Prenatal Exposure to Acetaminophen and Childhood Asthmatic Symptoms in a Population-Based Cohort in Los Angeles, California
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Acetaminophen Exposure
2.3. Asthmatic Outcomes
2.4. Statistical Analysis
2.5. Ethical Statement
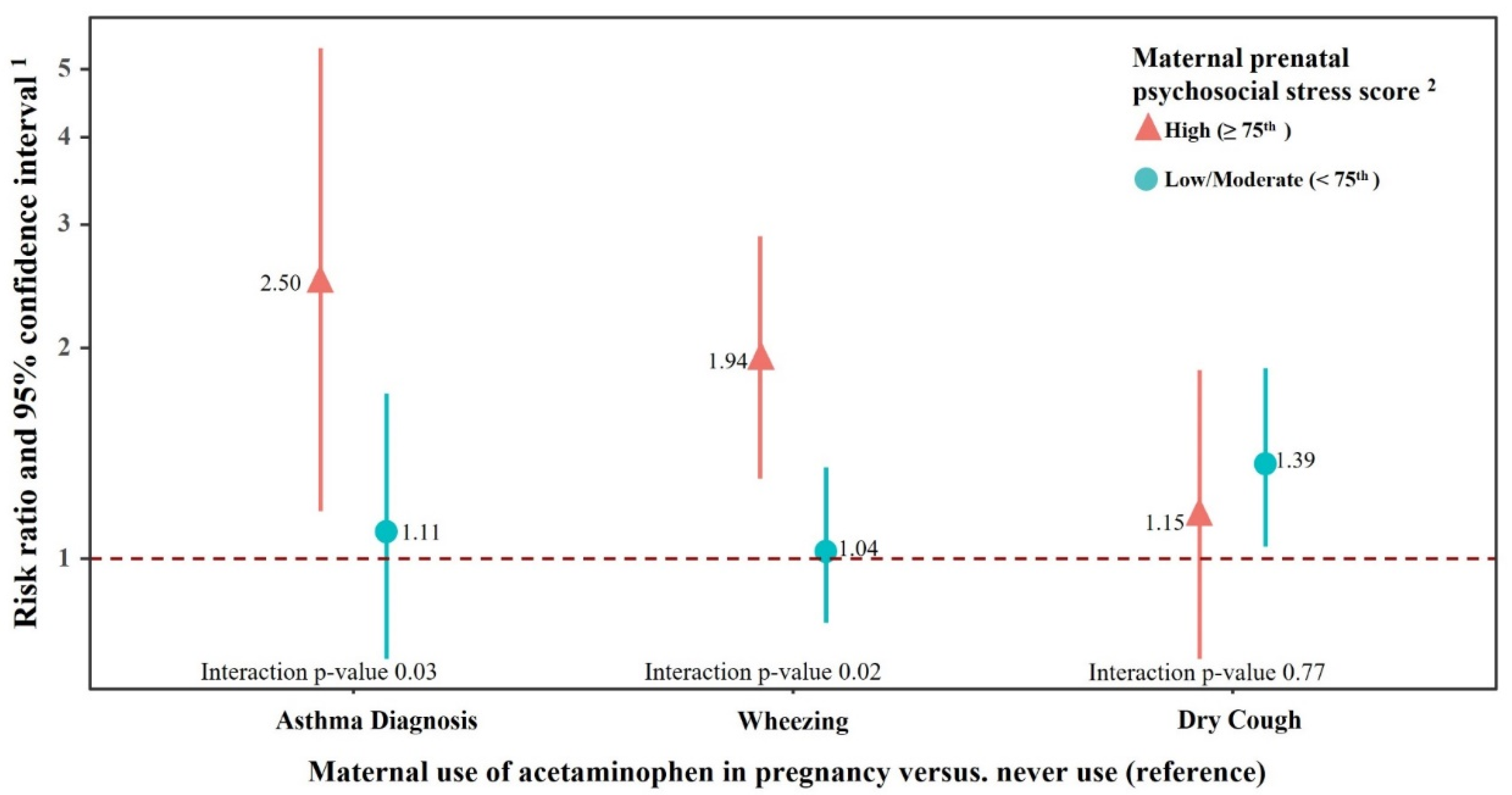
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werler, M.M.; Mitchell, A.A.; Hernandez-Diaz, S.; Honein, M.A. Use of Over-the-Counter Medications during Pregnancy. Am. J. Obstet. Gynecol. 2005, 193, 771–777. [Google Scholar] [CrossRef]
- Eyers, S.; Weatherall, M.; Jefferies, S.; Beasley, R. Paracetamol in Pregnancy and the Risk of Wheezing in Offspring: A Systematic Review and Meta-Analysis. Clin. Exp. Allergy 2011, 41, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Cheelo, M.; Lodge, C.J.; Dharmage, S.C.; Simpson, J.A.; Matheson, M.; Heinrich, J.; Lowe, A.J. Paracetamol Exposure in Pregnancy and Early Childhood and Development of Childhood Asthma: A Systematic Review and Meta-Analysis. Arch. Dis. Child. 2015, 100, 81–89. [Google Scholar] [CrossRef]
- Fan, G.; Wang, B.; Liu, C.; Li, D. Prenatal Paracetamol Use and Asthma in Childhood: A Systematic Review and Meta-Analysis. Allergol. Immunopathol. 2017, 45, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Garrettson, L.K.; Soda, D.M. Letter: Evidence of Placental Transfer of Acetaminophen. Pediatrics 1975, 55, 895. [Google Scholar]
- Horowitz, R.S.; Dart, R.C.; Jarvie, D.R.; Bearer, C.F.; Gupta, U. Placental Transfer of N-Acetylcysteine Following Human Maternal Acetaminophen Toxicity. J. Toxicol. Clin. Toxicol. 1997, 35, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, H.; Stewart, A.; Mitchell, E.; Crane, J.; Eyers, S.; Weatherall, M.; Beasley, R. The Role of Paracetamol in the Pathogenesis of Asthma. Clin. Exp. Allergy 2010, 40, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Akinbami, L. Centers for Disease Control and Prevention National Center for Health Statistics The State of Childhood Asthma, United States, 1980–2005. Adv. Data 2006, 12, 1–24. [Google Scholar]
- Babey, S.H.; Hastert, T.A.; Meng, Y.-Y.; Brown, E.R. Low-Income Californians Bear Unequal Burden of Asthma; Policy Brief; Center Health Policy Research UCLA: Los Angeles, CA, USA, 2007; pp. 1–7. [Google Scholar]
