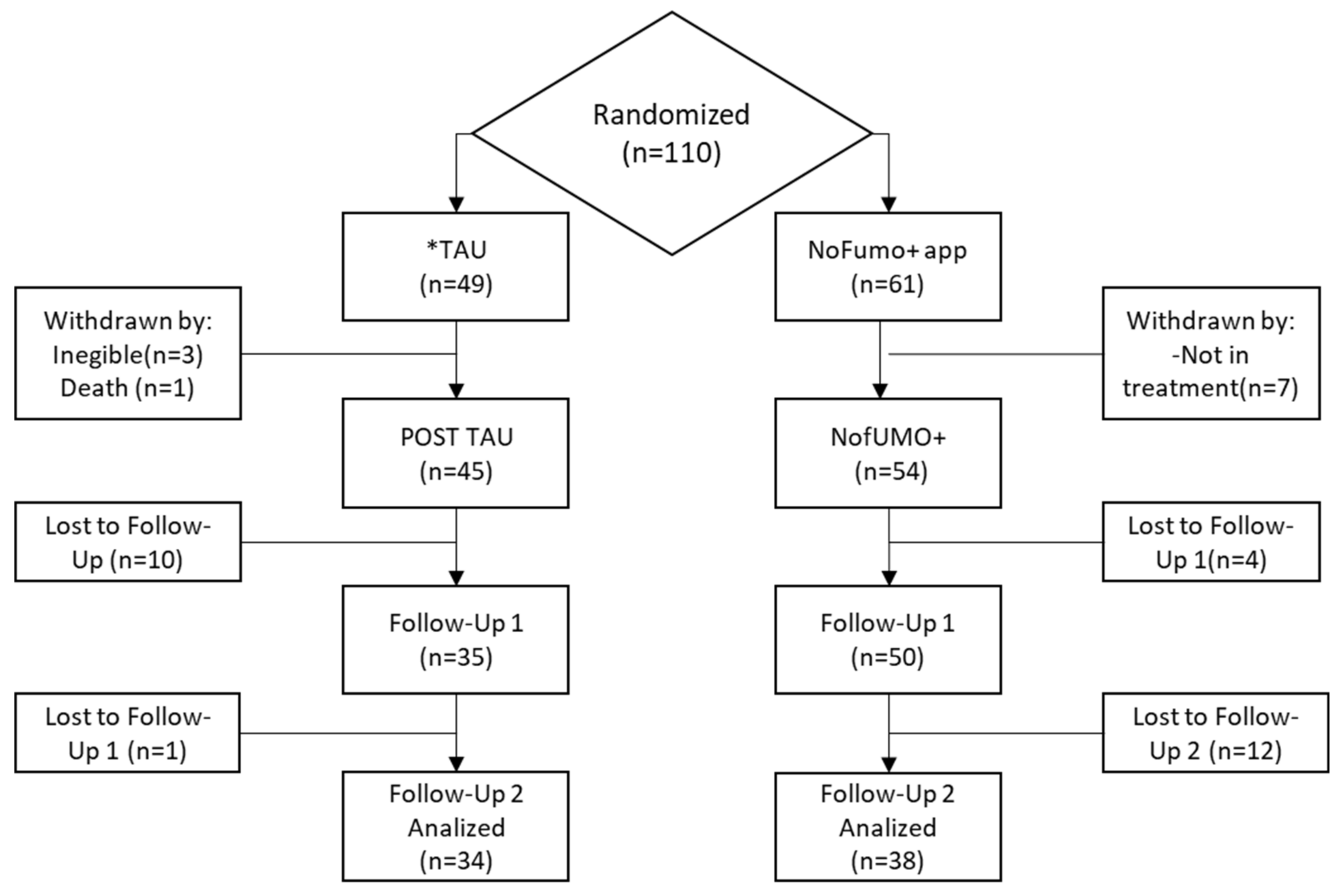
2.2. Participants
2.3. Intervention
2.3.1. NoFumo+
2.3.2. Treatment-As-Usual (TAU)
2.4. Procedure
2.5. Measures
2.5.1. Initial Interview
2.5.2. Instruments
2.5.3. The App Interface
2.5.4. Control and Follow-Up Interviews
2.6. Data Analysis
3. Results
3.1. Efficacy of the App at Post-Treatment and Follow-Up
3.2. Adherence Using the App
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| General Advice |
|
| Contact Your Health Center for Help with Your Detoxification. |
| Decalogue (* SEPAR) |
| Preparation: 1. Read information about smoking (e.g., think about why you smoke every time you light up a cigarette). 2. Write down a list of your reasons for not smoking (e.g., look for your reasons to quit smoking). 3. Choose smoke-free environments (e.g., avoid situations that you associate with smoking). 4. Inform family and friends (e.g., decide what day you are going to quit smoking). |
| Action Plan: What Should I Do on the First Day? |
| 5. Get up a little earlier and do physical exercise. Drink plenty of fluids (water, juices). 6. Be as active as possible throughout the day. 7. Make up your mind not to smoke, even if it is only for today. |
| How Do I Stay Smoke-Free? |
| 8. Practice some physical activity (better in a group) and eat a diet rich in fruits and vegetables. Reread your list of reasons to quit smoking. 9. If you feel a strong desire to smoke, don’t worry: relax, take a deep breath, and concentrate on what you are doing. 10. Don’t give in, even for one cigarette—Congratulate yourself for each day you go without smoking. |
References
- Global Report: Mortality Attributable to Tobacco. Available online: https://www.who.int/tobacco/publications/surveillance/en/ (accessed on 26 November 2020).
