Abstract
Mucormycosis, a serious and rare fungal infection, has recently been reported in COVID-19 patients worldwide. This study aims to map all the emerging evidence on the COVID-19-associated mucormycosis (CAM) with a special focus on clinical presentation, treatment modalities, and patient outcomes. An extensive literature search was performed in MEDLINE (Ovid), Embase (Ovid), Cochrane COVID-19 Study Register, and WHO COVID-19 database till 9 June 2021. The primary outcome was to summarize the clinical presentation, treatment modalities, and patient outcomes of CAM. Data were summarized using descriptive statistics and presented in tabular form. This evidence mapping was based on a total of 167 CAM patients with a mean age of 51 ± 14.62 years, and 56.28% of them were male. Diabetes mellitus (73.65% (n = 123)), hypertension (22.75% (n = 38)), and renal failure (10.77% (n = 18)) were the most common co-morbidities among CAM patients. The most common symptoms observed in CAM patients were facial pain, ptosis, proptosis, visual acuity, and vision loss. Survival was higher in patients who underwent both medical and surgical management (64.96%). Overall mortality among CAM patients was found to be 38.32%. In conclusion, this study found a high incidence of CAM with a high mortality rate. Optimal glycemic control and early identification of mucormycosis should be the priority to reduce the morbidity and mortality related to CAM.
Keywords:
COVID-19; diabetes; epidemiology; evidence; mortality; mucormycosis; mycoses; public health 1. Introduction
The coronavirus disease (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 228 million people globally, with about 4.7 million deaths as of 21 September 2021 [1]. The novel COVID-19 strains that have emerged this year are more severe variants of the disease and have resulted in higher intensive care unit (ICU) admissions, need for mechanical ventilation, and mortality [2,3]. This, consequently, has increased the burden on healthcare systems globally [4].
COVID-19 patients often have several comorbidities, including diabetes [5]. Ample evidence has found patients with comorbidities to be at higher risk of ICU admissions and mortality [5,6,7]. Study findings by Liu et al. from Wuhan Union Hospital found a more intense level of lymphocytopenia and cytokine storm in patients with severe COVID-19 compared to that in patients with mild disease [8]. Despite the colossal impact of this pandemic gripping the world, there are limited treatment options for it. COVID-19 patients in severe or critical stages (admitted to ICUs) are prescribed high doses of steroids as a life-saving measure [9]. Steroids suppress the immune system (decrease in CD4 + T and CD8 + T cells) to fight against the inflammation caused by the virus, thereby creating a favorable environment for other opportunistic infections [9,10]. This can make the immunocompromised COVID-19 patients more susceptible to a range of viral, bacterial, fungal, and other microbial co-infections [11]. Multiple studies have confirmed that patients with severe COVID-19 admitted to ICUs have a high occurrence of secondary infections and relatively infrequent bacterial co-infection [12,13,14].
Mucormycosis, a serious and rare fungal infection, has occurred concurrently in COVID-19 patients globally [15]. COVID-19-associated mucormycosis (CAM) notably created havoc in the second wave of COVID-19 in India. Mucormycosis, also known as black fungus, is an invasive fungal infection most commonly caused by species of the genus Rhizopus [16]. Other species causing this fungal infection include those belonging to the genera Apophysomyces, Absidia, Mucor, and others. Amongst the various types of mucormycosis, rhino-orbital-cerebral is the most common one [17]. Risk factors associated with the development of fungal infection among COVID-19 patients include diabetes, neutropenia, hematological malignancy, stem cell transplant recipients, patients receiving corticosteroid treatment, and individuals in the immunocompromised state [18,19]. Mucormycosis is associated with a high risk of all-cause mortality (54%), with mortality depending on body site infected, fungus type, and the patient’s overall condition [20].
This deadly fungal infection is clinically challenging and expensive to treat and puts a high toll on public health and a humanistic and economic burden on individuals and healthcare systems [21,22]. Low- and middle-income countries such as India witnessed a massive number of CAM cases in the second wave of COVID-19, leading to a collapse of the health system in the midst of the pandemic. The Indian government (state governments) declared mucormycosis as an outbreak in May 2021 [23]. Evidence from previous published studies was based on fewer cases and limited information [24,25].
Presently, more detailed evidence on the clinical presentation, treatment modalities, and patient outcomes is required. The preliminary search for mapping existing evidence was performed on 25 May 2021, in Epistemonikos, the international prospective register of systematic reviews (PROSPERO), Open Science Framework (OSF), Cochrane Library, and Jonna Briggs Institute (JBI) Evidence Synthesis, and no previous evidence mapping was identified. Therefore, we conducted this study with an objective to map all the emerging evidence on the CAM with a particular focus on each minute detail of clinical presentation, treatment modalities, and patient outcomes.
2. Materials and Methods
The proposed study was developed by adhering to the JBI methodology for evidence mapping and is reported as per the Preferred Reporting Items for Systematic Reviews and Meta-analyses for Scoping Reviews (PRISMA-ScR) [26,27]. Compliance with the PRISMA-ScR is presented in Supplementary Table S1.