- Magnus, M.C.; Karlstad, Ø.; Håberg, S.E.; Nafstad, P.; Davey Smith, G.; Nystad, W. Prenatal and Infant Paracetamol Exposure and Development of Asthma: The Norwegian Mother and Child Cohort Study. Int. J. Epidemiol. 2016, 45, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Rebordosa, C.; Kogevinas, M.; Sørensen, H.T.; Olsen, J. Pre-Natal Exposure to Paracetamol and Risk of Wheezing and Asthma in Children: A Birth Cohort Study. Int. J. Epidemiol. 2008, 37, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, S.O.; Newson, R.B.; Smith, G.D.; Henderson, A.J. Prenatal Paracetamol Exposure and Asthma: Further Evidence against Confounding. Int. J. Epidemiol. 2010, 39, 790–794. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Marcos, L.; Sanchez-Solis, M.; Perez-Fernandez, V.; Pastor-Vivero, M.D.; Mondejar-Lopez, P.; Valverde-Molina, J. Is the Effect of Prenatal Paracetamol Exposure on Wheezing in Preschool Children Modified by Asthma in the Mother? Int. Arch. Allergy Immunol. 2009, 149, 33–37. [Google Scholar] [CrossRef]
- Sordillo, J.E.; Scirica, C.V.; Rifas-Shiman, S.L.; Gillman, M.W.; Bunyavanich, S.; Camargo, C.A.; Weiss, S.T.; Gold, D.R.; Litonjua, A.A. Prenatal and Infant Exposure to Acetaminophen and Ibuprofen and the Risk for Wheeze and Asthma in Children. J. Allergy Clin. Immunol. 2015, 135, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Perzanowski, M.S.; Miller, R.L.; Tang, D.; Ali, D.; Garfinkel, R.S.; Chew, G.L.; Goldstein, I.F.; Perera, F.P.; Barr, R.G. Prenatal Acetaminophen Exposure and Risk of Wheeze at Age 5 Years in an Urban Low-Income Cohort. Thorax 2010, 65, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Persky, V.; Piorkowski, J.; Hernandez, E.; Chavez, N.; Wagner-Cassanova, C.; Vergara, C.; Pelzel, D.; Enriquez, R.; Gutierrez, S.; Busso, A. Prenatal Exposure to Acetaminophen and Respiratory Symptoms in the First Year of Life. Ann. Allergy Asthma Immunol. 2008, 101, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.M.; Lundsberg, L.S.; Illuzzi, J.L.; Bracken, M.B. Prenatal Exposure to Acetaminophen and Asthma in Children. Obstet. Gynecol. 2009, 114, 1295–1306. [Google Scholar] [CrossRef] [Green Version]
- Bandoli, G.; von Ehrenstein, O.; Ghosh, J.K.C.; Flores, M.E.S.; Dunkel Schetter, C.; Ritz, B. Prenatal Maternal Stress and the Risk of Lifetime Wheeze in Young Offspring: An Examination by Stressor and Maternal Ethnicity. J. Immigr. Minor Health 2016, 18, 987–995. [Google Scholar] [CrossRef] [Green Version]