- Rahmah, S.; Oktamianti, P. Smoking Cessation Clinic, Hospital’s Participation in Supporting a Quit Smoking Program: A Systematic Review. KnE Life Sci. 2018, 4, 101–118. [Google Scholar] [CrossRef]
- Goodchild, M.; Nargis, N.; D’Espaignet, E.T. Global economic cost of smoking-attributable diseases. Tob. Control 2018, 27, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.B.; Graham, A.L.; Levy, D.T.; Mabry, P.L.; Tracy Orleans, D. Boosting Population Quits Throught Evidence-Based Cessation Treatment and Policy. Am. J. Prev. Med. 2011, 38, 351–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babb, S.; Malarcher, A.; Schauer, G.; Asman, K.; Ahmed, J. Quitting smoking among adults-United States, 2001–2010. Morbility Mortal. Wkly. Rep. 2017, 65, 1457–1464. [Google Scholar] [CrossRef]
- Fiore, M.C.; Jaén, C.R.; Baker, T.B.; Bailey, W.; Benowitz, N.; Curry, S.; Leitzke, C. Treating Tobacco Use and Dependence: 2008 Update. Public Health Service Clinical Practice Guideline Executive Summary. Respir. Care 2008, 53, 1217–1222. [Google Scholar]
- NICE: Stop Smoking Interventions and Services. Available online: https://www.nice.org.uk/guidance/ng92/resources/stop-smoking-interventions-and-services-pdf-1837751801029 (accessed on 20 November 2020).
- SEPAR:Tratamiento del Tabaquismo en Pacientes Hospitalizados. Available online: https://www.dropbox.com/s/thgavysthjyw082/Normativa71.PDF?dl=0 (accessed on 23 June 2019).
- Stead, L.F.; Carroll, A.J.; Lancaster, T. Group behaviour therapy programmes for smoking cessation (Review). Cochrane Database Syst. Rev. 2017, 3. [Google Scholar] [CrossRef]
- Stead, L.F.; Koilpillai, P.; Fanshawe, T.R.; Lancaster, T. Combined pharmacotherapy and behavioural interventions for smoking cessation (Review). Cochrane Database Syst. Rev. 2016, 3. [Google Scholar] [CrossRef]
- Andres, A.; Castellano, Y.; Fu, M.; Feliu, A.; Ballbè, M.; Antón, L.; Baena, A.; Fernández, E.; Martínez, C. Exploring individual and contextual factors contributing to tobacco cessation intervention implementation. Addict. Behav. 2018, 88, 163–168. [Google Scholar] [CrossRef]
- Lan, A.; Lee, A.; Munroe, K.; McRae, C.; Kaleis, L.; Keshavjee, K.; Guergachi, A. Review of cognitive behavioural therapy mobile apps using a reference architecture embedded in the patient-provider relationship. Biomed. Eng. Online 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Whittaker, R.; McRobbie, H.; Bullen, C.; Rodgers, A.; Gu, Y.; Dobson, R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2019, 10, CD006611. [Google Scholar] [CrossRef]
- Whittaker, R.; McRobbie, H.; Bullen, C.; Rodgers, A.; Gu, Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2016, 4, CD006611. [Google Scholar] [CrossRef]
- Regmi, K.; Kassim, N.; Ahmad, N.; Tuah, N.A. Effectiveness of Mobile Apps for Smoking Cessation: A Review. Tob. Prev. Cessat. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, A.H.; Philip, N.Y. A framework for smoking cessation in the Kingdom of Saudi Arabia using smart mobile phone technologies (Smoke Mind). In Proceedings of the 2015 Second International Conference on Computer Science, Computer Engineering, and Social Media (CSCESM), Lodz, Poland, 21–23 September 2015; pp. 96–102. [Google Scholar] [CrossRef]
- Finkelstein, J.; Cha, E.M.; Schindler-Ruwisch, J.; Heminger, C.; Boyd, A. Using a Mobile App to Promote Smoking Cessation in Hospitalized Patients. JMIR mHealth uHealth 2016, 4, e59. [Google Scholar] [CrossRef]
- García-Pazo, P.; Fornés-Vives, J.; Sesé, A.; Pérez-Pareja, F.J. Apps para dejar de fumar mediante Terapia Cognitivo Conductual. Una revisión sistemática. Adicciones 2020. [Google Scholar] [CrossRef] [PubMed]
