When Movement Moves: Study Protocol for a Multi-Method Pre/Post Evaluation Study of Two Programmes; the Danish Team Twin and Cycling Without Age
Abstract
1. Introduction
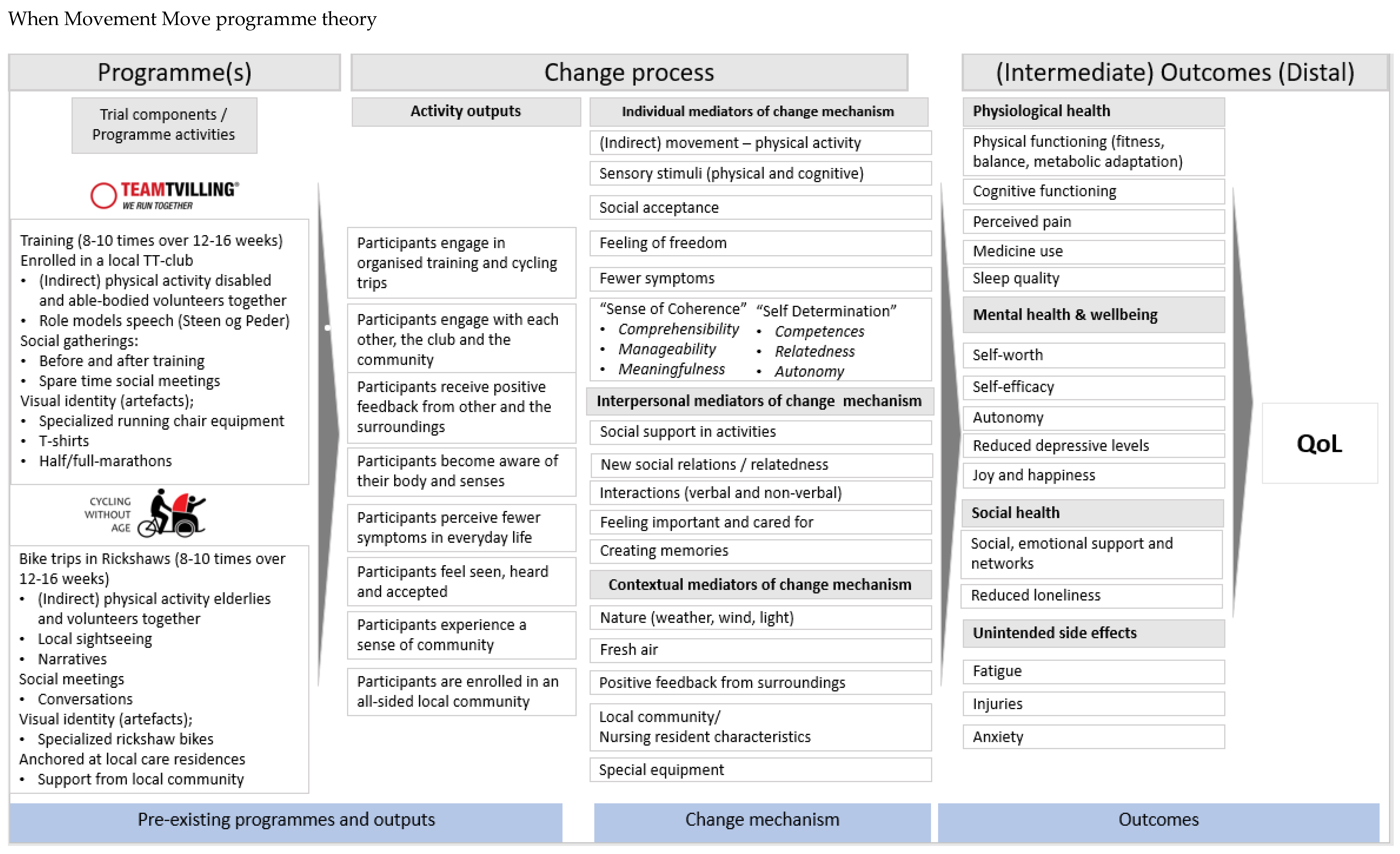
1.1. Presenting the Programmes
1.1.1. Team Twin—Association and Members
1.1.2. Cycling Without Age—Association and Members
1.1.3. Interrelated Dependency
2. Methods
2.1. Study Aims
- (1)
- How does being moved by others affect the QoL among handiathletes (disabled people—TT) and passengers (elderly—CWA)?
- (2)
- Does indirect PA lead to improved physiological health among handiathletes (disabled people—TT) and passengers (elderly—CWA)?
- (3)
- How does physically moving others affect the volunteers’ perceived physical and mental health and their QoL?
- (4)
- What does it mean for relatives and nursing staff that handiathletes and nursing home residents are affiliated with TT and CWA, respectively?
