Green Line Hospital-Territory Study: A Single-Blind Randomized Clinical Trial for Evaluation of Technological Challenges of Continuous Wireless Monitoring in Internal Medicine, Preliminary Results
Abstract
1. Introduction
2. Study Objective
3. Materials and Methods
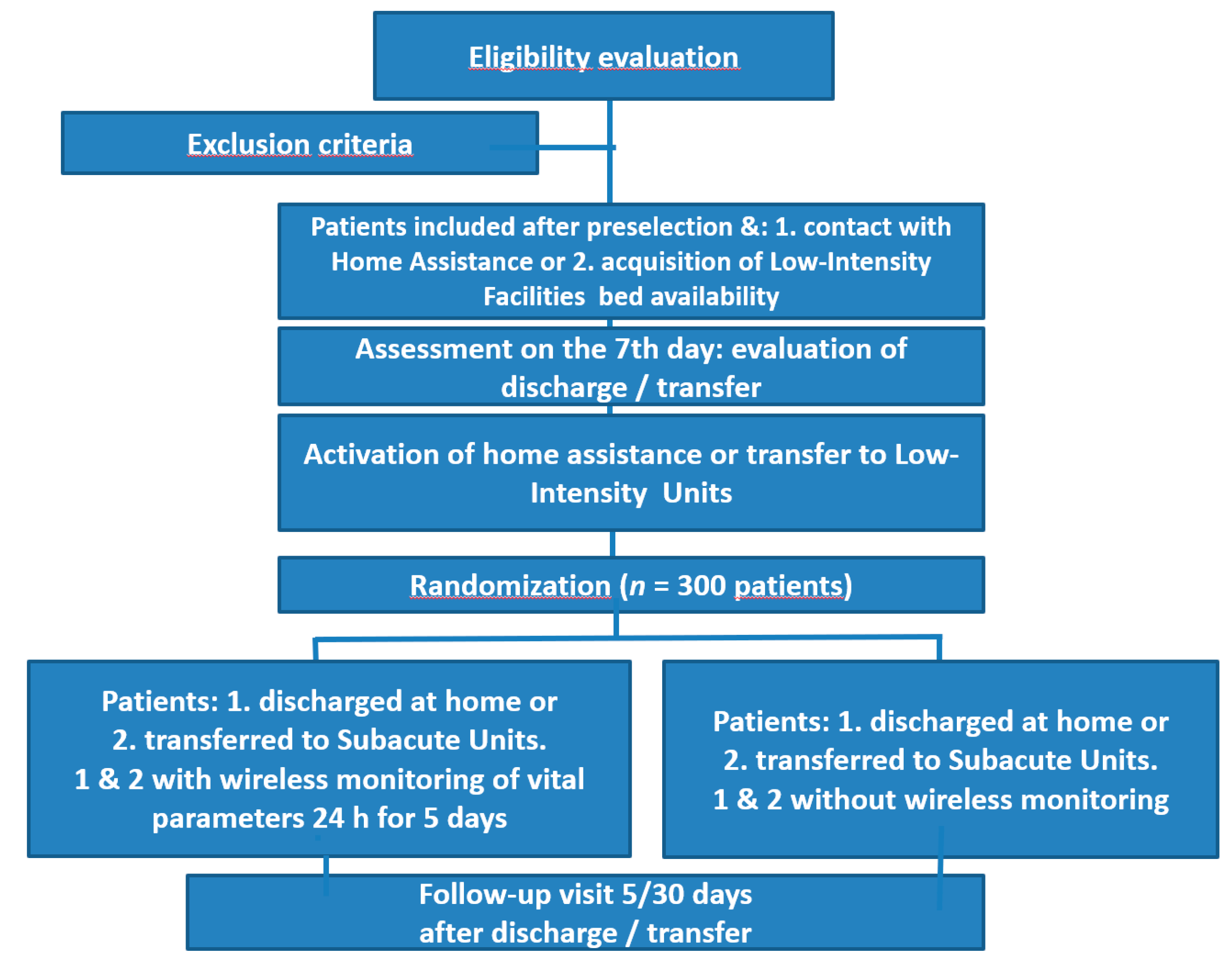
3.1. Study Design
3.2. Study Arms
- (1)
- Experimental group: all patients with inclusion criteria randomized by trial software.