- Heffner, J.L.; Vilardaga, R.; Mercer, L.D.; Kientz, J.A.; Bricker, J.B. Feature-level analysis of a novel smartphone application for smoking cessation. Am. J. Drug Alcohol Abus. 2015, 41, 68–73. [Google Scholar] [CrossRef]
- Haskins, B.L.; Lesperance, D.; Gibbons, P.; Boudreaux, E.D. A systematic review of smartphone applications for smoking cessation. Transl. Behav. Med. 2017, 7, 292–299. [Google Scholar] [CrossRef]
- Tratamiento del Tabaquismo en Pacientes Hospitalizados. Available online: https://www.sogapar.info/wp-content/uploads/2016/12/13-Tabaquismo-en-ingresados.pdf (accessed on 20 December 2020).
- Michie, S.; Hyder, N.; Walia, A.; West, R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict. Behav. 2011, 36, 315–319. [Google Scholar] [CrossRef]
- NICE Behaviour change: Digital and Mobile Health Interventions. 2020. Available online: https://www.nice.org.uk/guidance/indevelopment/gid-ng10101 (accessed on 20 January 2020).
- Torous, J.; Andersson, G.; Bertagnoli, A.; Christensen, H.; Cuijpers, P.; Firth, J.; Haim, A.; Hsin, H.; Hollis, C.; Lewis, S.; et al. Towards a consensus around standards for smartphone apps and digital mental health. World Psychiatry 2019, 18, 97–98. [Google Scholar] [CrossRef]
- Menezes, L.; Dias, Â.C.; Albino, D.; De Macêdo, D.; Lanzieri, I.O.; Richter, K.P.; Cristina, I.; Leite, G. Promoting cessation in hospitalized smoking patients: A systematic review. Rev. Assoc. Med. Bras. 2020, 66, 849–860. [Google Scholar]
- Rigotti, N.A.; Clair, C.; Munafo, M.; Stead, L.F. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst. Rev. 2012, 5, CD001837. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pérez, F.; Alonso-Cardeñoso, C.; García-González, J.V.; Fraile-Cobos, J.M.; Lobo-Llorente, N.; Secades-Villa, R. Efectividad de un programa multicomponente para dejar de fumar aplicado en atención primaria. Gac. Sanit. 2014, 28, 222–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Pareja, F.J.; Sesé, A.; Romo, A.F.; Palmer, A.; Tomás, M. Influencia de las Emociones Negativas (Ansiedad, Depresión e Ira) sobre la Eficacia de un Programa de Tratamiento Cognitivo Conductual de Deshabituación al Tabaco. Clínica Salud 2010, 21, 9–19. [Google Scholar] [CrossRef]
- Almaraz, D.A.; Alonso, M.M. Terapia Cognitivo Conductual para Dejar de Fumar: Revisión Sistemática. Enfermería Comunitaria 2018, 14, 1–9. [Google Scholar]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerstrom, K.-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- Richmond, R.; Kehoe, L.A.; Webster, I.W. Multivariate models for predicting abstention following intervention to stop smoking by general practitioners. Addiction 1993, 88, 1127–1135. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E.; Buela-Casal, G.; Guillén-Riquelme, N.S.C. Inventario de Ansiedad Estado-Rasgo, 9th ed.; TEA Ediciones: Madrid, Spain, 2015; ISBN 978-84-167231-15-7. [Google Scholar]
- Sanz, J.; Vázquez, C. Adaptación española del Inventario de Depresión de Beck-II (BDI-II); Pearson Education, S.A.: Madrid, Spain, 2011. [Google Scholar]
- Tobal, J.J.M.; Casado, M.I.; Cano, A.; Spielberger, C.D. STAXI-2 Inventario de Expresión de Ira Estado-Rasgo; TEA: Barcelona, Spain, 2014. [Google Scholar]
- Benowitz, N.L.; Iii, P.J.; Ahijevych, K.; Jarvis, M.; Hall, S.; Lehouezec, J.; Hansson, A.; Lichtenstein, E.; Henningfield, J.; Tsoh, J.; et al. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002, 4, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Carpenter, M.J. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob. Res. 2006, 8, 739–749. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Windows; Version 27.0; IBM Corp: Armonk, NY, USA, 2021.
