Socioeconomic Inequalities in Metabolic Syndrome by Age and Gender in a Spanish Working Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Variables
2.2. Statistical Analyses
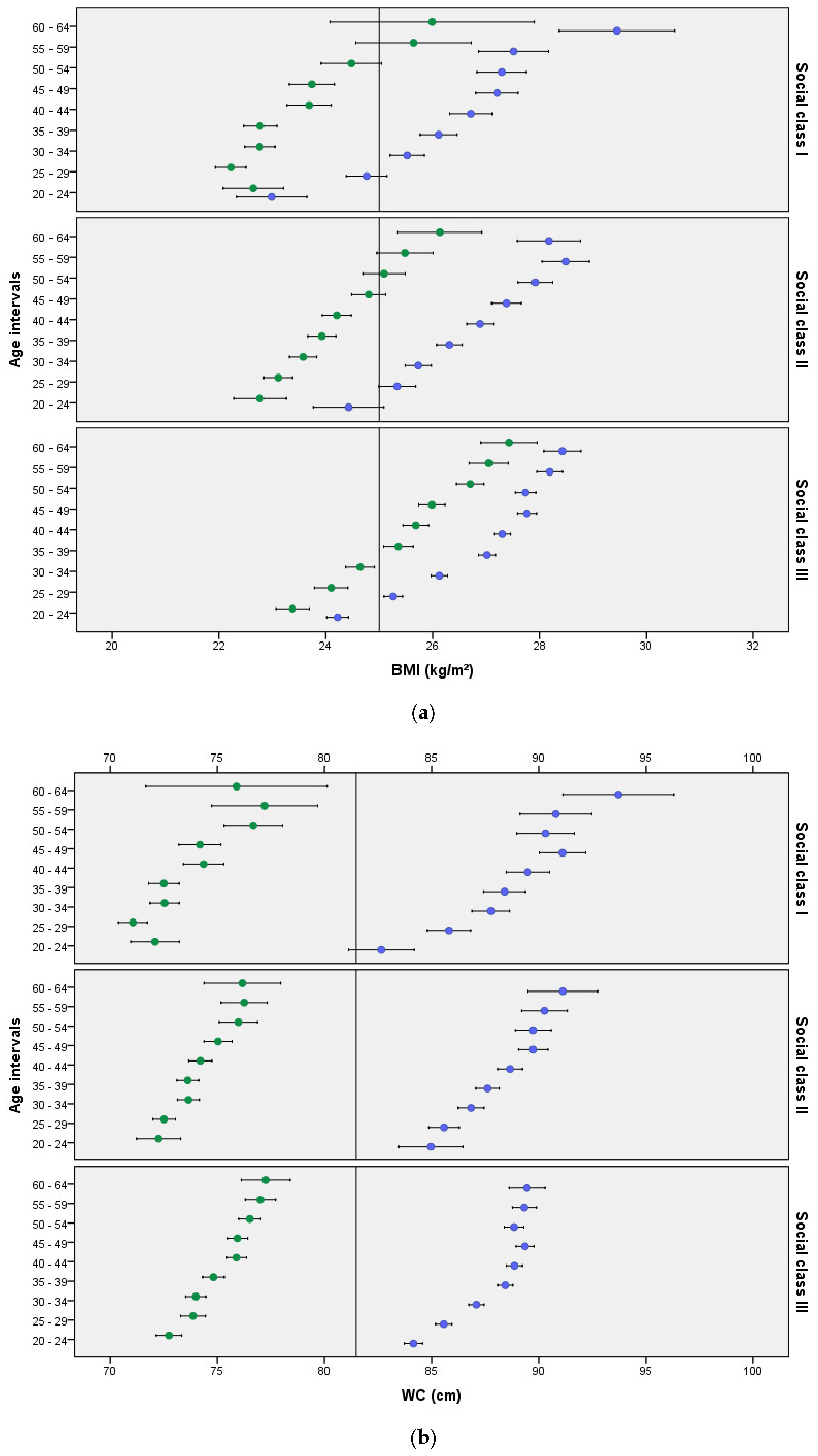
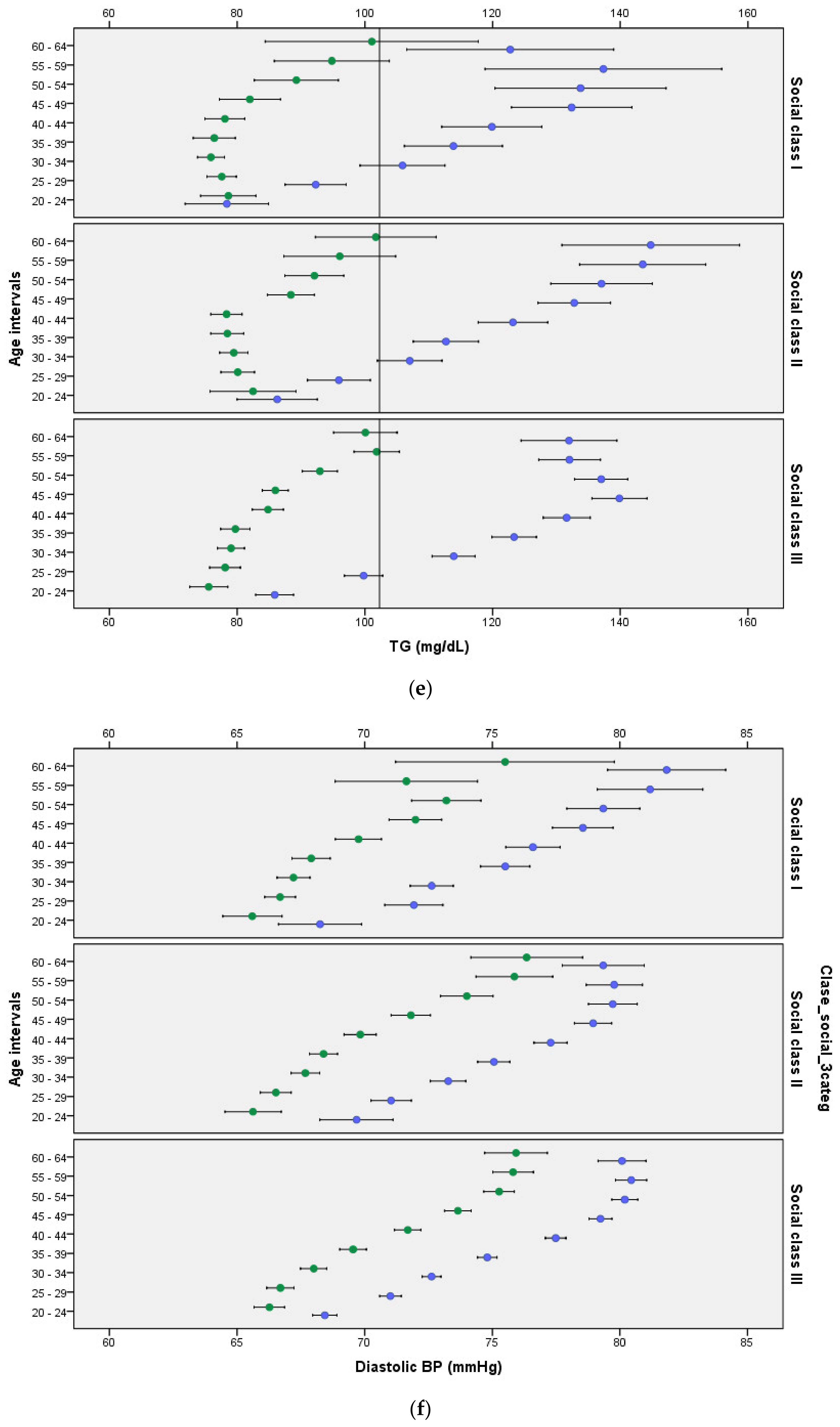
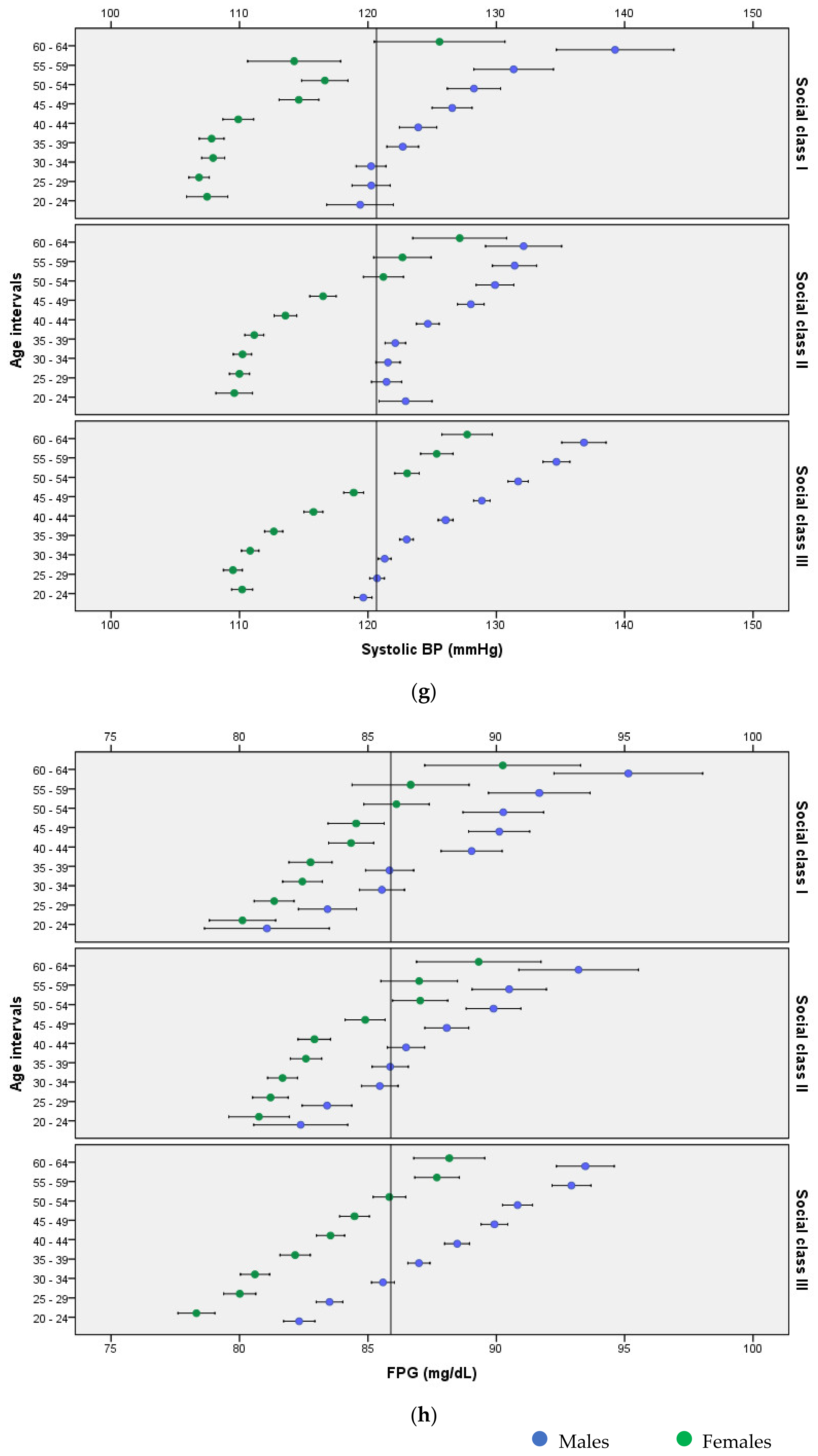
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, L.; Williams, J.; Townsend, N.; Mikkelsen, B.; Roberts, N.; Foster, C.; Wickramasinghe, K. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: A systematic review. Lancet Glob. Health 2017, 5, e277–e289. [Google Scholar] [CrossRef] [Green Version]
- Wagstaff, A. Poverty and health sector inequalities. Bull. World Health Organ. 2002, 80, 97–105. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Sanchez-Chaparro, M.A.; Calvo-Bonacho, E.; Gonzalez-Quintela, A.; Fernandez-Labandera, C.; Cabrera, M.; Sainz, J.C.; Fernandez-Meseguer, A.; Banegas, J.R.; Ruilope, L.M.; Valdivielso, P.; et al. Occupation-related differences in the prevalence of metabolic syndrome. Diabetes Care 2008, 31, 1884–1885. [Google Scholar] [CrossRef] [Green Version]
- Alegría, E.; Cordero, A.; Laclaustra, M.; Grima, A.; León, M.; Casasnovas, J.A.; Luengo, E.; del Río, A.; Ferreira, I. Prevalence of Metabolic Syndrome in the Spanish Working Population: MESYAS Registry. Rev. Española Cardiol. 2005, 58, 797–806. [Google Scholar] [CrossRef]
- Buckland, G.; Salas-Salvadó, J.; Roure, E.; Bulló, M.; Serra-Majem, L. Sociodemographic risk factors associated with metabolic syndrome in a Mediterranean population. Public Health Nutr. 2008, 11, 1372–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adedoyin, R.A.; Afolabi, A.; Adegoke, O.O.; Akintomide, A.O.; Awotidebe, T.O. Relationship between socioeconomic status and metabolic syndrome among Nigerian adults. Diabetes Metab. Syndr. Clin. Res. Rev. 2013, 7, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.D.; Paquet, C.; Howard, N.J.; Coffee, N.T.; Adams, R.; Taylor, A.; Daniel, M. Area-level socioeconomic characteristics and incidence of metabolic syndrome: A prospective cohort study. BMC Public Health 2013, 13, 681. [Google Scholar] [CrossRef] [Green Version]