- Ritz, B.; Wilhelm, M.; Hoggatt, K.J.; Ghosh, J.K.C. Ambient Air Pollution and Preterm Birth in the Environment and Pregnancy Outcomes Study at the University of California, Los Angeles. Am. J. Epidemiol. 2007, 166, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and Methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.C. Multiple Imputation for Missing Data: Concepts and New Development. Available online: https://support.sas.com/rnd/app/stat/papers/multipleimputation.pdf (accessed on 30 September 2020).
- Wu, J.; Ren, C.; Delfino, R.J.; Chung, J.; Wilhelm, M.; Ritz, B. Association between Local Traffic-Generated Air Pollution and Preeclampsia and Preterm Delivery in the South Coast Air Basin of California. Environ. Health Perspect. 2009, 117, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Ritz, B. Residential Proximity to Traffic and Adverse Birth Outcomes in Los Angeles County, California, 1994–1996. Environ. Health Perspect. 2003, 111, 207–216. [Google Scholar] [CrossRef]
- Garcia, E.; Berhane, K.T.; Islam, T.; McConnell, R.; Urman, R.; Chen, Z.; Gilliland, F.D. Association of Changes in Air Quality With Incident Asthma in Children in California, 1993–2014. JAMA 2019, 321, 1906–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kynyk, J.A.; Mastronarde, J.G.; McCallister, J.W. Asthma, the Sex Difference. Curr. Opin. Pulm. Med. 2011, 17, 6–11. [Google Scholar] [CrossRef]
- Prescott, L.F. Kinetics and Metabolism of Paracetamol and Phenacetin. Br. J. Clin. Pharmacol. 1980, 10, 291S–298S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Dharmage, S.C.; Abramson, M.J.; Erbas, B.; Bennett, C.M.; Svanes, C.; Hui, J.; Axelrad, C.; Lowe, A.J.; Lodge, C.J. Early Life Acetaminophen Exposure, Glutathione S-Transferase Genes, and Development of Adolescent Asthma in a High-Risk Birth Cohort. J. Allergy Clin. Immunol. 2020, 146, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J.M.; Clark, L.E.; Herrera, J.L. Acetaminophen Overdose in Pregnancy. South. Med. J. 2005, 98, 1118–1122. [Google Scholar] [CrossRef]
- Cookson, H.; Granell, R.; Joinson, C.; Ben-Shlomo, Y.; Henderson, A.J. Mothers’ Anxiety during Pregnancy Is Associated with Asthma in Their Children. J. Allergy Clin. Immunol. 2009, 123, 847–853.e11. [Google Scholar] [CrossRef] [Green Version]
- Merlot, E.; Couret, D.; Otten, W. Prenatal Stress, Fetal Imprinting and Immunity. Brain Behav. Immun. 2008, 22, 42–51. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.-J.C.; Sernia, C.; Lavidis, N.A. Activation of the Hypothalamic-Pituitary-Adrenal Stress Axis Induces Cellular Oxidative Stress. Front. Neurosci. 2014, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Grainge, C.L.; Davies, D.E. Epithelial Injury and Repair in Airways Diseases. Chest 2013, 144, 1906–1912. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Andrè, E.; Sartiani, L.; Aldini, G.; Trevisani, M.; Carnini, C.; Massi, D.; Pedretti, P.; Carini, M.; et al. Acetaminophen, via Its Reactive Metabolite N-Acetyl-p-Benzo-Quinoneimine and Transient Receptor Potential Ankyrin-1 Stimulation, Causes Neurogenic Inflammation in the Airways and Other Tissues in Rodents. FASEB J. 2010, 24, 4904–4916. [Google Scholar] [CrossRef] [PubMed]
- Konstandi, M.; Johnson, E.O.; Lang, M.A. Consequences of Psychophysiological Stress on Cytochrome P450-Catalyzed Drug Metabolism. Neurosci. Biobehav. Rev. 2014, 45, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.A.; Zeng, Z.; Wold, C.M.; Haddock, W.; Fielding, J.E. Prevalence of Childhood Asthma and Associated Morbidity in Los Angeles County: Impacts of Race/Ethnicity and Income. J. Asthma 2003, 40, 535–543. [Google Scholar] [CrossRef]
- Liew, Z.; Ernst, A. Intrauterine Exposure to Acetaminophen and Adverse Developmental Outcomes: Epidemiological Findings and Methodological Issues. Curr. Environ. Health Rep. 2021, 8, 23–33. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Maternal Ever Use of Acetaminophen during Pregnancy | |||
|---|---|---|---|---|
| Yes (N = 393) | No (N = 808) | |||
| n | % | n | % | |
| Child’s sex | ||||
| Male | 191 | 48.6 | 418 | 51.7 |
| Female | 202 | 51.4 | 390 | 48.3 |
| Maternal age (years) | ||||
| ≤24 | 70 | 17.8 | 217 | 26.9 |
| 25–29 | 99 | 25.2 | 200 | 24.8 |
| 30–34 | 129 | 32.9 | 226 | 28.0 |
| ≥35 | 95 | 24.2 | 165 | 20.4 |
| Maternal race/ethnicity | ||||
| White, non-Hispanic/Latina | 136 | 34.6 | 162 | 20.0 |
| Hispanic/Latina | 203 | 51.7 | 515 | 63.7 |
| Black/African American | 20 | 5.1 | 51 | 6.3 |
| Asian/Pacific Islanders and others 1 | 33 | 8.4 | 72 | 8.9 |
| Missing | 1 | 0.2 | 8 | 1.0 |
| Maternal education (years) | ||||
| <12 | 70 | 17.8 | 250 | 30.9 |
| 12 | 89 | 22.6 | 188 | 23.3 |
| 13–15 | 77 | 19.6 | 136 | 16.8 |
| ≥16 | 151 | 38.4 | 215 | 26.6 |
| Missing | 6 | 1.5 | 19 | 2.4 |
| Parity | ||||
| Nulliparous | 154 | 39.2 | 337 | 41.7 |
| Parous | 239 | 60.8 | 471 | 58.3 |
| Household income (dollars) | ||||
| 10–30 K | 104 | 26.5 | 299 | 37.0 |
| 30–50 K | 72 | 18.3 | 164 | 20.3 |
| >50 K | 178 | 45.3 | 245 | 30.3 |
| Missing | 39 | 9.9 | 100 | 12.4 |
| Mother’s U.S. born status | ||||
| U.S. born | 221 | 56.2 | 348 | 43.1 |
| Non-U.S. born | 170 | 43.3 | 460 | 56.9 |
| Missing | 2 | 0.5 | 0 | 0 |