- Lin, Y.; Tudor-Sfetea, C.; Siddiqui, S.; Sherwani, Y.; Ahmed, M.; Eisingerich, A.B. Effective Behavioral Changes through a Digital mHealth App: Exploring the Impact of Hedonic Well-Being, Psychological Empowerment and Inspiration. JMIR mHealth uHealth 2018, 6, e10024. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Sfetea, C.; Rabee, R.; Najim, M.; Amin, N.; Chadha, M.; Jain, M.; Karia, K.; Kothari, V.; Patel, T.; Suseeharan, M.; et al. Evaluation of Two Mobile Health Apps in the Context of Smoking Cessation: Qualitative Study of Cognitive Behavioral Therapy (CBT) Versus Non-CBT-Based Digital Solutions. JMIR mHealth uHealth 2018, 6, e98. [Google Scholar] [CrossRef]
- Bricker, J.B.; Mull, K.; Kientz, J.A.; Vilardaga, R.; Mercer, L.D.; Akioka, K.J.; Heffner, J.L. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend. 2014, 143, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bricker, J.B.; Copeland, W.; Mull, K.; Zeng, E.Y.; Watson, N.L.; Akioka, K.J.; Heffner, J.L. Single-arm trial of the second version of an acceptance & commitment therapy smartphone application for smoking cessation. Drug Alcohol Depend. 2017, 170, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Baskerville, N.B.; Struik, L.L.; Dash, D. Crush the Crave: Development and Formative Evaluation of a Smartphone App for Smoking Cessation. JMIR mHealth uHealth 2018, 6, e52. [Google Scholar] [CrossRef]
- Lee, M.; Lee, H.; Kim, Y.; Kim, J.; Cho, M.; Jang, J.; Jang, H. Mobile App-Based Health Promotion Programs: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2018, 15, 2838. [Google Scholar] [CrossRef]
- Granado-Font, E.; Ferré-Grau, C.; Rey-Reñones, C.; Pons-Vigués, M.; Ribera, E.P.; Berenguera, A.; Barrera-Uriarte, M.L.; Basora, J.; Valverde-Trillo, A.; Duch, J.; et al. Coping Strategies and Social Support in a Mobile Phone Chat App Designed to Support Smoking Cessation: Qualitative Analysis. JMIR mHealth uHealth 2018, 6, e11071. [Google Scholar] [CrossRef] [PubMed]
- Zeng, E.Y.; Vilardaga, R.; Heffner, J.L.; Mull, K.E.; Bricker, J.B. Predictors of Utilization of a Novel Smoking Cessation Smartphone App. Telemed. e-Health 2015, 21, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Mussulman, L.M.; Scheuermann, T.S.; Faseru, B.; Nazir, N.; Richter, K.P. Rapid relapse to smoking following hospital discharge. Prev. Med. Rep. 2019, 15, 100891. [Google Scholar] [CrossRef]
- Rigotti, N.A.; Arnsten, J.H.; McKool, F.M.; Wood-reid, K.M.; Pasternak, R.C.; Singer, D.E. Efficacy of a Smoking Cessation Program for Hospita Patients. JAMA Intern. Med. 1997, 157. [Google Scholar] [CrossRef]

| Box | Information (Text/Audio) | Contents (Short Videos and Leaflets) | Activities (Interactive and Notes) | Links (Internet/Web Pages) |
|---|---|---|---|---|
| 1 | Treatment plan (Establish objectives). Stimulus control (“Golden Rules”). | Tobacco components; nicotine; controlled breathing. | Relaxation training; encouraging social support (friend/family); recording benefits for those who quit smoking now. | Recommendations to stop smoking. |
| 2 | The analysis of smoking behavior. Prevention of response. Advice about change of routine. | Health effects of tobacco, pharmacological advice. | Write down reasons to stop smoking, encourage social support. | Information on how tobacco affects health. |