- Blanquet, M.; Debost-Legrand, A.; Gerbaud, L.; De La Celle, C.; Brigand, A.; Mioche, L.; Sass, C.; Hazart, J.; Aw, A. Metabolic syndrome and social deprivation: Results of a French observational multicentre survey. Fam. Pract. 2016, 33, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khambaty, T.; Schneiderman, N.; Llabre, M.M.; Elfassy, T.; Moncrieft, A.E.; Daviglus, M.; Talavera, G.A.; Isasi, C.R.; Gallo, L.C.; Reina, S.A.; et al. Elucidating the Multidimensionality of Socioeconomic Status in Relation to Metabolic Syndrome in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Int. J. Behav. Med. 2020, 27, 188–199. [Google Scholar] [CrossRef]
- Yang, Y.C.; Gerken, K.; Schorpp, K.; Boen, C.; Harris, K.M. Early-Life Socioeconomic Status and Adult Physiological Functioning: A Life Course Examination of Biosocial Mechanisms. Biodemography Soc. Biol. 2017, 63, 87–103. [Google Scholar] [CrossRef]
- Santos, A.C.; Ebrahim, S.; Barros, H. Gender, socio-economic status and metabolic syndrome in middle-aged and old adults. BMC Public Health 2008, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.C.; Schorpp, K.; Boen, C.; Johnson, M.; Harris, K.M. Socioeconomic Status and Biological Risks for Health and Illness Across the Life Course. J. Gerontol. Ser. B 2020, 75, 613–624. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol. 2006, 95, 136–147, Erratum in: 2006, 95, 147. [Google Scholar] [CrossRef]
- Krieger, N. Genders, sexes, and health: What are the connections—And why does it matter? Int. J. Epidemiol. 2003, 32, 652–657. [Google Scholar] [CrossRef] [PubMed]
- López-González, Á.A.; Bennasar-Veny, M.; Tauler, P.; Aguilo, A.; Tomàs-Salvà, M.; Yáñez, A. Desigualdades socioeconómicas y diferencias según sexo y edad en los factores de riesgo cardiovascular. Gac. Sanit. 2015, 29, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Choue, R.; Nguyen, T.; Wang, Y. Sociodemographic disparities in the composition of metabolic syndrome components among adults in South Korea. Diabetes Care 2012, 35, 2028–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Zhao, N.; Wang, H.; Zhang, J.; He, Q.; Su, D.; Zhao, M.; Wang, L.; Zhang, X.; Gong, W.; et al. Association between socioeconomic status and obesity in a Chinese adult population. BMC Public Health 2013, 13, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.F.; Tam, T.; Jin, L.; Lao, X.Q.; Chung, R.Y.N.; Su, X.F.; Zee, B. Age, gender, and socioeconomic gradients in metabolic syndrome: Biomarker evidence from a large sample in Taiwan, 2005–2013. Ann. Epidemiol. 2017, 27, 315–322.e2. [Google Scholar] [CrossRef]