- (2)
- Control group: all patients with inclusion criteria randomized by trial software.
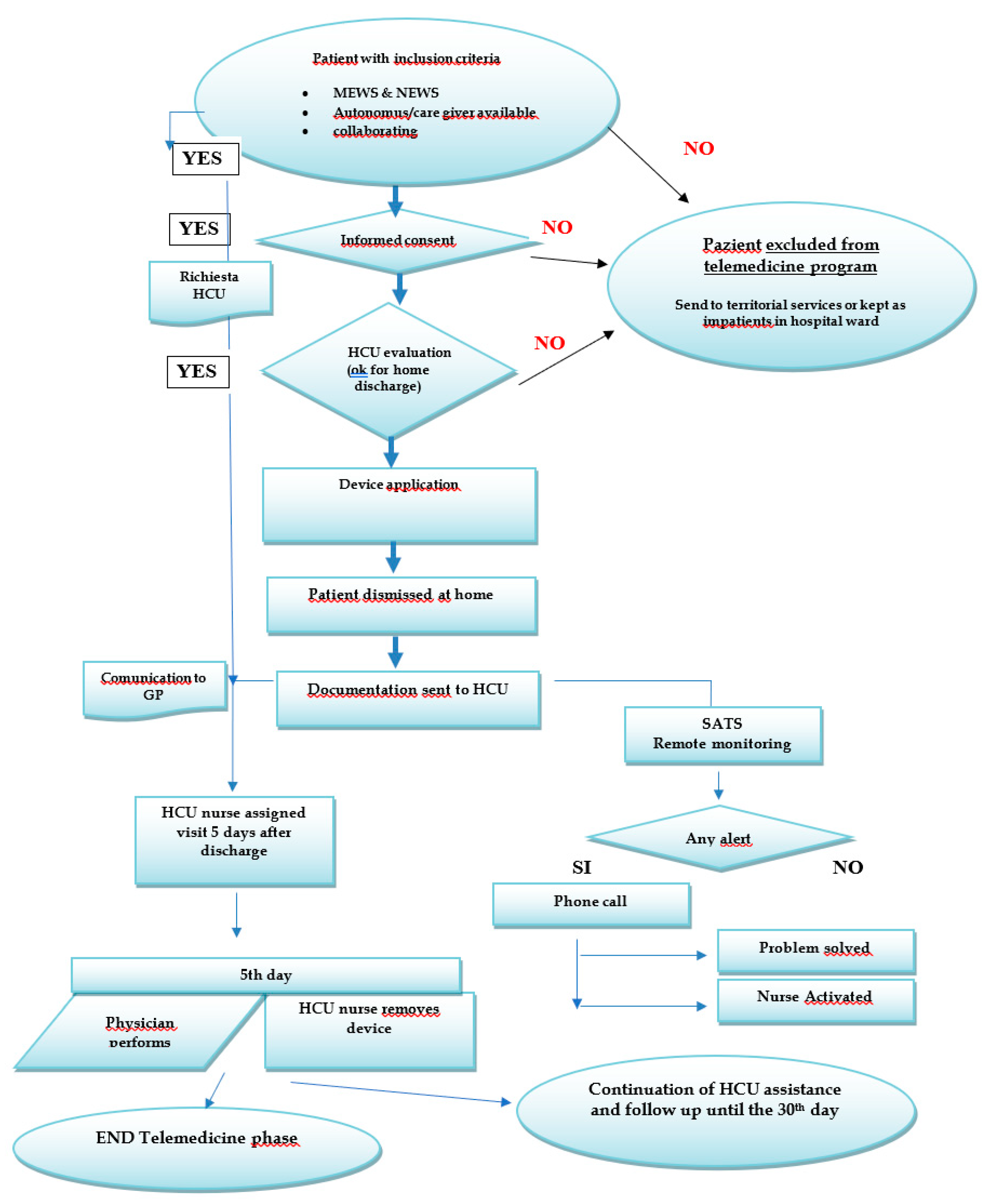
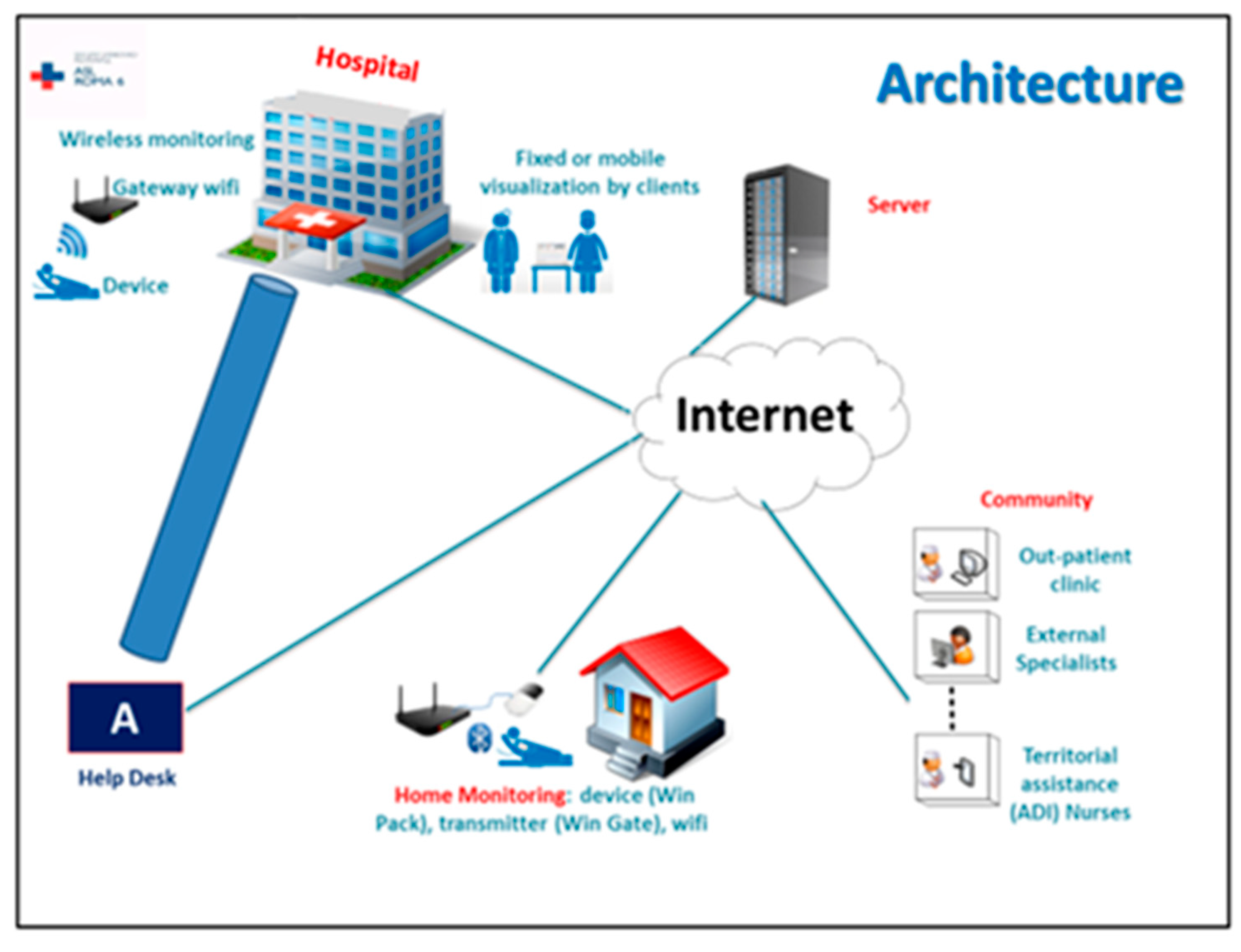
3.3. Identification of the Monitoring Tool and Procedure
3.4. Safety Assessment
3.5. Time Frame
3.6. Primary Outcomes
3.7. Secondary Outcomes
3.8. Statistical Methodology
3.9. Statistical Analysis
3.10. The Rationale for Monitoring
3.11. Wireless Monitoring System
- possible reduction in length of hospital stay and safe early discharge;
- reduction in ICU transfers;
- early transfer of patients from areas with higher-intensity care to areas with lower-intensity care and an estimated savings of €290 per day for each ordinary patient who is transferred from an intensive ward to a lower-intensity ward;
- a nurse reduction in time dedicated to the detection of vital parameters and the potential employ of the nurse’s skill in more valuable activities.