Furthermore, this review was conducted by adhering to our protocol registered prospectively at OSF with an identification number (osf.io/438sm) and published as a preprint at the Preprint Server for Health Sciences (medRxiv) [28]. There were slight deviations from the protocol; firstly, the critical appraisal was skipped as it is not mandatory as per the JBI guidelines. The second deviation was the inclusion of suspected COVID-19 cases with confirmed mucormycosis, as patients developed mucormycosis after recovery from COVID-19.
2.1. Eligibility Criteria
2.1.1. PCC Elements
According to the JBI reviewer’s manual, the following PCC (Population, Concept, and Context) elements were used for this review.
- (a)
- Participants: patients with confirmed COVID-19 (RT-PCR) and mucormycosis (either histologically or microbiologically confirmed) based on the definition of Centers for Disease Control and Prevention were included in the study. We also included studies with suspected COVID-19 patients (based on the included studies assessment) who had confirmed mucormycosis.
- (b)
- Concept and context: this review included all studies that described the clinical presentation, treatment modalities, and patient outcomes of CAM.
2.1.2. Types of Sources
We included analytical observational studies (cohort, case–control) and descriptive observational studies (case report, case series, cross-sectional).
2.1.3. Exclusion Criteria
- (a)
- Non-English language studies;
- (b)
- studies with no confirmed mucormycosis; and
- (c)
- systematic reviews, narrative reviews, editorials, opinions, and study protocols were excluded.
2.2. Information Sources and Search Strategy
A three-step search strategy was utilized to identify published, unpublished, or ongoing studies with no language restrictions. An initial limited search was undertaken in MEDLINE (Ovid), followed by analyzing the text words in the title and abstract and the index terms assigned to the articles. Slightly modified Ovid Expert Searches for COVID-19 were combined with keywords and index terms related to mucormycosis to perform the searches in MEDLINE (Ovid) [29] and Embase (Ovid) [30] (Appendix A).
On 9 June 2021, we conducted a second search in MEDLINE (Ovid), Embase (Ovid), Cochrane COVID-19 Study Register, and the World Health Organization (WHO) COVID-19 database.
Complete search strategies are presented in Supplementary Table S2 for each database with their respective hits. Third, the manual search of reference lists of all included studies and relevant systematic reviews was screened for any potentially eligible studies. Citation tracking was also performed for all the included articles.
2.3. Selection Process
Two independent reviewers (S.H. and H.B.) screened all the retrieved articles against the eligibility criteria. We included all those articles describing the mucormycosis case (diagnosed either based on histopathology, culture, or stain) in COVID-19-positive patients.
In the initial screening phase, articles were selected based on the title and abstract scanning. In the second phase, full-text screening was performed for the final inclusion of articles. Any confusion regarding study inclusion was resolved by discussion with the third reviewer (M.K.). A detailed description of the study selection process is shown using the PRISMA flow diagram in Figure 1.
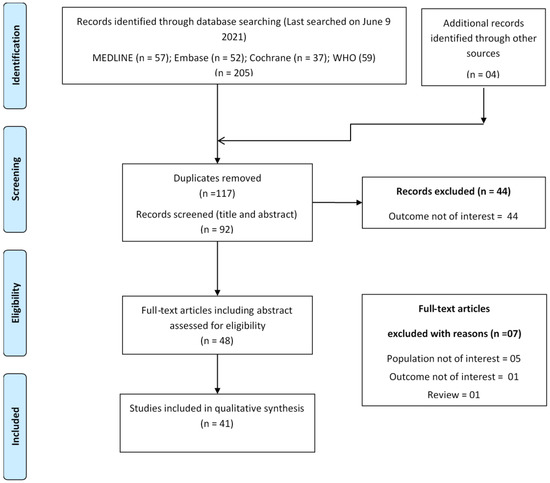
Figure 1.
PRISMA flowchart showing study selection process.
2.4. Data Extraction
Two reviewers (S.H. and H.B.) independently extracted the data in a pre-designed data extraction template. The following information was extracted from all the eligible studies qualified for inclusion: study author, year of publication, country, study design, demographic characteristics of the population (age and sex), sample size, comorbidities, treatment for COVID-19, symptoms of mucormycosis, diagnosis of mucormycosis, identification of fungal species, treatment for mucormycosis, and patient outcomes. The included studies are described using descriptive statistics and presented in a tabular form.
3. Results
A total of 209 articles were identified by searching the selected sources. After removing duplicates, only 92 articles were found to be unique. After the full-text screening, 37 studies [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] qualified for inclusion in this evidence mapping study. Four additional articles [68,69,70,71] were identified by hand search during bibliography screening and citation tracking. Finally, a total of 41 articles were included in this review [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. Refer to Supplementary Table S3 for the list of articles excluded during full-text screening with reason.