- Phillips, S.P.; Hamberg, K. Women’s relative immunity to the socio-economic health gradient: Artifact or real? Glob. Health Action 2015, 8, 27259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Bergés, D.; Cabrera de León, A.; Sanz, H.; Elosua, R.; Guembe, M.J.; Alzamora, M.; Vega-Alonso, T.; Félix-Redondo, F.J.; Ortiz-Marrón, H.; Rigo, F.; et al. Metabolic Syndrome in Spain: Prevalence and Coronary Risk Associated With Harmonized Definition and WHO Proposal. DARIOS Study. Rev. Española Cardiol. 2012, 65, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Santander, N.; Espinola, M.; Cartanyà-Hueso, À.; Lidón-Moyano, C.; González-Marrón, A.; Martín-Sánchez, J.C.; Cainzos-Achirica, M.; Martínez Sánchez, J.M. Prevalence and determinants of metabolic syndrome in Spanish salaried workers: Evidence from 15,614 men and women. J. Public Health 2020, 42, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Tauler, P.; Bennasar-Veny, M.; Morales-Asencio, J.M.; Lopez-Gonzalez, A.A.; Vicente-Herrero, T.; De Pedro-Gomez, J.; Royo, V.; Pericas-Beltran, J.; Aguilo, A. Prevalence of Premorbid Metabolic Syndrome in Spanish Adult Workers Using IDF and ATPIII Diagnostic Criteria: Relationship with Cardiovascular Risk Factors. PLoS ONE 2014, 9, e89281. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.K.; Alberti, K.G.; Gale, E.A.; Colagiuri, S.; Tuomilehto, J.; Qiao, Q.; Ramachandran, A.; Tajima, N.; Mirchov, I.B.; Ben-Nakhi, A.; et al. The metabolic syndrome: Useful concept or clinical tool? Report of a WHO Expert Consultation. Diabetologia 2010, 53, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Salvany, A.; Regidor, E.; Alonso, J.; Alvarez-Dardet, C. Proposal for a social class measure. Working Group of the Spanish Society of Epidemiology and the Spanish Society of Family and Community Medicine. Aten. Primaria 2000, 25, 350–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C. Propuestas de clase social neoweberiana y neomarxista a partir de la Clasificación Nacional de Ocupaciones 2011. Gac. Sanit. 2013, 27, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfell-Jones, M.J.; Stewart, A.D.; de Ridder, J.H. ISAK Manual, International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Wellington, New Zealand, 2012; ISBN 9780620362078. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Solis, A.L.; Datta Banik, S.; Méndez-González, R.M. Prevalence of Metabolic Syndrome in Mexico: A Systematic Review and Meta-Analysis. Metab. Syndr. Relat. Disord. 2018, 16, 395–405. [Google Scholar] [CrossRef]