2.2. Programme Theory Development
3. Study Design and Evaluation
3.1. Shared Outcomes and Outcome Measures
3.2. Sample Size and Power
3.3. Qualitative Approach
- (1)
- Individual and/or focus group interviews with representatives from all target groups, both primary and secondary, from both programmes.
- (2)
- Participant observations during both TT and CWA activities.
3.4. Team Twin—Recruitment and Data Collection
3.4.1. Recruitment Process
3.4.2. Handiathletes
- (1)
- The HA must be affiliated with a TT local club.
- (2)
- The HA must be competent in legal matters (i.e., manage own life).
3.4.3. Runners
3.4.4. Relatives
3.5. Quantitative Approach—Material and Instruments
3.5.1. Clinical Examination
- Fasting must be initiated at least 8 h prior to testing.
- No exercise 36 h prior to testing.
- No caffeine 24 h prior to testing.
- No alcohol 48 h prior to testing.
- No smoking 8 h prior to testing.
- No Antacida, NSAIDs, paracetamol, or Proton Pump Inhibitors PPIs 24 h prior to testing.
3.5.2. Medical Examination, Blood Samples and Blood Pressure
3.5.3. Oral Glucose Tolerance Test
3.5.4. Body Anthropometrics
3.5.5. Bio Tracking PA and Sleep Pattern
3.5.6. Trip Registration—TT Activities
3.5.7. Outcomes
- Outcomes and measures in the Team Twin programme evaluation
- Outcomes of the WMM study—Team Twin
| Measurement (Outcome) | What (Operational) | How (Instrument) | When (Timing of Collection) | Who (Data Source) |
|---|---|---|---|---|
| PRIMARY OUTCOME | ||||
| Quality of life (QOL) | Cantril Ladder of Life Scale [62] | Web and interview-based questionnaires | Pre, post | HA, Runners, Relatives |
| SECONDARY OUTCOMES | ||||
| Autonomy | The perceived feeling of being in control over ones own life | Web-based questionnaire | Pre, post | HA |
| Sleep | Sleep quality and sleep quantity | Web-based questionnaire Bio tracking (HA) SMS-Survey (HA) | Baseline, during (HA), follow-up | HA, Runners |
| Well-being | WHO-five Well-being Index [59] | Web-based questionnaire | Pre, post | HA, Runners |
| Loneliness | A perceived feeling of loneliness and lack of network and support [54,63] | Web-based questionnaire | Pre, post | HA, Runners |
| EXPLORATIVE OUTCOMES | ||||
| Self-perceived health | Subjectively perceived Health [64] | Web-based questionnaire | Pre, post | HA, Runners |
| Perceived pain | Mental and physical pain/discomfort [64] | Web-based questionnaire | Pre, post | HA |
| Self-perceived Physical performance | Subjectively perceived Physical performance [65] | Web-based questionnaire | Pre, post | Runners |
| Epileptic seizures | Reduced epileptic seizures (adjusted version [66]) | Paper-based questionnaire | Pre, post | HA |
| Self-efficacy | General self-efficacy [56,67] | Web-based questionnaire | Pre, post | HA, Runners |
| Self-worth | Perceived feeling of acceptance [55] | Web-based questionnaire | Pre, post | HA, Runners |
| Social/emotional support and network | Contact and support with friends, family and others. The perceived feeling of being valued, respected and accepted by others [64] | Web-based questionnaire | Pre, post | HA |
| UNINTENDED SIDE EFFECTS | ||||
| Fatigue | The perceived feeling of fatigue related to voluntariness or programme activity | Web-based questionnaire | Post | HA, Runners |
| Anxiety | The perceived feeling of anxiety trigged by the programme activity | Web-based questionnaire | Post | HA, Runners |
| Injuries | Amount of injuries by participation | Web-based questionnaire | Post | HA, Runners |
| Objective clinical data (only measures for HA TT) | ||||
| Body anthropometrics |
| Dual X-ray absorptiometry | Pre1, Pre2 + Post | HA |