3.1. Studies Characteristics
Out of 41 studies, the majority of studies (n = 15) were from India with 82 mucormycosis cases, 9 studies with 9 cases of mucormycosis were from the USA, while only 3 studies were from Iran but with 17 mucormycosis cases. Most of the included studies were case reports (n = 27) followed by case series (n = 9), and the rest were of other study designs. Diabetes mellitus (73.65% (n = 123)), hypertension (22.75% (n = 38)), and renal failure (10.77% (n = 18)) were the most common co-morbidities among CAM patients. Diabetic ketoacidosis was observed in one-tenth of the diabetic patients.
3.2. Clinical Presentation
This evidence mapping was based on a total of 167 CAM patients with a mean age of 51 ± 14.62 years, of which 56.28% of them were male. COVID-19 was confirmed through the RT-PCR test in approximately three-fourth (74%) of the included studies.
The majority of the patients (76.04%) were treated using steroids, while only 11.64% of patients were treated with remdesivir to manage COVID-19. Most patients who developed mucormycosis had severe (based on included studies’ categorization) or critical COVID-19 (defined based on ICU status/mechanical ventilation).
Twenty-nine (17.57%) patients had concurrent CAM, while the remaining patients were diagnosed with CAM after an average of 19.24 days. Mucormycosis was diagnosed using stain (24 studies), culture (26 studies), or histopathology (30 studies), and nine studies diagnosed mucormycosis using all three diagnostic techniques. The Rhizopus species were the most common fungal species infecting CAM patients (13.77%).
Facial pain, ptosis, proptosis, visual acuity, and vision loss were the most common symptoms observed in CAM patients. Rhino-orbital (16%) followed by rhino-orbital-cerebral (11.3%) mucormycosis was the most common form of mucormycosis found in CAM patients (Table 1).

Table 1.
Summary of study characteristics and anamnestic, diagnostic, and treatment features of COVID-19-associated mucormycosis (CAM) cases.
3.3. Treatment Modalities and Outcomes
Liposomal amphotericin B in various doses (5 mg/kg/day) was the most commonly used drug for managing mucormycosis infection in 158 patients (35 studies). Adjunct surgery was performed on 142 patients, and surgical debridement was the most common surgical procedure performed. Only 23 CAM patients were managed without surgery, and most of them (18 CAM patients) died between 7 to 62 days after the diagnosis of mucormycosis.
Survival was higher in patients who underwent both medical and surgical management (64.96%) than in CAM patients who underwent medical management only (21.73%). Overall mortality among CAM patients in the included studies was 38.32% (n = 64). The patients died between 6 to 90 days after mucormycosis diagnosis (Table 2).

Table 2.
Treatment details and patient outcomes.
4. Discussion
To the best of our knowledge, this is the most comprehensive and up-to-date evidence mapping aimed to explore the published and unpublished evidence on the clinical presentation, treatment modalities, and patient outcomes of CAM. The current body of evidence was based on the 41 studies that met our inclusion criteria and discussed the association of COVID-19 with mucormycosis.
Mucormycosis is a rare opportunistic infection, and COVID-19 patients are at risk of developing mucormycosis because of pre-compromised immune systems. A growing body of evidence supports that comorbidities (diabetes, transplantation, malignancies) and medications (steroids) make the patients more vulnerable to CAM [5,6,7]. A recent case report found an invasive pulmonary mucormycosis case in a patient after a short course of steroids [72]. Likewise, Pan et al. found mucormycosis in a patient with AIDS receiving short-term systemic steroids [73]. In our study, we found that COVID-19 patients with comorbidities had a higher occurrence of mucormycosis.
Around 50% of CAM cases in our study were reported from India. A possible reason for this could be the deadly COVID-19 delta variant wave infecting around half a million people every day in recent months and a high prevalence of diabetes mellitus in CAM patients [74]. Diabetes mellitus is a predisposing factor for the development of mucormycosis [75,76]. The potential mechanism behind this could be the aggravation of the inflammatory state due to hyperglycemia and activation of antiviral immunity [77]. The risk of developing CAM increases significantly in patients with diabetic ketoacidosis, where Mucorales use free iron levels in the serum for pathogenesis [78].
In our study, the number of male mucormycosis patients was twice the number of female patients. These findings are aligned with a previously published study by Roden et al. [79] that found mucormycosis in 65% of male patients.
Rhino-orbital and rhino-orbital-cerebral were the most common forms of mucormycosis observed in this study. In both forms of infection, the fungus invades the nasal mucosa and orbital wall and leads to the occurrence of symptoms such as facial pain, vision loss, proptosis, apoptosis, and ophthalmoplegia [80,81]. CAM patients who underwent both surgical and medical management had a better survival rate than those with medical management alone. Published studies from different parts of the world have also found better outcomes in mucormycosis patients who underwent combined surgical and medical management [82,83]. However, despite the best management of CAM patients, the overall mortality was high, suggesting the need for the early identification of cases.
Our study findings suggest that clinical practitioners (intensivists and their teams) should be alerted about the increased possibility of CAM in critically ill COVID-19 patients; therefore, they should act proactively and monitor for potential fungal and bacterial co-infections and secondary infections among the COVID-19 cohorts, especially the immunocompromised and diabetic patients [84]. Moreover, these findings call drug regulators and health systems, especially in low- and lower-middle-income countries, to implement strict policies for steroid stewardship.