- Farmanfarma, K.K.; Kaykhaei, M.A.; Mohammadi, M.; Adineh, H.A.; Ansari-Moghaddam, A. The Prevalence and Trend of Metabolic Syndrome in the South-East of Iran. J. Med. Life 2020, 13, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Ivezić-Lalić, D.; Marković, B.B.; Kranjčević, K.; Ker, J.; Vrdoljak, D.; Vučak, J. Diversity of metabolic syndrome criteria in association with cardiovascular diseases—A family medicine-based investigation. Med. Sci. Monit. 2013, 19, 571–578. [Google Scholar] [CrossRef]
- Perel, P.; Langenberg, C.; Ferrie, J.; Moser, K.; Brunner, E.; Marmot, M. Household wealth and the metabolic syndrome in the Whitehall II study. Diabetes Care 2006, 29, 2694–2700. [Google Scholar] [CrossRef] [Green Version]
- Alkerwi, A.; Donneau, A.F.; Sauvageot, N.; Lair, M.L.; Albert, A.; Guillaume, M. Dietary, behavioural and socio-economic determinants of the metabolic syndrome among adults in Luxembourg: Findings from the ORISCAV-LUX study. Public Health Nutr. 2012, 15, 849–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallongeville, J.; Cottel, D.; Ferrières, J.; Arveiler, D.; Bingham, A.; Ruidavets, J.B.; Haas, B.; Ducimetière, P.; Amouyel, P. Household income is associated with the risk of metabolic syndrome in a sex-specific manner. Diabetes Care 2005, 28, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sygnowska, E.; Piwońska, A.; Waśkiewicz, A.; Broda, G. Socioeconomic factors and the risk of metabolic syndrome in the adult Polish population: The WOBASZ study. Kardiol. Pol. 2012, 70, 718–727. [Google Scholar]
- Hildrum, B.; Mykletun, A.; Hole, T.; Midthjell, K.; Dahl, A.A. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health 2007, 7, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishram, J.K.K.; Borglykke, A.; Andreasen, A.H.; Jeppesen, J.; Ibsen, H.; Jørgensen, T.; Palmieri, L.; Giampaoli, S.; Donfrancesco, C.; Kee, F.; et al. Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in Europeans. The MORGAM prospective cohort project. PLoS ONE 2014, 9, e107294. [Google Scholar] [CrossRef] [Green Version]