3.12. Inclusion/Exclusion Criteria
3.13. Randomization Criteria
4. Results
5. Discussion
Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pietrantonio, F.; Scotti, E. Internal Medicine Network: A New Way of Thinking Hospital-Territory Integration and Public-Private Partnership. Ital. J. Med. 2016. Available online: http://www.italjmed.org/index.php/ijm/article/view/itjm.2016.764 (accessed on 6 February 2021).
- Pietrantonio, F.; Orlandini, F.; Moriconi, L.; La Regina, M. Acute Complex Care Model: An organizational approach for the medical care of hospitalized acute complex patients. Eur. J. Intern. Med. 2015, 26, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Aperti, P.; Tonoli, L.; Tyndall, E.; Meneghetti, O. The correct setting to improve the quality of health care process: A retrospective study in Internal Medicine Department. Ital. J. Med. 2018, 12, 285–295. [Google Scholar] [CrossRef]
- Weinstock, R.S.; Xing, D.; Maahs, D.M.; Michels, A.; Rickels, M.R.; Peters, A.L.; Bergenstal, R.M.; Harris, B.; DuBose, S.N.; Miller, K.M.; et al. Severe Hypoglycemia and Diabetic Ketoacidosis in Adults with Type 1 Diabetes: Results from the T1D Exchange Clinic Registry. J. Clin. Endocrinol. Metab. 2013, 98, 3411–3419. [Google Scholar] [CrossRef] [PubMed]
- Charleer, S.; Mathieu, C.; Nobels, F.; De Block, C.; Radermecker, R.P.; Hermans, M.P.; Taes, Y.; Vercammen, C.; T’Sjoen, G.; Crenier, L.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control, Acute Admissions, and Quality of Life: A Real-World Study. J. Clin. Endocrinol. Metab. 2018, 103, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Blais, R.; Sears, N.A.; Doran, D.; Baker, G.R.; Macdonald, M.; Mitchell, L.; Thalès, S. Assessing adverse events among home care clients in three Canadian provinces using chart review. BMJ Qual. Saf. 2013, 22, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Sears, N.; Baker, G.R.; Barnsley, J.; Shortt, S. The incidence of adverse events among home care patients. Int. J. Qual. Health Care. 2013, 25, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Masotti, P.; McColl, M.A.; Green, M. Adverse events experienced by homecare patients: A scoping review of the literature. Int. J. Qual. Health Care 2010, 22, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Doran, D.; Hirdes, J.P.; Blais, R.; Baker, G.R.; Poss, J.W.; Li, X.; Dill, D.; Gruneir, A.; Heckman, G.; Lacroix, H.; et al. Adverse events associated with hospitalization or detected through the RAI-HC assessment among Canadian home care clients. Healthc. Policy 2013, 9, 76–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vincent, C.; Amalberti, R. Safer Healthcare: Strategies for the Real World, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–26. [Google Scholar]
- Zhu, Y.; Gu, X.; Xu, C. Effectiveness of telemedicine systems for adults with heart failure: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2020, 25, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Tchero, H.; Kangambega, P.; Briatte, C.; Brunet-Houdard, S.; Retali, G.R.; Rusch, E. Clinical Effectiveness of Telemedicine in Diabetes Mellitus: A Meta-Analysis of 42 Randomized Controlled Trials. Telemedicine and e-Health 2019, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Kraef, C.; van der Meirschen, M.; Free, C. Digital telemedicine interventions for patients with multimorbidity: A systematic review and meta-analysis. BMJ Open 2020, 10, e036904. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Schulz, K.F.; Altman, D.G. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel group randomised trials. Lancet 2001, 357, 1191–1194. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality (AHrQ). Health Care Quality Indicators. Available online: http://www.qualityindicators.ahrq.gov/ (accessed on 31 January 2021).