| Clinical blood samples | Blood glucose control:
| Standard clinical procedure | Pre1, Pre2 + Post | HA |
Blood lipids:
| ||||
| OGTT |
| Standard OGTT-procedure | Pre1, Pre2 + Post | HA |
| Fitness level |
| Estimated from haematocrit level | Pre1, Pre2 + Post | HA |
| Office blood pressure |
| Monitored by a standard procedure for office blood pressure | Pre1, Pre2 + Post | HA |
| Trip registration | ||||
| Objective observational data from every training/trip |
| Online trip registration (QR-code directing users to a short web-based questionnaire is scanned immediately after the TT) | During the programmes and after finishing a programme activity. | Context related data completed by a runner after each activity TT: Runners fulfil the online form |
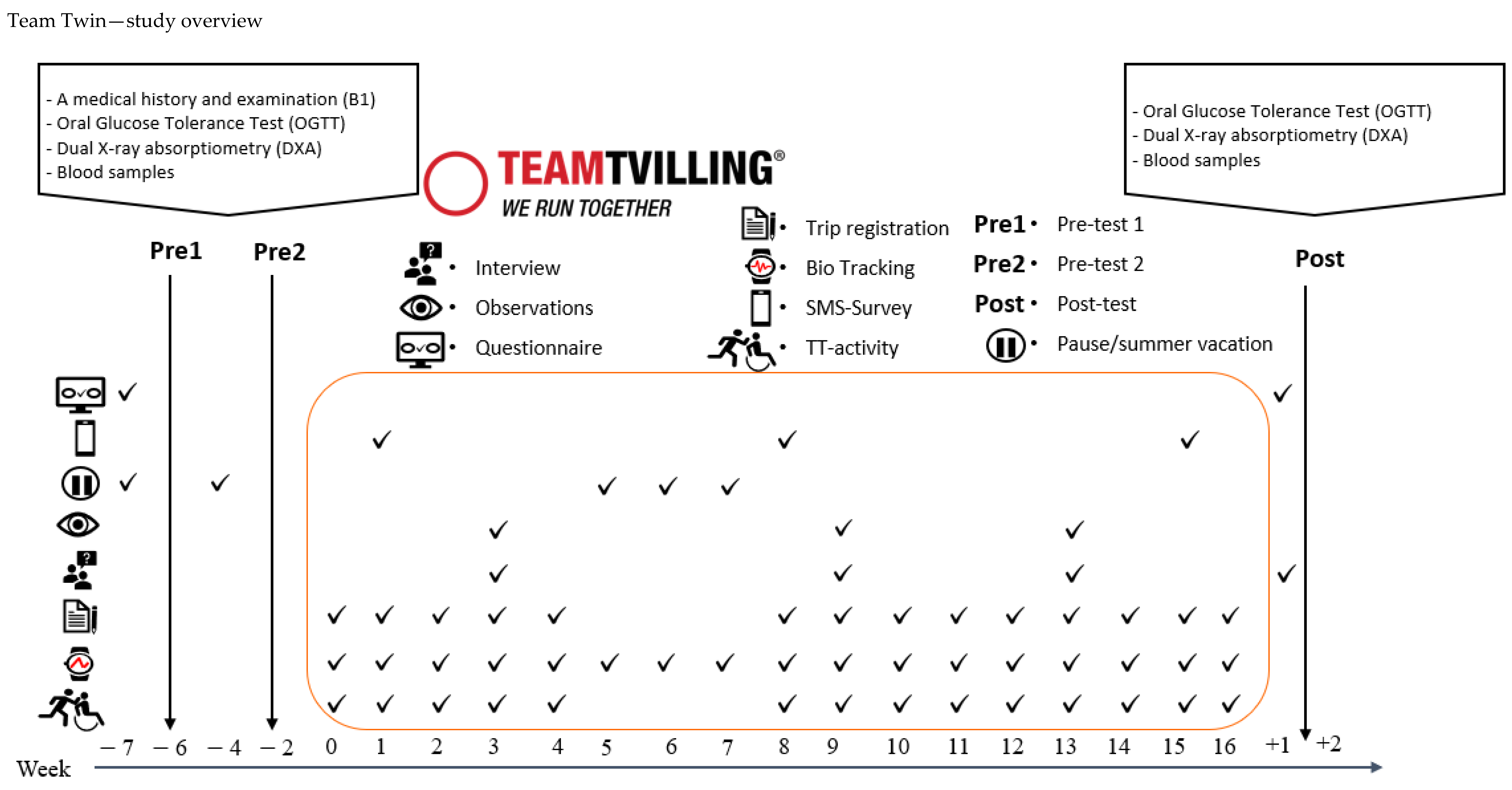
3.6. Cycling Without Age—Recruitment and Data Collection
3.6.1. Recruitment Process
3.6.2. Passengers
3.7. Quantitative Approach—Material and Instruments
3.7.1. Objective Functional and Cognitive Appraisal
3.7.2. Physical Function and Mobility
3.7.3. Muscle Strength
3.7.4. Trip Registration for CWA Programme
3.7.5. Outcomes
- Outcomes and measures in the Cycling Without Age evaluation
- Outcomes of the WMM study—Cycling Without Age
| Measurement (Outcome) | What (Operational) | How (Instrument) | When (Timing of Collection) | Who in CWA (Data Source) |
|---|---|---|---|---|
| PRIMARY OUTCOME | ||||
| Quality of life (QoL) | Cantril Ladder of Life Scale [62] | Web- and interview-based questionnaire | Pre, post | Passengers, Pilots, Nursing staff |
| SECONDARY OUTCOMES | ||||
| Autonomy | The perceived feeling of being in control over ones own life | Web-based questionnaire | Pre, post | Passengers |
| Sleep | Sleep quality and sleep quantity | Web-based questionnaire | Pre, post | Passengers, Pilots, |
| Well-being | CUA: Warwick-Edinburgh Mental Well-Being Scale (S)WEMWBS) [58,60] | Web-based questionnaire | Pre, post | Passengers, Pilots |
| Loneliness | A perceived feeling of loneliness and lack of network and support [54,63] | Web-based questionnaire | Pre, post | Passengers, Pilots |
| EXPLORATIVE OUTCOMES | ||||
| Self-perceived health | Subjectively perceived Health [64] | Web-based questionnaire | Pre, post | Passengers, Pilots, |
| Perceived pain | Mental and physical pain/discomfort [64] | Web-based questionnaire | Pre, post | Passengers, pilots |
| Self-perceived Physical performance | Subjectively perceived Physical performance [65] | Web-based questionnaire | Pre, post | Passengers, pilots |
| Self-efficacy | General self-efficacy [56,67] | Web-based questionnaire | Pre, post | Passengers, pilots |
| Self-worth | Perceived feeling of Acceptance [55] | Web-based questionnaire | Pre, post | Passengers, pilots |
| Autonomy | The perceived feeling of being in control over ones own life | Web-based questionnaire | Pre, post | Passengers, pilots |
| Social/emotional support and network | Contact and support with friends, family and others. The perceived feeling of being valued, respected and accepted by others [64] | Web-based questionnaire | Pre, post | Passengers, pilots |
| UNINTENDED SIDE EFFECTS | ||||
| Fatigue | The perceived feeling of fatigue related to voluntariness or programme activity | Web-based questionnaire | Post | Passengers, pilots |
| Anxiety | The perceived feeling of anxiety trigged by the programme activity | Web-based questionnaire | Post | Passengers, pilots |
| Injuries | Amount of injuries by participation | Web-based questionnaire | Post | Passengers, pilots |
| Objective functional and cognitive level (only for CWA; Passengers) | ||||