- Brunner, E.J.; Marmot, M.G.; Nanchahal, K.; Shipley, M.J.; Stansfeld, S.A.; Juneja, M.; Alberti, K.G.M.M. Social Inequality in Coronary Risk: Central Obesity and the Metabolic Syndrome. Evidence from the Whitehall II Study. Diabetologia 1997, 40, 1341–1349. [Google Scholar] [CrossRef] [Green Version]
- Langenberg, C.; Kuh, D.; Wadsworth, M.E.J.; Brunner, E.; Hardy, R. Social circumstances and education: Life course origins of social inequalities in metabolic risk in a prospective national birth cohort. Am. J. Public Health 2006, 96, 2216–2221. [Google Scholar] [CrossRef]
- Gustafsson, P.E.; Persson, M.; Hammarstrom, A. Life Course Origins of the Metabolic Syndrome in Middle-Aged Women and Men: The Role of Socioeconomic Status and Metabolic Risk Factors in Adolescence and Early Adulthood. Ann. Epidemiol. 2011, 21, 103–110. [Google Scholar] [CrossRef]
- Macleod, J.; Smith, G.D. Psychosocial factors and public health: A suitable case for treatment? J. Epidemiol. Community Health 2003, 57, 565–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scuteri, A.; Vuga, M.; Najjar, S.S.; Mehta, V.; Everson-Rose, S.A.; Sutton-Tyrrell, K.; Matthews, K.; Lakatta, E.G. Education eclipses ethnicity in predicting the development of the metabolic syndrome in different ethnic groups in midlife: The Study of Women’s Health Across the Nation (SWAN). Diabet. Med. 2008, 25, 1390–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.T.; Kim, H.Y.; Kim, J.K.; Linton, J.A.; Lee, Y.J. Employment is associated with a lower prevalence of metabolic syndrome in postmenopausal women based on the 2007–2009 Korean National Health Examination and Nutrition Survey. Menopause 2014, 21, 221–226. [Google Scholar] [CrossRef]
- Montez, J.K.; Bromberger, J.T.; Harlow, S.D.; Kravitz, H.M.; Matthews, K.A. Life-Course Socioeconomic Status and Metabolic Syndrome Among Midlife Women. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2016, 71, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Matthews, K.A.; Räikkönen, K.; Gallo, L.; Kuller, L.H. Association Between Socioeconomic Status and Metabolic Syndrome in Women: Testing the Reserve Capacity Model. Health Psychol. 2008, 27, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- INE. Encuesta de Población Activa 2011. In Estadística INd; Instituto Nacional de Estadística: Madrid, Spain, 2012. [Google Scholar]

| Men | Women | p | |
|---|---|---|---|
| n (%) | 23,956 (56.8) | 18,190 (43.2) | |