4.1. Limitations
Like every study, this evidence mapping has few limitations. Firstly, we could not differentiate the outcome based on glycemic-controlled status due to the lack of information on the glycosylated hemoglobin value of the CAM patients with diabetes in the included studies. Secondly, there was variability in the definition of severity of COVID-19 in the included studies. Lastly, limited information (fungal species identified, RT-PCR result) in a few included studies was also a drawback.
4.2. Strengths
The major strength of this review was a large number of exhaustive literature searches in major databases, a protocol-oriented approach, most up-to-date evidence with sound methodology, and the capture of each minute detail of 167 CAM patients to make this review a one-stop source of information for CAM.
5. Conclusions
This evidence mapping found a high incidence of CAM with a high mortality rate. Therefore, clinicians should cautiously use the steroids using the risk–benefit analysis approach. Optimal glycemic control and early identification of mucormycosis should be the priority to reduce the morbidity and mortality related to CAM.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph181910340/s1, Table S1: PRISMA-ScR checklist, Table S2: Search strategy, Table S3: List of excluded articles.
Author Contributions
Conceptualization, S.H. and M.K.; methodology, S.H. and M.K.; formal analysis, S.H.; investigation, S.H. and H.B.; resources, M.K.; data curation, S.H. and H.B.; writing—original draft preparation, S.H.; writing—review and editing, S.H., H.B., A.R., J.K., A.P., S.S., R.L., A.K.N. and M.K.; supervision, S.H. and M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
S.H. was supported by the Operational Program Research, Development and Education Project, Postdoc2MUNI (Nno. CZ.02.2.69/0.0/0.0/18_053/0016952). A.R., J.K., A.P., R.L. and M.K. were supported by the INTER-EXCELLENCE grant number LTC20031 toward an International Network for Evidence-based Research in Clinical Health Research in the Czech Republic. The work of A.R. was also supported by Masaryk University grants MUNI/IGA/1543/2020 and MUNI/A/1608/2020.
Institutional Review Board Statement
The study was exempted from ethical approval due to its observational nature and the use of publicly accessible data.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (S.H. or M.K.) upon reasonable request.
Acknowledgments
This work is dedicated to the more than 3 million worldwide fatalities and their families who have fallen victim to COVID-19.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. MEDLINE® ALL <1946 to 8 June 2021> (Ovid)
| # | Search String | No. of Results |
| 1 | exp Coronavirus/ | 77,269 |
| 2 | exp Coronavirus Infections/ | 94,303 |
| 3 | (coronavirus* or corona virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars-cov* or sarscov* or Sars-coronavirus* or Severe Acute Respiratory Syndrome Coronavirus* or “Kawasaki like paediatric inflammatory multisystem syndrome” or “Kawasaki like pediatric inflammatory multisystem syndrome” or “PIMS-TS” or “Kawa-COVID-19” or “MIS-C” or “multisystem inflammatory syndrome in children” or pediatric multisystem inflammatory disease).mp. | 159,987 |
| 4 | (or/1–3) and ((20191* or 202*).dp. or 20190101:20301231.(ep).) (147001) | 147,001 |
| 5 | 4 not (SARS or SARS-CoV or MERS or MERS-CoV or Middle East respiratory syndrome or camel * or dromedary* or equine or coronary or coronal or covidence* or covidien or influenza virus or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SADS-CoV or canine or CCov or zoonotic or avian influenza or H1N1 or H5N1 or H5N6 or IBV or murine corona*).mp. | 54,231 |
| 6 | ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019-ncov or sars*).mp. or exp pneumonia/) and Wuhan.mp. | 5278 |