| Maternal smoking | ||||
| Ever smoked during pregnancy | 22 | 5.6 | 31 | 3.8 |
| Ever smoked but not during pregnancy | 134 | 34.1 | 215 | 26.6 |
| Never smoked | 237 | 60.3 | 562 | 69.6 |
| Maternal BMI | ||||
| <18.5 | 15 | 3.8 | 35 | 4.3 |
| 18.5–24.9 | 202 | 51.4 | 391 | 48.4 |
| 25–29 | 74 | 18.8 | 174 | 21.5 |
| >29 | 70 | 17.8 | 116 | 14.4 |
| Missing | 32 | 8.1 | 92 | 11.4 |
| Maternal alcohol intake during pregnancy (yes) | 53 | 13.5 | 76 | 9.4 |
| Missing | 1 | 0.3 | 0 | 0 |
| Mother high fever during pregnancy (yes) | 125 | 31.9 | 186 | 23.0 |
| Missing | 1 | 0.3 | 1 | 0.1 |
| Maternal antibiotic use during pregnancy(yes) | 14 | 3.6 | 34 | 4.2 |
| Maternal aspirin use during pregnancy (yes) | 25 | 6.4 | 32 | 4.0 |
| Maternal ibuprofen use during pregnancy | 19 | 4.8 | 22 | 2.7 |
| Maternal Race/Ethnicity | Acetaminophen Use during Pregnancy | No. of All Children | Asthma Diagnosis | Wheezing | Dry Cough | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | RR 1 (95% CI) | Interaction p-Value 1 | N | RR 1 (95% CI) | Interaction p-Value 1 | N | RR 1 (95% CI) | Interaction p-Value 1 | |||
| Total population | Never | 808 | 71 | Ref | 186 | Ref | 147 | Ref | |||
| Ever | 393 | 47 | 1.39 (0.96, 2.00) | n/a | 118 | 1.25 (1.01, 1.54) | n/a | 96 | 1.35 (1.06, 1.73) | n/a | |
| White, not Hispanic | Never | 162 | 9 | Ref | 35 | Ref | 29 | Ref | |||
| Ever | 136 | 14 | 1.52 (0.68, 3.40) | 0.15 | 44 | 1.27 (0.86, 1.87) | 0.36 | 32 | 1.15 (0.74, 1.80) | 0.97 | |
| Hispanic/Latina | Never | 515 | 50 | Ref | 118 | Ref | 93 | Ref | |||
| Ever | 203 | 17 | 0.79 (0.45, 1.40) | Ref | 50 | 1.05 (0.78, 1.42) | Ref | 42 | 1.17 (0.82, 1.68) | Ref | |
| Black/African American | Never | 51 | 6 | Ref | 19 | Ref | 12 | Ref | |||
| Ever | 20 | 9 | 4.27 (1.93, 9.46) | <0.01 | 12 | 2.04 (1.23, 3.40) | 0.05 | 10 | 1.88 (0.93, 3.77) | 0.12 | |
| Asian/Pacific Islander and others 2 | Never | 72 | 6 | Ref | 12 | Ref | 12 | Ref | |||
| Ever | 33 | 7 | 2.55 (0.91, 7.16) | 0.08 | 11 | 2.18 (1.08, 4.40) | 0.23 | 12 | 2.66 (1.26, 5.63) | 0.16 | |
| Maternal Race/Ethnicity | Acetaminophen Use during Pregnancy | No. of All Children | Asthma Diagnosis | Wheezing | Dry Cough | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | RR 1 (95% CI) | p-Trend 2 | N | RR 1 (95% CI) | p-Trend 2 | N | RR 1 (95% CI) | p-Trend 2 | |||
| Total population | Never use | 808 | 71 | Ref | 186 | Ref | 147 | Ref | |||
| Once a month or less | 231 | 27 | 1.41 (0.90, 2.20) | 74 | 1.37 (1.08, 1.74) | 61 | 1.47 (1.11, 1.95) | ||||