| Cognitive function | Brief Assessment of Impaired Cognition Questionnaire (BASIC-Q) [68,80] | Interview-based case-finding survey | Pre, post | Passengers |
| Physical function and mobility | Short Physical Performance Battery (SPPB) [69,71] | Functional and physical test | Pre, post | Passengers |
| Muscle Strength | Grip Strength [81] | Physical test | Pre, post | Passengers |
| Gait endurance | 6-Min Walk Test [73,75] | Functional test | Pre, post | Passengers |
| Trip registration for CWA | ||||
| Objective observational data from every training/trip |
| Online trip registration (QR-code directing users to a short web-based questionnaire is scanned immediately after the CWA activity) | During the programme After finishing a CWA activity. | Context related data completed by one person after each activity Passengers pilots |

3.8. Planned Statistical Analyses
4. Discussion
4.1. Strengths
4.2. Limitations
4.3. Main Contribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | Physical activity |
| QoL | Quality of Life |
| WMM | When Movement Moves |
| TT | Team Twin |
| CWA | Cycling Without Age |
| HA | Handiathletes |
| CFAS | Centre for Physical Activity Research |
| BASIC-Q | Cognitive function Brief Assessment of Impaired Cognition Questionnaire |
| SOP | Standard operation procedure |
References
- World Health Organization. World Report on Disability 2011; 9241564180; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Van der Ploeg, H.P.; Van der Beek, A.J.; Van der Woude, L.H.; van Mechelen, W. Physical activity for people with a disability. Sports Med. 2004, 34, 639–649. [Google Scholar] [CrossRef]
- Dishman, R.K.; Heath, G.W.; Lee, I.-M. Physical Activity Epidemiology; Human Kinetics: Champaign, IL, USA, 2021. [Google Scholar]
- Rimmer, J.H.; Riley, B.; Wang, E.; Rauworth, A. Accessibility of health clubs for people with mobility disabilities and visual impairments. Am. J. Public Health 2005, 95, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, H.; Miyahara, M.; Nichols-Dunsmuir, A. Multiple perspectives on accessibility to physical activity for people with long-term mobility impairment. Scand. J. Disabil. Res. 2016, 19, 295–306. [Google Scholar] [CrossRef]
- Ryan, J.M.; Allen, E.; Gormley, J.; Hurvitz, E.A.; Peterson, M. The risk, burden, and management of non-communicable diseases in cerebral palsy: A scoping review. Dev. Med. Child Neurol. 2018, 60, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, C.K.; Davidsen, M.; Christensen, A.I. Ældres Sundhed og Trivsel: Ældreprofilen 2019 er Baseret på Sundheds-og Sygelighedsundersøgelserne, de Nationale Sundhedsprofiler og Udvalgte Register; 8770140758; Sundhedsstyrelsen: Copenhagen, Denmark, 2019. [Google Scholar]
- Amilon, A.; Larsen, L.B.; Østergaard, S.V.; Rasmussen, A.H. Personer Med Handicap: Hverdagsliv og Levevilkår 2016; 1396–1810; VIVE-Det Nationale Forsknings-og Analysecenter for Velfærd: Copenhagen, Denmark, 2017. [Google Scholar]
- Carroll, D.D.; Courtney-Long, E.A.; Stevens, A.C.; Sloan, M.L.; Lullo, C.; Visser, S.N.; Fox, M.H.; Armour, B.S.; Campbell, V.A.; Brown, D.R. Vital signs: Disability and physical activity—United States, 2009–2012. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 407. [Google Scholar] [PubMed]