| 7 | (2019-ncov or ncov19 or ncov-19 or 2019-novel CoV or sars-cov2 or sars-cov-2 or sarscov2 or sarscov-2 or SARS-2-nCoV or SARS-2-Cov or SARS-COV-19 or Sars-coronavirus2 or Sars-coronavirus-2 or SARS 2 coronavirus* or Severe Acute Respiratory Syndrome-CoV-2 or SARS-like coronavirus* or coronavirus-19 or covid19 or covid-19 or covid 2019 or ((novel or new or nouveau) adj2 (CoV or nCoV or covid or coronavirus* or corona virus or Pandemi*2)) or ((covid or covid19 or covid-19 or SARS-CoV-2) and pandemic*2) or (coronavirus* and pneumonia)).mp. | 144,923 |
| 8 | (COVID-19 or SARS-CoV-2).rx,px,ox,rn. or (COVID-19 or COVID-19 serotherapy or ORF7b protein, SARS-CoV-2 or ORF6 protein, SARS-CoV-2 or ORF8 protein, SARS-CoV-2 or pediatric multisystem inflammatory disease, COVID-19 related or envelope protein, SARS-CoV-2 or ORF7a protein, SARS-CoV-2 or spike protein, SARS-CoV-2 or ORF3a protein, SARS-CoV-2 or COVID-19 drug treatment or severe acute respiratory syndrome coronavirus 2 or membrane protein, SARS-CoV-2 or ORF1ab polyprotein, SARS-CoV-2 or nucleocapsid protein, Coronavirus or COVID-19 vaccine or COVID-19 diagnostic testing).os,ps,rn,rs. | 8460 |
| 9 | (“32185863” or “32172715” or “32227595” or “32140676” or “32246156” or “32267941” or “32176889” or “32169616” or “32265186” or “32253187” or “32152148” or “32053580” or “32179788” or “32213260” or “32205350” or “32188729” or “32152361” or “32277065” or “32088947” or “32240583” or “31917786” or “32127714” or “32047315” or “32020111” or “32240632” or “32243118” or “32267344” or “32239781” or “32396977” or “32402130” or “32243299” or “32807526” or “32344395” or “32403202” or “32389714” or “32416016” or “32405099” or “32976849” or “32685966” or “33221888” or “32379271” or “32188728” or “32221976” or “32417321” or “32489438” or “32332959” or “32943452” or “32807525” or “32826274” or “32898560” or “32293023” or “33159926” or “32919952” or “32835716” or “32619499” or “32663524” or “32392627” or “32392625” or “33037657” or “32777045” or “32521569” or “32492200” or “32930765” or “33075143” or “32237249” or “32683439” or “32495994” or “32344447” or “32896006” or “32240549” or “32438448” or “32425477” or “32951095” or “32274794” or “32750178” or “32463935” or “32428286” or “32491981” or “32930748” or “32119409” or “32432657” or “33003176” or “32459319” or “32822920” or “32878290” or “32270498” or “32250493” or “32512243” or “32837399” or “32426074” or “32199942” or “32839969” or “32639522” or “33073717” or “32502134” or “32334003” or “32510470” or “32819741” or “32309248” or “32243951” or “32378772” or “32835361” or “32962779” or “32916324” or “32785973” or “32272221” or “32299207” or “33044515” or “33134955” or “32970917” or “32407438” or “32513790” or “32439468” or “33063036” or “33077677” or “32406056” or “32716821” or “32588590” or “32239757” or “32829902” or “32807521” or “32379350” or “33125767” or “32829731” or “32988821” or “32780977” or “32648633” or “32829907” or “32330635” or “32692998” or “33013067” or “33010706” or “32502292” or “32780969” or “32998780” or “32754731” or “32639607” or “32233030” or “32953429” or “32246897” or “32955802” or “32425490” or “32418270” or “32445255” or “32775945” or “32775948” or “32775953” or “32407043”).ui. | 152 |
| 10 | or/5–9 | 147,637 |
| 11 | 10 and 20191201:20301231.(dt). | 145,485 |
| 12 | exp Zygomycosis/ | 4474 |
| 13 | mucormycos#s.mp. | 5053 |
| 14 | Mucormycose.mp. | 98 |
| 15 | mucoromycos#s.mp. | 6 |
| 16 | zygomycos#s.mp. | 1414 |
| 17 | (black fungus or black fungi).mp. | 192 |
| 18 | exp Mucorales/ | 6616 |
| 19 | Mucorales.mp. | 192 |
| 20 | mucoralean.mp. | 70 |
| 21 | Absidia.mp. | 562 |
| 22 | Cunninghamella.mp. | 768 |
| 23 | Mortierella.mp. | 751 |
| 24 | Mucor.mp. | 3382 |
| 25 | Apophysomyces.mp. | 147 |
| 26 | Saksenaea.mp. | 102 |
| 27 | Rhizopus.mp. | 4211 |
| 28 | Rhizomucor.mp. | 691 |
| 29 | Lichtheimia.mp. | 191 |
| 30 | Cokeromyces.mp. | 24 |
| 31 | Actinomucor.mp. | 58 |
| 32 | Syncephalastrum.mp. | 163 |
| 33 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 14,534 |
| 34 | 11 and 33 | 57 |
| Search was conducted on 9 June 2021 at 4:25 p.m. (CET). | ||
References
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 22 September 2021).