| 3 | Behavior analysis; the desire to smoke. Contents: Activities. | Abstinence syndrome. Advise on coping strategies. | Select and/or note optional activities. | Alternative coping strategies for withdrawal symptoms. |
| 4 | Maximize the self-rewarding experience. Contents | Physical benefits of quitting smoking. Social support. Distraction. Prevention of response. | Activities: Training in coping skills for risk situations. | Benefits of quitting smoking. |
| 5 | Analyze smoking behavior and desire to smoke, personal reasons to quit. | Identify risk situations. | Relaxation (Jacobson and autogenous). Feedback graphics. | Controlled breathing, self-guided relaxation technique (Jacobson). |
| 6 | Remember to control stimuli that remind you to smoke (e.g., situations, smoke-free spaces …). | Effective pharmacological treatments to stop smoking. | Activities to maintain abstinence. | Life without tobacco. |
| 7 | History of tobacco. Raising awareness in tobacco advertising. Assert yourself as a non-smoker. | Informing about the history of tobacco, psychological part of addiction. | Alternative activities for risk situations; Social support. | Harmful advertising about smoking. |
| 8 | Time management. Graphical feedback of behavior analysis. | Social support (chat). Distraction. | Smoking behavior analysis charts. | Common myths. |
| 9 | Negative thoughts. | Advice about errors in thinking and their modification; social chat. | Write down negative thoughts and modify them; talk to yourself. | Change in thinking. |
| 10 | Stress management. | The problem-solving technique. | Technical problem-solving training. | Problem-solving technique. |
| 11 | Advise about social skills as a technique. | Talking to oneself: Thought-stopping technique; social skills for cigarette refusal. | Training in social skills. | |
| 12 | Reinforcing abstinence as a reward and objective of the program. | Cognitive restructuring: True story (COPD). | Action in the event of a fall or/and relapse. | The reason for the fall and relapse. |
| 13 | Strengthen the ex-smoker’s identity (smoking is not an option). | The urge to smoke, the temptation. | Testimony of 2 ex-smoking patients (1 year and 25 years of abstinence). | Resources/testimonies from other ex-smokers. |
| 14 | Reinforcing alternative smoking behaviors. | Physical exercise of different intensities. Psychoeducation and behavioral activation. | Use a pedometer. | Program to promote physical exercise at different ages. |
| 15 | Reinforcement and re-objectives of the treatment in terms of monitoring in person and through the application. | Weight control. Training in planning (diet and exercise). | Harvard plate. | Healthy eating education and recipes. |
| Characteristics | Control Group | Experimental Group | p-Value | |
|---|---|---|---|---|
| n = 99 | 45.5% (45) | 53.5% (54) | ||
| Reason for admission | Respiratory | 48.8% (21) | 54.7% (29) | 0.55 |
| Cardiovascular | 18.6% (8) | 20.8% (11) | 1.00 | |
| Other | 32.6% (14) | 24.1% (13) | 0.50 | |
| Demographics | Age (Med, Q1–Q3) | 59.0, 48–67 | 54.0, 42.7–63.2 | 0.29 |
| Woman | 37.7% (17) | 25.9% (14) | 0.69 | |
| Man | 62.2% (28) | 55% (30) | 0.62 | |
| Married | 53.5% (23) | 51.9% (28) | 0.84 | |
| Educative level | Primary studies or less | 50.0% (21) | 46.0% (23) | 0.77 |