- Whitney, D.G.; Hurvitz, E.A.; Ryan, J.M.; Devlin, M.J.; Caird, M.S.; French, Z.P.; Ellenberg, E.C.; Peterson, M.D. Noncommunicable disease and multimorbidity in young adults with cerebral palsy. Clin. Epidemiol. 2018, 10, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Cremer, N.; Hurvitz, E.A.; Peterson, M.D. Multimorbidity in middle-aged adults with cerebral palsy. Am. J. Med. 2017, 130, 744.e9–744.e15. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Sullivan, R.O.; Caserotti, P.; Tully, M.A. Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scand. J. Med. Sci. Sports 2020, 30, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Bauman, A.; Merom, D.; Bull, F.C.; Buchner, D.M.; Singh, M.A.F. Updating the evidence for physical activity: Summative reviews of the epidemiological evidence, prevalence, and interventions to promote “active aging”. Gerontologist 2016, 56, S268–S280. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance; 9240014888; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ginis, K.A.M.; van der Ploeg, H.P.; Foster, C.; Lai, B.; McBride, C.B.; Ng, K.; Pratt, M.; Shirazipour, C.H.; Smith, B.; Vásquez, P.M.; et al. Participation of people living with disabilities in physical activity: A global perspective. Lancet 2021, 398, 443–455. [Google Scholar] [CrossRef]
- Molina-Cantero, A.J.; Merino-Monge, M.; Castro-García, J.A.; Pousada-García, T.; Valenzuela-Muñoz, D.; Gutiérrez-Párraga, J.; López-Álvarez, S.; Gómez-González, I.M. A study on physical exercise and general mobility in people with cerebral palsy: Health through costless routines. Int. J. Environ. Res. Public Health 2021, 18, 9179. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Maïano, C.; Hue, O.; Morin, A.J.S.; Lepage, G.; Tracey, D.; Moullec, G. Exercise interventions to improve balance for young people with intellectual disabilities: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2019, 61, 406–418. [Google Scholar] [CrossRef]
- Andersen, M.M. Significant experiences—Development processes for young people and adults with cerebral palsy through resilience-based social adapted physical activity interventions. Dev. Med. Child Neurol. 2020, 57, 1093–1104. [Google Scholar]
- Kissow, A.-M. Participation in physical activity and the everyday life of people with physical disabilities: A review of the literature. Scand. J. Disabil. Res. 2015, 17, 144–166. [Google Scholar] [CrossRef]
- Kissow, A.M.; Singhammer, J. Participation in physical activities and everyday life of people with disabilities. Eur. J. Adapt. Phys. Act. 2012, 5, 65–81. [Google Scholar] [CrossRef]
- Ravenek, K.E.; Ravenek, M.J.; Hitzig, S.L.; Wolfe, D.L. Assessing quality of life in relation to physical activity participation in persons with spinal cord injury: A systematic review. Disabil. Health J. 2012, 5, 213–223. [Google Scholar] [CrossRef]
- Diaz, R.; Miller, E.K.; Kraus, E.; Fredericson, M. Impact of adaptive sports participation on quality of life. Sports Med. Arthrosc. Rev. 2019, 27, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Côté-Leclerc, F.; Duchesne, G.B.; Bolduc, P.; Gélinas-Lafrenière, A.; Santerre, C.; Desrosiers, J.; Levasseur, M. How does playing adapted sports affect quality of life of people with mobility limitations? Results from a mixed-method sequential explanatory study. Health Qual. Life Outcomes 2017, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Vagetti, G.C.; Filho, V.B.; Moreira, N.B.; De Oliveira, V.; Mazzardo, O.; De Campos, W. Association between physical activity and quality of life in the elderly: A systematic review, 2000—2012. Rev. Bras. Psiquiatr. 2014, 36, 76–88. [Google Scholar] [CrossRef]
- Santini, Z.I.; Jose, P.E.; Koyanagi, A.; Meilstrup, C.; Nielsen, L.; Madsen, K.R.; Koushede, V. Formal social participation protects physical health through enhanced mental health: A longitudinal mediation analysis using three consecutive waves of the Survey of Health, Ageing and Retirement in Europe (SHARE). Soc. Sci. Med. 2020, 251, 112906. [Google Scholar] [CrossRef] [PubMed]