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ 2021, 372, n579. [Google Scholar]
- Vaidyanathan, G. Coronavirus variants are spreading in India—What scientists know so far. Nature 2021, 593, 321–322. [Google Scholar] [CrossRef]
- Miller, I.F.; Becker, A.D.; Grenfell, B.T.; Metcalf, C.J.E. Disease and healthcare burden of COVID-19 in the United States. Nat. Med. 2020, 26, 1212–1217. [Google Scholar] [CrossRef]
- Hussain, S.; Baxi, H.; Jamali, M.C.; Nisar, N.; Hussain, M.S. Burden of diabetes mellitus and its impact on COVID-19 patients: A meta-analysis of real-world evidence. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1595–1602. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef]
- Tian, W.; Jiang, W.; Yao, J.; Nicholson, C.J.; Li, R.; Sigurslid, H.; Wooster, L.; Rotter, J.I.; Guo, X.; Malhotra, R. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J. Med. Virol. 2020, 92, 1875–1883. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Singh, A.K.; Majumdar, S.; Singh, R.; Misra, A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.I.; Singanayagam, A. Immunosuppression for hyperinflammation in COVID-19: A double-edged sword? Lancet 2020, 395, 1111. [Google Scholar] [CrossRef]
- Chen, X.; Liao, B.; Cheng, L.; Peng, X.; Xu, X.; Li, Y.; Hu, T.; Li, J.; Zhou, X.; Ren, B. The microbial co-infection in COVID-19. Appl. Microbiol. Biotechnol. 2020, 1–9. [Google Scholar] [CrossRef]
- Feldman, C.; Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Abdoli, A. Helminths and COVID-19 Co-Infections: A Neglected Critical Challenge. ACS Pharmacol. Transl. Sci. 2020, 3, 1039–1041. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- El-Herte, R.I.; Baban, T.A.; Kanj, S.S. Mucormycosis: A review on environmental fungal spores and seasonal variation of human disease. Adv. Infect. Dis 2012, 2, 76–81. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Wali, U.; Balkhair, A.; Al-Mujaini, A. Cerebro-rhino orbital mucormycosis: An update. J. Infect. Public Health 2012, 5, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.; Kong, D.C.; Chen, S.C.-A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Mucormycosis Statistics. Available online: https://www.cdc.gov/fungal/diseases/mucormycosis/statistics.html (accessed on 10 June 2021).
- Kontoyiannis, D.P.; Yang, H.; Song, J.; Kelkar, S.S.; Yang, X.; Azie, N.; Harrington, R.; Fan, A.; Lee, E.; Spalding, J.R. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: A retrospective study. BMC Infect. Dis. 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Heimann, S.M.; Vehreschild, M.J.; Cornely, O.A.; Heinz, W.J.; Grüner, B.; Silling, G.; Kessel, J.; Seidel, D.; Vehreschild, J.J. Healthcare burden of probable and proven invasive mucormycosis: A multi-centre cost-of-illness analysis of patients treated in tertiary care hospitals between 2003 and 2016. J. Hosp. Infect. 2019, 101, 339–346. [Google Scholar] [CrossRef]
- ‘Black Fungus’ Declared an Epidemic in 4 States, 1 UT. Available online: http://timesofindia.indiatimes.com/articleshow/82804720.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst (accessed on 10 June 2021).
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102146. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Singh, B.; Bhadada, S.K.; Banerjee, M.; Bhogal, R.S.; Hage, N.; Kumar, A. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses 2021. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Godfrey, C.; McInerney, P.; Soares, C.B.; Khalil, H.; Parker, D. Methodology for JBI scoping reviews. In The Joanna Briggs Institute Reviewers Manual 2015; Joanna Briggs Institute: Adelaide, Australia, 2015; pp. 3–24. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Perters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Baxi, H.; Riad, A.; Kulgarova, J.; Licenik, R.; Klugar, M. COVID-19 Associated Mucormycosis: Scoping Review Protocol. medRxiv 2021. [Google Scholar] [CrossRef]
- Expert Search Coronavirus (Covid-19) 2019-nCoV on MEDLINE. 2020. Available online: https://tools.ovid.com/ovidtools/expertsearches.html (accessed on 9 June 2021).
- Expert Search COVID-19 Embase 1974 to Present. 2020. Available online: https://tools.ovid.com/coronavirus/ (accessed on 9 June 2021).
- Alekseyev, K.; Didenko, L.; Chaudhry, B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J. Med. Cases 2021, 12, 85. [Google Scholar] [CrossRef]
- Arana, C.; Ramírez, R.E.C.; Xipell, M.; Casals, J.; Moreno, A.; Herrera, S.; Bodro, M.; Cofan, F.; Diekmann, F.; Esforzado, N. Mucormycosis associated with covid19 in two kidney transplant patients. Transpl. Infect. Dis. 2021, 23, e13652. [Google Scholar] [CrossRef]
- Ashour, M.M.; Abdelaziz, T.T.; Ashour, D.M.; Askoura, A.; Saleh, M.I.; Mahmoud, M.S. Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients: A case series and a review of literature. J. Neuroradiol. 2021, 48, 319–324. [Google Scholar] [CrossRef]