| Secondary studies | 09.5% (4) | 12.0% (6) | 1.00 | |
| Bachelor | 14.3% (6) | 12.0% (6) | 0.77 | |
| Vocational training | 16.7% (7) | 18.0% (9) | 0.79 | |
| University | 09.5% (4) | 12.0% (6) | 0.63 | |
| Tobacco History | Smoking family; Yes | 47.5% (19) | 48.0% (24) | 0.62 |
| Consumption start age | 15.0, 13.0–19.0 | 16.0, 14.0–18.0 | 0.40 | |
| Previous attempts; No | 78.6% (33) | 84.6% (44) | 0.21 | |
| Cigarettes per day | 21.5, 13.5–30.0 | 20.0, 10.0–20.0 | 0.45 | |
| Smoking years (Med, Q1–Q3) | 42.0, 34.0–51.0 | 40.0, 26.0–45.0 | 0.40 | |
| Cumulative dose/year (Med, Q1–Q3) | 33.0, 17.5–63.0 | 32.1, 12.3–45.2 | 0.62 | |
| Co-oximetry (CO) (Med, Q1–Q3) | 3.0, 2.0–6.0 | 4.0, 1–41 | 0.37 | |
| Smoking family, Yes | 47.5% (19) | 48.0% (24) | 0.62 | |
| Pharmacotherapy | None | 69.0% (20) | 67.3% (35) | 0.93 |
| TSN 1 | 13.8% (4) | 11.5% (6) | 0.89 | |
| Vareniclina | 17.2% (5) | 21.2% (11) | 0.72 | |
| Questionnaires (Med, Q1–Q3) | T. Fageström 2 | 4.00, 3–6 | 5, 3.25–6 | 0.30 |
| T.Richmond 3 | 7.50, 6–9.25 | 7.0, 5–9 | 0.30 | |
| BDI-II 4 | 9.00, 5–20.50 | 11.0, 6–16.50 | 0.55 | |
| STAI 5: State | 19.0, 10.75–31.0 | 21.0, 12.5–30.0 | 0.77 | |
| Traits | 21.0, 15.0–31.75 | 18.0, 13.0–29.0 | 0.25 | |
| STAXI 6: State | 10.0, 10–12 | 10.0, 10–12 | 0.99 | |
| Traits | 15.0, 12.0, 19.0 | 14.0, 12.0–18.0 | 0.89 |
| Questionnaires | GC Median (Q1–Q3) | GE2 Median (Q1–Q3) | p-Value * | |
|---|---|---|---|---|
| Post-treatment 2 months | BDI-II 1 | 11.0 (11.0–11.5) | 7.0 (3.5–15.25) | 0.68 |
| STAI 2-Estate | 12.5 (7.2–19.5) | 8.5 (2.5–22.25) | 0.68 | |
| STAXI 3-Estate | 10.0 (10.0–10.0) | 10.0 (10.0–10.75) | 0.62 | |
| Following 6 months | BDI-II 1 | 11.0 (6.00–13.5) | 4.0 (3.0–14.0) | 0.37 |
| STAI 2-Estate | 14.5 (8.70–30.5) | 14.0 (4.0–19.0) | 0.64 | |
| STAXI 3-Estate | 11.0 (10.0–12.7) | 10.0 (10.0–10.0) | 0.79 |
| Engagement | GE2 Med (Q1–Q3) |
|---|---|
| Days used | 42.00 (30.00–56.00) |
| Chat Interactions | 19.00 (3.25–54.25) |
| Days chat | 16.00 (3.70–24.50) |
| Chat duration (minutes) | 21.65 (4.70–70.50) |
| Emergency Interactions | 15.00 (7.20–40.00) |
| Send e-mail (frequency) | 0.00 (0.00–1.20) |
| Games online | 0.00 (0.00–0.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pazo, P.; Sesé, A.; Llabrés, J.; Fornés-Vives, J. NoFumo+: A Clinical Trial of an mHealth for Smoking Cessation with Hospitalized Patients. Int. J. Environ. Res. Public Health 2021, 18, 10476. https://doi.org/10.3390/ijerph181910476
García-Pazo P, Sesé A, Llabrés J, Fornés-Vives J. NoFumo+: A Clinical Trial of an mHealth for Smoking Cessation with Hospitalized Patients. International Journal of Environmental Research and Public Health. 2021; 18(19):10476. https://doi.org/10.3390/ijerph181910476
Chicago/Turabian StyleGarcía-Pazo, Patricia, Albert Sesé, Jordi Llabrés, and Joana Fornés-Vives. 2021. "NoFumo+: A Clinical Trial of an mHealth for Smoking Cessation with Hospitalized Patients" International Journal of Environmental Research and Public Health 18, no. 19: 10476. https://doi.org/10.3390/ijerph181910476
APA StyleGarcía-Pazo, P., Sesé, A., Llabrés, J., & Fornés-Vives, J. (2021). NoFumo+: A Clinical Trial of an mHealth for Smoking Cessation with Hospitalized Patients. International Journal of Environmental Research and Public Health, 18(19), 10476. https://doi.org/10.3390/ijerph181910476