- Ginis, K.M.; Ma, J.K.; Latimer, A.; Rimmer, J.H. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health Psychol. Rev. 2016, 10, 478–494. [Google Scholar] [CrossRef]
- Singh, M.A.F. Exercise comes of age: Rationale and recommendations for a geriatric exercise prescription. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M262–M282. [Google Scholar] [CrossRef]
- Rimmer, J.H.; Chen, M.-D.; McCubbin, J.A.; Drum, C.; Peterson, J. Exercise intervention research on persons with disabilities: What we know and where we need to go. Am. J. Phys. Med. Rehabil. 2010, 89, 249–263. [Google Scholar] [CrossRef]
- Winther, H.; Kissow, A.-M.; Pedersen, M.T.; Sandahl, C. Forundersøgelse om Team Tvilling: Når Bevægelse Bevæger: Om Betydningen af Positive Kropsoplevelser, Ligeværdige Relationer og Identitetsudviklende Fællesskaber for Mennesker Med Store Bevægelsesbegrænsninger; University of Copenhagen: Copenhagen, Denmark, 2018. [Google Scholar]
- Cotnam, V. Exploring the Effects of the Cycling Without Age Program on Older Adults Living in Long-Term Care. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2020. [Google Scholar]
- Gow, A.J.; Bell, C.; Biggar, J. Cycling Without Age-Evaluation Report 2018; Heriot Watt University: Edinburgh, UK, 2019. [Google Scholar]
- Gravely, E.; Dutta, P.; Vithyananthan, M.; David, K. Starting and Operating a Cycling Without Age Chapter in a Care Facility; McMaster University: Hamilton, ON, Canada, 2019. [Google Scholar]
- Gray, R.; Gow, A.J. Cycling without age: Assessing the impact of a cycling-based initiative on mood and wellbeing. Gerontol. Geriatr. Med. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- McNiel, P.; Westphal, J. Cycling without age program: The impact for residents in long-term care. West. J. Nurs. Res. 2020, 42, 728–735. [Google Scholar] [CrossRef]
- Salas, K. Impact of “Cycling Without Age” on the Health of the Elderly. 2018. Available online: http://communitylighteldernetwork.org/wp-content/uploads/Impact-study-on-health-benefits-and-well-being.pdf (accessed on 18 September 2021).
- Team Twin—We Run Together. Available online: https://teamtvilling.dk/ (accessed on 7 January 2021).
- Cycling Without Age—The Right to Wind in Your Hair. Available online: https://cyklingudenalder.dk (accessed on 18 January 2021).
- Damgaard, M.; Steffensen, T.; Bengtsson, S. Hverdagsliv og Levevilkår for Mennesker Med Funktionsnedsættelse: En Analyse af Sammenhængen Mellem Hverdagsliv, Samliv, Udsathed, og Type og Grad af Funktionsnedsættelse; 8771191895; VIVE: Copenhagen, Denmark, 2013. [Google Scholar]
- Michelsen, S.I.; Flachs, E.M.; Damsgaard, M.T.; Parkes, J.; Parkinson, K.; Rapp, M.; Arnaud, C.; Nystrand, M.; Colver, A.; Fauconnier, J.; et al. European study of frequency of participation of adolescents with and without cerebral palsy. Eur. J. Paediatr. Neurol. 2014, 18, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.E.; Raina, P.; Levasseur, M.; Sohel, N.; Payette, H.; Tuokko, H.; van den Heuvel, E.; Wister, A.; Gilsing, A.; Patterson, C. Functional disability and social participation restriction associated with chronic conditions in middle-aged and older adults. J. Epidemiol. Community Health 2016, 71, 381–389. [Google Scholar] [CrossRef]
- Johnsen, N.F.; Davidsen, M.; Michelsen, S.I.; Juel, K. Health profile for Danish adults with activity limitation: A cross-sectional study. BMC Public Health 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- ldreministeriet, S.-o. National Undersøgelse af Forholdene på Plejecentre. Available online: https://sum.dk/~/media/Filer2016 (accessed on 5 May 2021).