- Smith, G.B.; Prytherch, D.R.; Meredith, P.; Schmidt, P.E.; Featherstone, P.I. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation 2013, 84, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Nardi, R.; Scanelli, G.; Borioni, D.; Grandi, M.; Sacchetti, C.; Parenti, M.; Fiorino, S.; Iori, I.; Di Donato, C.; Agostinelli, P.; et al. The assessment of complexity in internal medicine patients. The FADOI Medicomplex Study. Eur. J. Intern. Med. 2007, 18, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Fueglistaler, P.; Adamina, M.; Guller, U. Non-Inferiority Trials in Surgical Oncology. Ann. Surg. Oncol. 2007, 14, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, R.; Albolino, S.; Bellandi, T.; Bianchini, E.; Biggeri, A.; Fabbro, G.; Bevilacqua, L.; Dell’Erba, A.; Privitera, G.; Sommella, L.; et al. Adverse events and preventable consequences: Retrospective study in five large Italian hospitals. Epidemiol Prev. 2012, 36, 151–161. [Google Scholar] [PubMed]
- Vincent, C. La Sicurezza del Paziente; Springer: Mailand, Italy, 2011. [Google Scholar] [CrossRef]
- Kohn, L.; Corrigan, J.; Donaldson, M. To Err Is Human: Building a Safer Health System; National Academies Press: Washington, DC, USA, 2000; Available online: http://www.nap.edu/catalog/9728 (accessed on 6 February 2021).
- Continuous Remote Monitoring Using the Sensium System Positively Impacts Length of Stay and Admission to Critical Care. Sensium. 2020. Available online: https://www.sensium.co.uk/news/study-continuous-remote-monitoring-using-the-sensium-system-positively-impacts-length-of-stay-and-admission-to-critical-care (accessed on 29 September 2021).