| Age (y) | 39.54 (10.39) | 38.87 (10.13) | <0.001 |
| BMI (kg/m2) | 26.68 (4.13) | 24.60 (4.68) | <0.001 |
| Systolic BP (mmHg) | 125.20 (15.18) | 113.99 (14.52) | <0.001 |
| Diastolic BP (mmHg) | 75.61 (10.68) | 70.08 (10.09) | <0.001 |
| T-Chol (mg/dL) | 193.96 (38.12) | 189.75 (35.60) | <0.001 |
| HDL-C (mg/dL) | 49.98 (6.99) | 54.21 (9.13) | <0.001 |
| FPG (mg/dL) | 87.34 (12.13) | 83.01 (10.74) | <0.001 |
| TG (mg/dL) | 120.33 (85.59) | 82.97 (42.59) | <0.001 |
| WC (cm) | 7.97 (9.41) | 74.45 (9.06) | <0.001 |
| Social class (n (%)) | <0.001 | ||
| I | 2195 (9.2) | 3036 (16.7) | |
| II | 4849 (20.2) | 5674 (31.3) | |
| III | 16,912 (70.6) | 9480 (42.1) | |
| Weight status (n (%)) | <0.001 | ||
| Underweight | 155 (0.6) | 588 (3.1) | |
| Normal weight | 8097 (36.8) | 11,185 (59.9) | |
| Overweight | 10,628 (43.9) | 4633 (24.8) | |
| Obesity | 4521 (18.7) | 2275 (12.2) | |
| Smoking habits (n (%)) | <0.001 | ||
| Current smokers | 8972 (37.1) | 6235 (33.4) | |
| Non-smokers | 10,536 (43.5) | 9935 (53.2) | |
| Ex-smokers | 4703 (19.4) | 2511 (13.4) | |
| MS prevalence (n (%)) | <0.001 | ||
| ATP-III | 2261 (9.4) | 693 (3.8) | |
| IDF | 2948 (12.3) | 1045 (5.7) |
| I | II | III | p | |
|---|---|---|---|---|
| n (%) | 5231 (12.4) | 10,523 (25.0) | 26,392 (62.6) | |
| Gender (n (%)) | <0.001 | |||
| Men | 2195 (42.0) | 4849 (46.1) | 16,912 (64.1) | |
| Women | 3036 (58.0) | 5674 (53.9) | 9480 (35.9) | |
| Age (y) | 37.19 (9.48) | 39.27 (9.45) | 39.65 (10.70) | <0.001 |
| BMI (kg/m2) | 24.38 (4.12) | 25.24 (4.31) | 26.29 (4.56) | <0.001 |
| Systolic BP (mmHg) | 115.45 (14.82) | 118.64 (15.27) | 122.02 (16.08) | <0.001 |
| Diastolic BP (mmHg) | 71.43 (10.18) | 72.43 (10.7) | 73.89 (10.88) | <0.001 |
| T-Chol (mg/dL) | 188.23 (34.69) | 193.01 (35.88) | 192.58 (38.01) | <0.001 |
| HDL-C (mg/dL) | 54.43 (10.50) | 52.04 (8.20) | 51.19 (7.66) | <0.001 |
| FPG (mg/dL) | 84.77 (10.39) | 84.77 (11.09) | 85.89 (12.23) | <0.001 |
| TG (mg/dL) | 93.96 (58.39) | 99.43 (65.17) | 108.13 (77.67) | <0.001 |
| WC (cm) | 79.57 (12.09) | 80.53 (11.28) | 83.29 (11.19) | <0.001 |
| Weight status (n (%)) | <0.001 | |||
| Underweight | 170 (3.2) | 191 (1.8) | 360 (1.4) | |
| Normal weight | 3061 (58.5) | 5494 (52.2) | 11,102 (42.1) | |
| Overweight | 1527 (29.2) | 3490 (33.2) | 10,043 (38.1) | |
| Obesity | 473 (9.0) | 1348 (12.8) | 4887 (18.5) | |