- Funnell, S.C.; Rogers, P.J. Purposeful Program Theory: Effective Use of Theories of Change and Logic Models; John Wiley & Sons: San Francisco, CA, USA, 2011; Volume 31. [Google Scholar]
- AskovFonden. Vind i Håret Giver Livsglæde—Og Positive tal på Bundlinjen; Struenseegade: Copenhagen, Denmark, 2018; p. 20. [Google Scholar]
- Montero, I.; León, O.G. A guide for naming research studies in psychology. Int. J. Clin. Health Psychol. 2007, 7, 847–862. [Google Scholar]
- Levin, K.A.; Currie, C. Reliability and validity of an adapted version of the cantril ladder for use with adolescent samples. Soc. Indic. Res. 2014, 119, 1047–1063. [Google Scholar] [CrossRef]
- Palmore, E.; Kivett, V. Change in life satisfaction: A longitudinal study of persons aged 46–70. J. Gerontol. 1977, 32, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.I.; Lau, C.J.; Kristensen, P.L.; Johnsen, S.B.; Wingstrand, A.; Friis, K.; Davidsen, M.; Andreasen, A.H. The Danish National Health Survey: Study design, response rate and respondent characteristics in 2010, 2013 and 2017. Scand. J. Public Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.E.; Waite, L.J.; Hawkley, L.C.; Cacioppo, J.T. A short scale for measuring loneliness in large surveys: Results from two population-based studies. Res. Aging 2004, 26, 655–672. [Google Scholar] [CrossRef]
- Jespersen, L.N. Measuring Quality of Life and Participation in a Population with Diverse Disabilities. Ph.D. Thesis, Syddansk Universitet, Copenhagen, Denmark, 2018. [Google Scholar]
- Schwarzer, R.; Jerusalem, M. Generalized self-efficacy scale. Measures in health psychology: A user’s portfolio. Causal Control Beliefs 1995, 1, 35–37. [Google Scholar]
- Meilstrup, C.; Thygesen, L.C.; Nielsen, L.; Koushede, V.; Cross, D.; Holstein, B.E. Does self-efficacy mediate the association between socioeconomic background and emotional symptoms among schoolchildren? Int. J. Public Health 2016, 61, 505–512. [Google Scholar] [CrossRef]
- Tennant, R.; Hiller, L.; Fishwick, R.; Platt, S.; Joseph, S.; Weich, S.; Parkinson, J.; Secker, J.; Stewart-Brown, S. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): Development and UK validation. Health Qual. Life Outcomes 2007, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Bech, P. Subjective positive well-being. World Psychiatry 2012, 11, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Koushede, V.; Lasgaard, M.; Hinrichsen, C.; Meilstrup, C.; Nielsen, L.; Rayce, S.B.; Torres-Sahli, M.; Gudmundsdottir, D.G.; Stewart-Brown, S.; Santini, Z.I. Measuring mental well-being in Denmark: Validation of the original and short version of the Warwick-Edinburgh mental well-being scale (WEMWBS and SWEMWBS) and cross-cultural comparison across four European settings. Psychiatry Res. 2018, 271, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Castleberry, A.; Nolen, A. Thematic analysis of qualitative research data: Is it as easy as it sounds? Curr. Pharm. Teach. Learn. 2018, 10, 807–815. [Google Scholar] [CrossRef]
- Zubaida, S.D.; Cantril, H. The pattern of human concerns. Br. J. Sociol. 1967, 18, 212. [Google Scholar] [CrossRef]
- Russell, D.; Peplau, L.A.; Cutrona, C.E. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. J. Personal. Soc. Psychol. 1980, 39, 472. [Google Scholar] [CrossRef]
- Jensen, H.A.R.; Davidsen, M.; Ekholm, O.; Christensen, A.I. Danskernes Sundhed-Den Nationale Sundhedsprofil 2017; 8771049576; National Institute of Public Health: Copenhagen, Denmark, 2018. [Google Scholar]
- Grimby, G.; Börjesson, M.; Jonsdottir, I.; Schnohr, P.; Thelle, D.; Saltin, B. The “Saltin–Grimby physical activity level scale” and its application to health research. Scand. J. Med. Sci. Sports 2015, 25, 119–125. [Google Scholar] [CrossRef]
- Midtjylland, N.A.-R. Spørgeskema om din Epilepsi. Available online: https://docplayer.dk/16414548-Spoergeskema-om-din-epilepsi.html (accessed on 15 July 2021).
- Nielsen, L.; Hinrichsen, C.; Santini, Z.I.; Koushede, V. Måling af Mental Sundhed: En Baggrundsrapport for Spørgeskemaundersøgelsen Danskernes Trivsel 2016; 8778993709; University of Southern Denmark: Copenhagen, Denmark, 2017. [Google Scholar]
- Jørgensen, K.; Nielsen, T.R.; Nielsen, A.; Waldorff, F.B.; Waldemar, G. Brief assessment of Impaired Cognition Questionnaire (BASIC-Q)—Development and validation of a new tool for identification of cognitive impairment in community settings. Int. J. Geriatr. Psychiatry 2020, 35, 693–701. [Google Scholar] [CrossRef]
- Short Physical Performance Battery (SPPB). Available online: https://www.nia.nih.gov/research/labs/leps/short-physical-performance-battery-sppb (accessed on 21 June 2021).
- Gill, T.M.; Allore, H.G.; Hardy, S.E.; Guo, Z. The dynamic nature of mobility disability in older persons. J. Am. Geriatr. Soc. 2006, 54, 248–254. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Newton, P.; Salamonson, Y.; Carrieri-Kohlman, V.L.; Davidson, P.M. A review of the six-minute walk test: Its implication as a self-administered assessment tool. Eur. J. Cardiovasc. Nurs. 2009, 8, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Knak, K.L.; Andersen, L.K.; Witting, N.; Vissing, J. Reliability of the 2-and 6-min walk tests in neuromuscular diseases. J. Rehabil. Med. 2017, 49, 362–366. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Physiotherapy Rehabilitation of Osteoporotic Vertebral Fracture. Short Physical Performance Battery (SPPB)—Protocol. Available online: https://research.ndorms.ox.ac.uk/prove/documents/assessors/outcomeMeasures/SPPB_Protocol.pdf (accessed on 22 June 2021).