- Report MEDMAL 10^ Edizione. STUDIO Sull’andamento del Rischio da Medical Malpractice Nella Sanità Italiana Pubblica e Privata. Marsch & McLennan Companies. 2019. Available online: https://www.marsh.com/it/it/insights/research-briefings/report-medmal-italia-un-sinistro-ogni-dieci-giorni-per-struttura-pubblica.html (accessed on 29 September 2021).
- Pietrantonio, F.; Bussi, A.R.; Amadasi, S.; Bresciani, E.; Caldonazzo, A.; Colombini, P.; Giovannini, M.S.; Grifi, G.; Lanzini, L.; Migliorati, P.; et al. Technological Challenges set up by Continuous Wireless Monitoring designed to Improve Management of Critically Ill Patients in an Internal Medicine Unit (LIMS study): Study Design and Preliminary Results. J. Community Prev. Med. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Cangemi, A.; Turchetti, B. Miglioramento della qualità della vita dei pazienti e riduzione del costo per il SSN attraverso l’uso di un sistema wireless di monitoraggio multi-parametrico dei parametri fisiologici. Case study sull’adozione del sistema di monitoraggio fisiologico WIN@Hospital presso l’Ospedale Campo di Marte, Lucca. In Proceedings of the Europe Health Summit, Berlino, Berlin, Germany, 4–8 May 2014. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Rosiello, F.; Alessi, E.; Pascucci, M.; Rainone, M.; Cipriano, E.; Di Berardino, A.; Vinci, A.; Ruggeri, M.; Ricci, S. Burden of COVID-19 on Italian Internal Medicine Wards: Delphi, SWOT, and Performance Analysis after Two Pandemic Waves in the Local Health Authority “Roma 6” Hospital Structures. Int. J. Environ. Res. Public Health 2021, 18, 5999. [Google Scholar] [CrossRef] [PubMed]

| Major Complications | |
|---|---|
| 1. | Unexpected death |
| 2. | Cardiac arrest |
| 3. | Healthcare-related infections (urinary tract infections, pneumonia, sepsis, etc.) |
| 4. | Cardiac arrhythmias, acute coronary syndrome |
| 5. | Acute renal failure |