| More than once a month | 136 | 19 | 1.56 (0.96, 2.52) | 0.07 | 39 | 1.18 (0.87, 1.60) | 0.18 | 29 | 1.17 (0.81, 1.70) | 0.39 | |
| White, not Hispanic | Never use | 162 | 9 | Ref | 35 | Ref | 29 | Ref | |||
| Once a month or less | 86 | 8 | 1.41 (0.62, 3.22) | 28 | 1.27 (0.84, 1.93) | 20 | 1.10 (0.67, 1.82) | ||||
| More than once a month | 40 | 5 | 1.67 (0.52, 5.34) | 0.07 | 14 | 1.32 (0.74, 2.33) | 0.06 | 9 | 1.13 (0.57, 2.23) | 0.63 | |
| Hispanic/Latina | Never use | 515 | 50 | Ref | 118 | Ref | 93 | Ref | |||
| Once a month or less | 115 | 10 | 0.86 (0.43, 1.72) | 32 | 1.24 (0.88, 1.75) | 28 | 1.37 (0.92, 2.04) | ||||
| More than once a month | 76 | 7 | 0.80 (0.37, 1.76) | 0.41 | 16 | 0.83 (0.51, 1.36) | 0.38 | 13 | 0.92 (0.52, 1.63) | 0.56 | |
| Others 3 | Never use | 123 | 12 | Ref | 31 | Ref | 24 | Ref | |||
| Once a month or less | 29 | 9 | 3.54 (1.56, 8.03) | 13 | 2.18 (1.32, 3.57) | 13 | 2.61 (1.47, 4.66) | ||||
| More than once a month | 20 | 7 | 5.00 (2.33, 10.71) | <0.001 | 9 | 2.00 (1.20, 3.33) | <0.001 | 7 | 1.89 (0.96, 3.71) | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, Z.; Yuan, Y.; Meng, Q.; von Ehrenstein, O.S.; Cui, X.; Flores, M.E.S.; Ritz, B. Prenatal Exposure to Acetaminophen and Childhood Asthmatic Symptoms in a Population-Based Cohort in Los Angeles, California. Int. J. Environ. Res. Public Health 2021, 18, 10107. https://doi.org/10.3390/ijerph181910107
Liew Z, Yuan Y, Meng Q, von Ehrenstein OS, Cui X, Flores MES, Ritz B. Prenatal Exposure to Acetaminophen and Childhood Asthmatic Symptoms in a Population-Based Cohort in Los Angeles, California. International Journal of Environmental Research and Public Health. 2021; 18(19):10107. https://doi.org/10.3390/ijerph181910107
Chicago/Turabian StyleLiew, Zeyan, Yuying Yuan, Qi Meng, Ondine S. von Ehrenstein, Xin Cui, Marie E. S. Flores, and Beate Ritz. 2021. "Prenatal Exposure to Acetaminophen and Childhood Asthmatic Symptoms in a Population-Based Cohort in Los Angeles, California" International Journal of Environmental Research and Public Health 18, no. 19: 10107. https://doi.org/10.3390/ijerph181910107
APA StyleLiew, Z., Yuan, Y., Meng, Q., von Ehrenstein, O. S., Cui, X., Flores, M. E. S., & Ritz, B. (2021). Prenatal Exposure to Acetaminophen and Childhood Asthmatic Symptoms in a Population-Based Cohort in Los Angeles, California. International Journal of Environmental Research and Public Health, 18(19), 10107. https://doi.org/10.3390/ijerph181910107