- Bayram, N.; Ozsaygılı, C.; Sav, H.; Tekin, Y.; Gundogan, M.; Pangal, E.; Cicek, A.; Özcan, I. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn. J. Ophthalmol. 2021, 65, 515–525. [Google Scholar] [CrossRef]
- Bellanger, A.-P.; Navellou, J.-C.; Lepiller, Q.; Brion, A.; Brunel, A.-S.; Millon, L.; Berceanu, A. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect. Dis. Now 2021, 51, 633–635. [Google Scholar]
- Dallalzadeh, L.O.; Ozzello, D.J.; Liu, C.Y.; Kikkawa, D.O.; Korn, B.S. Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit 2021, 1–4. [Google Scholar] [CrossRef]
- Do Monte Junior, E.S.; Dos Santos, M.E.L.; Ribeiro, I.B.; de Oliveira Luz, G.; Baba, E.R.; Hirsch, B.S.; Funari, M.P.; de Moura, E.G.H. Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report. Clin. Endosc. 2020, 53, 746–749. [Google Scholar] [CrossRef]
- El-Kholy, N.A.; Abd El-Fattah, A.M.; Khafagy, Y.W. Invasive fungal sinusitis in post COVID-19 patients: A new clinical entity. Laryngoscope 2021. [Google Scholar] [CrossRef]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus disease (Covid-19) associated mucormycosis (CAM): Case report and systematic review of literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.; Naresh, K.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253. [Google Scholar] [CrossRef]
- Johnson, A.K.; Ghazarian, Z.; Cendrowski, K.D.; Persichino, J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med. Mycol. Case Rep. 2021, 32, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.; Jordan, A.; Olewiler, S.; Wehberg, K.; Cortes, M.; Jackson, B.R. A fatal case of Rhizopus azygosporus pneumonia following COVID-19. J. Fungi 2021, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Galougahi, M.; Arastou, S.; Haseli, S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2021, 11, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Chang, K.-M.; Berlinrut, I.; Wallach, F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient—Case report and review of literature. J. Med. Mycol. 2021, 31, 101125. [Google Scholar] [CrossRef]
- Krishna, D.S.; Raj, H.; Kurup, P.; Juneja, M. Maxillofacial Infections in Covid-19 Era—Actuality or the Unforeseen: 2 Case Reports. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–4. [Google Scholar] [CrossRef]
- Krishna, V.; Morjaria, J.; Jalandari, R.; Omar, F.; Kaul, S. Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection. IDCases 2021, 25, e01172. [Google Scholar] [CrossRef]
- Maini, A.; Tomar, G.; Khanna, D.; Kini, Y.; Mehta, H.; Bhagyasree, V. Sino-orbital mucormycosis in a COVID-19 patient: A case report. Int. J. Surg. Case Rep. 2021, 82, 105957. [Google Scholar] [CrossRef]
- Mehta, S.; Pandey, A. Rhino-orbital mucormycosis associated with COVID-19. Cureus 2020, 12, e10726. [Google Scholar] [CrossRef]
- Mekonnen, Z.K.; Ashraf, D.C.; Jankowski, T.; Grob, S.R.; Vagefi, M.R.; Kersten, R.C.; Simko, J.P.; Winn, B.J. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e40. [Google Scholar] [CrossRef]
- Meshram, H.S.; Kute, V.B.; Chauhan, S.; Desai, S. Mucormycosis in post-COVID-19 renal transplant patients: A lethal complication in follow-up. Transpl. Infect. Dis. 2021, 23, e13663. [Google Scholar] [CrossRef]
- Moorthy, A.; Gaikwad, R.; Krishna, S.; Hegde, R.; Kale, P.G.; Rao, P.S.; Haldipur, D.; Bonanthaya, K. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids—An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. J. Maxillofac. Oral Surg. 2021, 20, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Nehara, H.R.; Puri, I.; Singhal, V.; Ih, S.; Bishnoi, B.R.; Sirohi, P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian J. Med Microbiol. 2021, 39, 380–383. [Google Scholar] [CrossRef]
- Pakdel, F.; Ahmadikia, K.; Salehi, M.; Tabari, A.; Jafari, R.; Mehrparvar, G.; Rezaie, Y.; Rajaeih, S.; Alijani, N.; Barac, A.; et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicenter study from Iran. Mycoses 2021, 64, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Pasero, D.; Sanna, S.; Liperi, C.; Piredda, D.; Branca, G.P.; Casadio, L.; Simeo, R.S.; Buselli, A.; Rizzo, D.; Bussu, F.; et al. A challenging complication following SARS-CoV-2 infection: A case of pulmonary mucormycosis. Infection 2020, 1–6. [Google Scholar] [CrossRef]
- Pauli, M.A.; de Melo Pereira, L.; Monteiro, M.L.; de Camargo, A.R.; Rabelo, G.D. Painful palatal lesion in a COVID-19 patient. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Placik, D.A.; Taylor, W.L.; Wnuk, N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 2020, 15, 2378–2381. [Google Scholar] [CrossRef]
- Rabagliati, R.; Rodríguez, N.; Núñez, C.; Huete, A.; Bravo, S.; Garcia, P. COVID-19–Associated Mold Infection in Critically Ill Patients, Chile. Emerg. Infect. Dis. 2021, 27, 1454. [Google Scholar] [CrossRef]