| 6. | Acute respiratory failure |
| 7. | Onset and evolution of neurological deficits not present at admission |
| 8. | Deep vein thrombosis and pulmonary thromboembolism |
| 9. | Gastrointestinal hemorrhage |
| 10. | Pressure ulcers |
| 11. | Injury/fall |
| 12. | Allergic reactions |
| 13. | Unexpected transfer from a general care ward to a more addictive/intensive care ward |
| 14. | Unplanned new hospitalization within 21 days of discharge |
| 15. | Episodes of glucose decompensation (hypo/hyperglycemia) in patients with diabetes mellitus. |
| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Items | Preliminary Results | Observations |
|---|---|---|
| Patients enrolled | 110 (56 M/54 F) | Enrollments in progress |
| Mean Age | 76.2 years (range 43–92)— STD 75.9, WMON 76.7 | 45 patients (42.5%) > 80 years |
| Comorbidity | CIRS CI 3.93 CIRS SI 1.93 | High patient complexity |
| BRASS ≥ 20 | 20 patients (18.9%) | Indicating need for step-down care |
| Barthel Score | 65.2 (range 0–100) | Mean value |
| Exton–Smith scale | 16.5 (range 7–20) | Mean value |
| Charlson Index | 3.8 | Mean value |
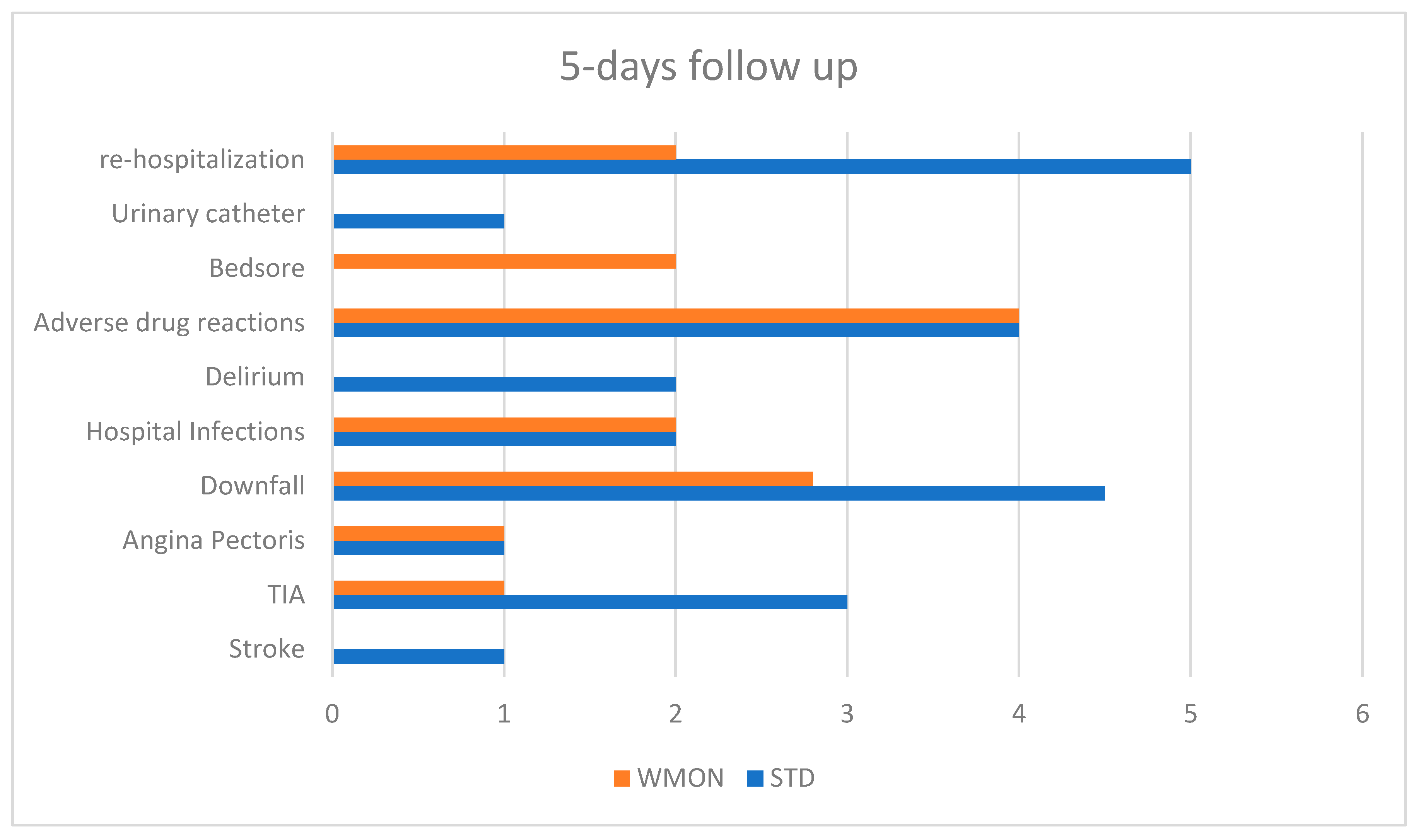
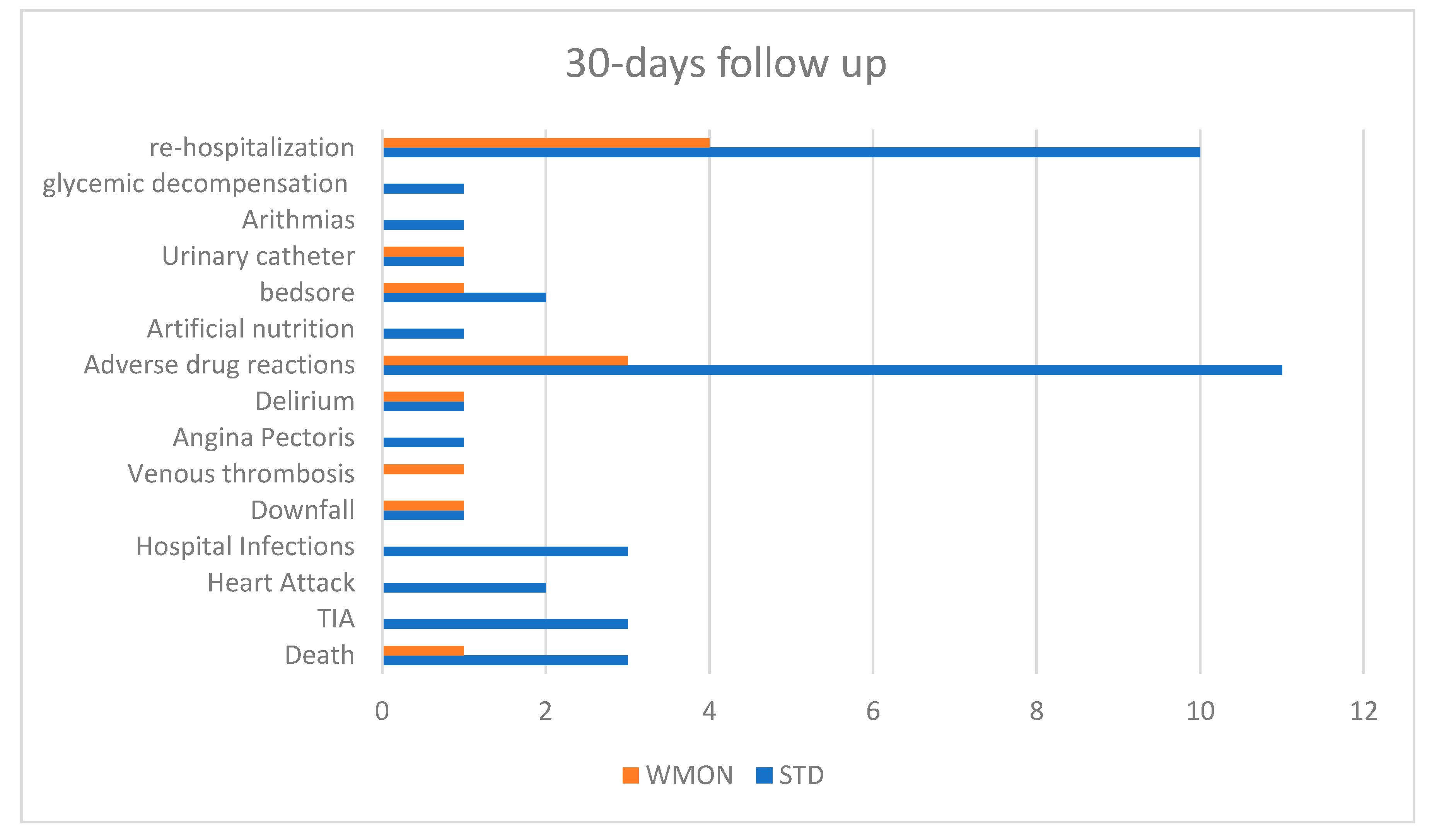
| Major Complications at 5 days of follow up | 24 (14 STD, 10 WMON) | 25% STD, 19% WMON Favors intervention |
| Major Complications at 30 days of follow up | 39 (27 STD, 12 WMON) | 48% STD, 22% WMON |
| Re-hospitalization in 5 days | 7 (5 STD, 2 WMON) | Favors intervention |
| Re-hospitalization in 30 days | 14 (10 STD, 4 WMON) | WMON arm has half hospitalization rate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrantonio, F.; Vinci, A.; Rosiello, F.; Alessi, E.; Pascucci, M.; Rainone, M.; Delli Castelli, M.; Ciamei, A.; Montagnese, F.; D’Amico, R.; et al. Green Line Hospital-Territory Study: A Single-Blind Randomized Clinical Trial for Evaluation of Technological Challenges of Continuous Wireless Monitoring in Internal Medicine, Preliminary Results. Int. J. Environ. Res. Public Health 2021, 18, 10328. https://doi.org/10.3390/ijerph181910328