- Mijnarends, D.M.; Meijers, J.M.; Halfens, R.J.; ter Borg, S.; Luiking, Y.C.; Verlaan, S.; Schoberer, D.; Cruz-Jentoft, A.J.; van Loon, L.J.; Schols, J.M. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: A systematic review. J. Am. Med. Dir. Assoc. 2013, 14, 170–178. [Google Scholar] [CrossRef]
- Balogun, J.; Akomolafe, C.T.; Amusa, L.O. Grip strength: Effects of testing posture and elbow position. Arch. Phys. Med. Rehabil. 1991, 72, 280–283. [Google Scholar]
- Barut, C.; Demirel, P. Influence of testing posture and elbow position on grip strength. Med. J. Islamic World Acad. Sci. 2012, 109, 94–97. [Google Scholar]
- Jørgensen, K.; Nielsen, T.R.; Nielsen, A.; Waldorff, F.B.; Waldemar, G. Validation of the brief assessment of impaired cognition and the brief assessment of Impaired Cognition Questionnaire for identification of mild cognitive impairment in a memory clinic setting. Int. J. Geriatr. Psychiatry 2020, 35, 907–915. [Google Scholar] [CrossRef]
- Suetta, C.; Haddock, B.; Alcazar, J.; Noerst, T.; Hansen, O.M.; Ludvig, H.; Kamper, R.S.; Schnohr, P.; Prescott, E.; Andersen, L.L. The Copenhagen Sarcopenia Study: Lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J. Cachexia Sarcopenia Muscle 2019, 10, 1316–1329. [Google Scholar] [CrossRef]
- Holstein, B. Triangulering–metoderedskab og validitetsinstrument. In Humanistisk Forskning Inden for Sundhedsvidenskab: Kvalitative Metoder, 1. udgave, 3. oplag ed.; Inga Marie Lunde, P.R., Ed.; Akademisk Forlag: Copenhagen, Denmark, 2003; pp. 330–333. [Google Scholar]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and Evaluating Complex Interventions: The New Medical Research Council Guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef]
- Beatty, P.C.; Willis, G.B. Research synthesis: The practice of cognitive interviewing. Public Opin. Q. 2007, 71, 287–311. [Google Scholar] [CrossRef]
- Crabtree, B.F.; Miller, W.L. Clinical research: A multimethod typology and qualitative roadmap. Doing Qual. Res. 1999, 2, 3–30. [Google Scholar]
- Friedman, L.M.; Furberg, C.D.; DeMets, D.L.; Reboussin, D.M.; Granger, C.B. Fundamentals of Clinical Trials, 5th ed.; Springer International Publishing Switzerland: Cham, Switzerland, 2015. [Google Scholar]
- Ato, M.; Lopez, J.J.; Benavente, A. A classification system for research designs in psychology. An. Psicol. 2013, 29, 1038–1059. [Google Scholar]
- World_Medical_Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/wp-content/uploads/2018/07/DoH-Oct2008.pdf (accessed on 2 October 2020).

| Target Group | Primary | Secondary | ||
|---|---|---|---|---|
| Programme | ||||
| Team Twin | Handiathletes | Runners | Relatives | |
| Cycling Without Age | Passengers | Pilots | Nursing staff | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jørgensen, A.; Petersen, C.B.; Eghøj, M.; Toftager, M. When Movement Moves: Study Protocol for a Multi-Method Pre/Post Evaluation Study of Two Programmes; the Danish Team Twin and Cycling Without Age. Int. J. Environ. Res. Public Health 2021, 18, 10008. https://doi.org/10.3390/ijerph181910008
Jørgensen A, Petersen CB, Eghøj M, Toftager M. When Movement Moves: Study Protocol for a Multi-Method Pre/Post Evaluation Study of Two Programmes; the Danish Team Twin and Cycling Without Age. International Journal of Environmental Research and Public Health. 2021; 18(19):10008. https://doi.org/10.3390/ijerph181910008
Chicago/Turabian StyleJørgensen, Andreas, Christina Bjørk Petersen, Martin Eghøj, and Mette Toftager. 2021. "When Movement Moves: Study Protocol for a Multi-Method Pre/Post Evaluation Study of Two Programmes; the Danish Team Twin and Cycling Without Age" International Journal of Environmental Research and Public Health 18, no. 19: 10008. https://doi.org/10.3390/ijerph181910008
APA StyleJørgensen, A., Petersen, C. B., Eghøj, M., & Toftager, M. (2021). When Movement Moves: Study Protocol for a Multi-Method Pre/Post Evaluation Study of Two Programmes; the Danish Team Twin and Cycling Without Age. International Journal of Environmental Research and Public Health, 18(19), 10008. https://doi.org/10.3390/ijerph181910008