- Rao, R.; Shetty, A.; Nagesh, C. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: Clinico-radiological features. Indian J. Ophthalmol. 2021, 69, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Ravani, S.A.; Agrawal, G.A.; Leuva, P.A.; Modi, P.H.; Amin, K.D. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J. Ophthalmol. 2021, 69, 1563–1568. [Google Scholar] [PubMed]
- Revannavar, S.M.; Supriya, P.; Samaga, L.; Vineeth, V. COVID-19 triggering mucormycosis in a susceptible patient: A new phenomenon in the developing world? BMJ Case Rep. CP 2021, 14, e241663. [Google Scholar]
- Saldanha, M.; Reddy, R.; Vincent, M.J. Of the article: Paranasal mucormycosis in COVID-19 patient. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–4. [Google Scholar] [CrossRef]
- Sarkar, S.; Gokhale, T.; Choudhury, S.S.; Deb, A.K. COVID-19 and orbital mucormycosis. Indian J. Ophthalmol. 2021, 69, 1002. [Google Scholar]
- Sen, M.; Lahane, S.; Lahane, T.P.; Parekh, R.; Honavar, S.G. Mucor in a viral land: A tale of two pathogens. Indian J. Ophthalmol. 2021, 69, 244. [Google Scholar]
- Veisi, A.; Bagheri, A.; Eshaghi, M.; Rikhtehgar, M.H.; Kanavi, M.R.; Farjad, R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: A case report. Eur. J. Ophthalmol. 2021, 11206721211009450. [Google Scholar] [CrossRef]
- Waizel-Haiat, S.; Guerrero-Paz, J.A.; Sanchez-Hurtado, L.; Calleja-Alarcon, S.; Romero-Gutierrez, L. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus 2021, 13, e13163. [Google Scholar]
- Werthman-Ehrenreich, A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg Med. 2021, 42, 264.e5–264.e8. [Google Scholar] [CrossRef]
- Zurl, C.; Hoenigl, M.; Schulz, E.; Hatzl, S.; Gorkiewicz, G.; Krause, R.; Eller, P.; Prattes, J. Autopsy Proven Pulmonary Mucormycosis Due to Rhizopus microsporus in a Critically Ill COVID-19 Patient with Underlying Hematological Malignancy. J. Fungi 2021, 7, 88. [Google Scholar] [CrossRef]
- Mishra, N.; Mutya, V.S.S.; Thomas, A.; Rai, G.; Reddy, B.; Mohanan, A.A. A case series of invasive mucormycosis in patients with COVID-19 infection. Int. J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 867–870. [Google Scholar] [CrossRef]
- Satish, D.; Joy, D.; Ross, A. Balasubramanya. Mucormycosis co-infection associated with global COVID-19: A case series from India. Int. J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 815–820. [Google Scholar] [CrossRef]
- Evert, K.; Dienemann, T.; Brochhausen, C.; Lunz, D.; Lubnow, M.; Ritzka, M.; Keil, F.; Trummer, M.; Scheiter, A.; Salzberger, B.; et al. Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Arch. 2021, 479, 97–108. [Google Scholar] [CrossRef]
- Khan, N.; Gutierrez, C.G.; Martinez, D.V.; Proud, K.C. A case report of COVID-19 associated pulmonary mucormycosis. Arch. Clin. Cases 2021, 7, 46–51. [Google Scholar] [CrossRef]
- Hoang, K.; Abdo, T.; Reinersman, J.M.; Lu, R.; Higuita, N.I.A. A case of invasive pulmonary mucormycosis resulting from short courses of corticosteroids in a well-controlled diabetic patient. Med. Mycol. Case Rep. 2020, 29, 22–24. [Google Scholar] [CrossRef]
- Pan, A.S.; Srinath, L. Mucormycosis in a patient with AIDS receiving systemic steroids. J. Am. Osteopath. Assoc. 2013, 113, 708–711. [Google Scholar] [CrossRef][Green Version]
- India Accounts for 1 in 3 New Covid Cases Being Recorded. Available online: https://www.cnbc.com/2021/05/03/india-covid-crisis-charts-show-the-severity-of-the-second-wave.html (accessed on 10 June 2021).
- Corzo-León, D.E.; Chora-Hernández, L.D.; Rodríguez-Zulueta, A.P.; Walsh, T.J. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med. Mycol. 2018, 56, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Erener, S. Diabetes, infection risk and COVID-19. Mol. Metabolism. 2020, 39, 101044. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Morales-Franco, B.; Nava-Villalba, M.; Medina-Guerrero, E.O.; Sánchez-Nuño, Y.A.; Davila-Villa, P.; Anaya-Ambriz, E.J.; Charles-Niño, C.L. Host-Pathogen Molecular Factors Contribute to the Pathogenesis of Rhizopus spp. in Diabetes Mellitus. Curr. Trop. Med. Rep. 2021, 8, 6–17. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef]
- Uğurlu, K.; Selim, S.; Kopar, A.; Songu, M. Rhino-orbital mucormycosis: Clinical findings and treatment outcomes of four cases. Turk. J. Ophthalmol. 2015, 45, 169. [Google Scholar] [CrossRef] [PubMed]
- Rhino-Orbital-Cerebral Mucormycosis. Available online: https://eyewiki.aao.org/Rhino-Orbital-Cerebral_Mucormycosis (accessed on 10 June 2021).
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 2018, 56, S93–S101. [Google Scholar] [CrossRef] [PubMed]
- Characterization of Fungal Infections in COVID-19 Infected and Mechanically Ventilated Patients in ICU (MY-CO-VID). Available online: https://clinicaltrials.gov/ct2/show/NCT04368221 (accessed on 25 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).