| Smoking habits (n (%)) | <0.001 | |||
| Current smokers | 1404 (26.8) | 2912 (27.7) | 10,649 (40.3) | |
| Non-smokers | 3214 (61.4) | 5593 (53.2) | 11,163 (42.3) | |
| Ex-smokers | 613 (11.7) | 2018 (19.2) | 4580 (17.4) | |
| MS prevalence (n (%)) | <0.001 | |||
| ATP-III | 217 (4.1) | 642 (6.1) | 2095 (7.9) | |
| IDF | 311 (5.9) | 867 (8.2) | 2815 (10.7) |
| Social Class | Total | p | PR (95% CI) * | |||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| Men (n (%)) | 2195 (9.7) | 4849 (20.2) | 16,912 (71.1) | 23,956 | ||
| Weight status (n (%)) | ||||||
| Underweight | 3 (0.1) | 13 (0.3) | 135 (0.8) | 151 (0.6) | <0.001 | 5.84 (1.89 to 18.1) |
| Normal weight | 885 (40.3) | 1765 (36.4) | 6143 (36.3) | 8793 (36.7) | <0.001 | 0.90 (0.86 to 0.95) |
| Overweight | 1010 (46.0) | 2231 (46.0) | 7290 (43.1) | 10,531 (44.0) | <0.001 | 0.94 (0.90 to 0.98) |
| Obesity | 297 (13.5) | 840 (17.3) | 3344 (19.8) | 4481 (18.7) | <0.001 | 1.46 (1.31 to 1.62) |
| Women (n (%)) | 3036 (16.7) | 5674 (31.2) | 9480 (52.1) | 18,190 | ||
| Weight status (n (%)) | ||||||
| Underweight | 167 (5.5) | 178 (3.1) | 225 (2.4) | 570 (3.1) | <0.001 | 0.43 (0.37 to 0.5) |
| Normal weight | 2176 (71.7) | 3729 (65.7) | 4959 (52.3) | 10,864 (59.7) | <0.001 | 0.73 (0.71 to 0.75) |
| Overweight | 517 (17.0) | 1259 (22.2) | 2753 (29.0) | 4529 (24.9) | <0.001 | 1.71 (1.58 to 1.84) |
| Obesity | 176 (5.8) | 508 (9.0) | 1543 (16.3) | 2227 (12.2) | <0.001 | 2.81 (2.43 to 3.24) |
| Social Class | Total | p | PR (95% CI) * | |||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| Men (n (%)) | 2195 (9.2) | 4849 (20.2) | 16,912 (70.6) | 23,956 | ||
| MS prevalence (n (%)) | ||||||
| ATP-III | 176 (8.0) | 423 (8.7) | 1662 (9.8) | 2261 (9.4) | 0.004 | 1.23 (1.06 to 1.41) |
| IDF | 241 (11.0) | 561 (11.6) | 2146 (12.7) | 2948 (12.3) | 0.016 | 1.15 (1.03 to 1.30) |
| Women (n (%)) | 3036 (16.7) | 5674 (31.2) | 9480 (52.1) | 18,190 | ||
| MS prevalence (n (%)) | ||||||
| ATP-III | 41 (1.4) | 219 (3.9) | 433 (4.6) | 693 (3.8) | <0.001 | 3.38 (2.50 to 4.58) |
| IDF | 70 (2.3) | 306 (5.4) | 669 (7.1) | 1045 (5.7) | <0.001 | 3.06 (2.43 to 3.86) |
| Age Groups (y) | n | MS by ATP-III Criteria | MS by IDF Criteria | ||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| 20–24 | 2987 | 1.60 (0.23 to 11.23) | 0.28 (0.04 to 1.98) | 2.29 (0.33 to 16.05) | 0.35 (0.13 to 0.92) |