Pietrantonio F, Vinci A, Rosiello F, Alessi E, Pascucci M, Rainone M, Delli Castelli M, Ciamei A, Montagnese F, D’Amico R, et al. Green Line Hospital-Territory Study: A Single-Blind Randomized Clinical Trial for Evaluation of Technological Challenges of Continuous Wireless Monitoring in Internal Medicine, Preliminary Results. International Journal of Environmental Research and Public Health. 2021; 18(19):10328. https://doi.org/10.3390/ijerph181910328
Chicago/Turabian StylePietrantonio, Filomena, Antonio Vinci, Francesco Rosiello, Elena Alessi, Matteo Pascucci, Marianna Rainone, Michela Delli Castelli, Angela Ciamei, Fabrizio Montagnese, Roberto D’Amico, and et al. 2021. "Green Line Hospital-Territory Study: A Single-Blind Randomized Clinical Trial for Evaluation of Technological Challenges of Continuous Wireless Monitoring in Internal Medicine, Preliminary Results" International Journal of Environmental Research and Public Health 18, no. 19: 10328. https://doi.org/10.3390/ijerph181910328
APA StylePietrantonio, F., Vinci, A., Rosiello, F., Alessi, E., Pascucci, M., Rainone, M., Delli Castelli, M., Ciamei, A., Montagnese, F., D’Amico, R., Valerio, A., & Manfellotto, D. (2021). Green Line Hospital-Territory Study: A Single-Blind Randomized Clinical Trial for Evaluation of Technological Challenges of Continuous Wireless Monitoring in Internal Medicine, Preliminary Results. International Journal of Environmental Research and Public Health, 18(19), 10328. https://doi.org/10.3390/ijerph181910328