| 25–29 | 5269 | 1.59 (0.72 to 3.50) | 2.78 (0.90 to 8.59) | 1.61 (0.81 to 3.19) | 2.56 (1.07 to 6.14) |
| 30–34 | 6913 | 1.35 (0.87 to 2.09) | 3.55 (1.15 to 10.98) | 1.44 (0.97 to 2.15) | 2.75 (1.32 to 5.75) |
| 35–39 | 7124 | 1.80 (1.19 to 2.73) | 1.77 (0.93 to 3.38) | 1.41 (1.02 to 1.96) | 2.39 (1.33 to 4.29) |
| 40–44 | 6504 | 1.25 (0.90 to 1.75) | 2.83 (1.36 to 5.90) | 1.31 (0.98 to 1.76) | 2.66 (1.52 to 4.64) |
| 45–49 | 5578 | 1.10 (0.83 to 1.47) | 2.25 (1.02 to 4.96) | 1.07 (0.84 to 1.37) | 2.18 (1.19 to 4.01) |
| 50–54 | 4175 | 0.94 (0.68 to 1.30) | 1.65 (0.87 to 3.13) | 0.94 (0.71 to 1.25) | 1.46 (0.90 to 2.37) |
| 55–59 | 2525 | 1.29 (0.82 to 2.04) | 4.34 (1.11 to 16.98) | 1.08 (0.76 to 1.53) | 4.02 (1.33 to 12.13) |
| 60–64 | 1071 | 0.77 (0.48 to 1.24) | 3.12 (0.46 to 21.23) | 0.63 (0.44 to 0.88) | 1.54 (0.54 to 4.45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbate, M.; Pericas, J.; Yañez, A.M.; López-González, A.A.; De Pedro-Gómez, J.; Aguilo, A.; Morales-Asencio, J.M.; Bennasar-Veny, M. Socioeconomic Inequalities in Metabolic Syndrome by Age and Gender in a Spanish Working Population. Int. J. Environ. Res. Public Health 2021, 18, 10333. https://doi.org/10.3390/ijerph181910333
Abbate M, Pericas J, Yañez AM, López-González AA, De Pedro-Gómez J, Aguilo A, Morales-Asencio JM, Bennasar-Veny M. Socioeconomic Inequalities in Metabolic Syndrome by Age and Gender in a Spanish Working Population. International Journal of Environmental Research and Public Health. 2021; 18(19):10333. https://doi.org/10.3390/ijerph181910333
Chicago/Turabian StyleAbbate, Manuela, Jordi Pericas, Aina M. Yañez, Angel A. López-González, Joan De Pedro-Gómez, Antoni Aguilo, José M. Morales-Asencio, and Miquel Bennasar-Veny. 2021. "Socioeconomic Inequalities in Metabolic Syndrome by Age and Gender in a Spanish Working Population" International Journal of Environmental Research and Public Health 18, no. 19: 10333. https://doi.org/10.3390/ijerph181910333
APA StyleAbbate, M., Pericas, J., Yañez, A. M., López-González, A. A., De Pedro-Gómez, J., Aguilo, A., Morales-Asencio, J. M., & Bennasar-Veny, M. (2021). Socioeconomic Inequalities in Metabolic Syndrome by Age and Gender in a Spanish Working Population. International Journal of Environmental Research and Public Health, 18(19), 10333. https://doi.org/10.3390/ijerph181910333










