Effects of Air Pollutants on Airway Diseases
Abstract
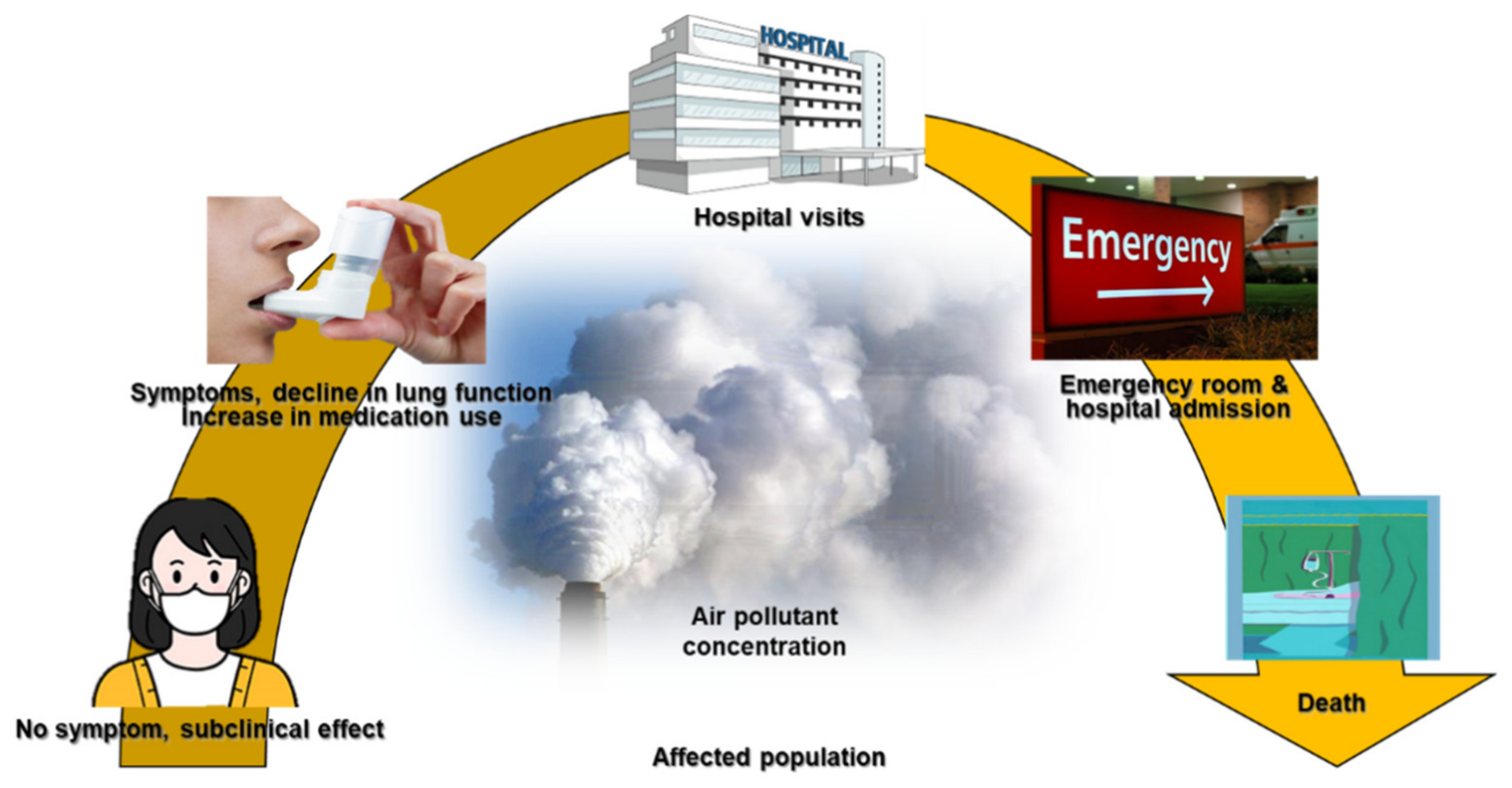
:1. Introduction
1.1. The Effects of Air Pollutants on Asthma and COPD, Upper Airway Disease
1.2. Airway Toxicity Mechanisms Related to Air Pollutants
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCormack, M.C.; Breysse, P.N.; Hansel, N.N.; Matsui, E.C.; Tonorezos, E.S.; Curtin-Brosnan, J.; Williams, D.L.; Buckley, T.J.; Eggleston, P.A.; Diette, G.B. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore preschool children. Environ. Res. 2008, 106, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Di Filippo, P.; Pomata, D.; Riccardi, C.; Buiarelli, F.; Castellani, F.; Calitri, G.; Simonetti, G.; Sonego, E.; Bruni, E.; Uccelletti, D. Concentrations of bacteria and bacterial and fungal spores calculated from chemical tracers associated with size-segregated aerosol in a composting plant. Air Qual. Atmos. Health 2020, 13, 469–476. [Google Scholar] [CrossRef]
- Douglas, P.; Robertson, S.; Gay, R.; Hansell, A.L.; Gant, T.W. A systematic review of the public health risks of bioaerosols from intensive farming. Int. J. Hyg. Environ. Health 2018, 221, 134–173. [Google Scholar] [CrossRef] [PubMed]
- Humbal, C.; Gautam, S.; Trivedi, U. A review on recent progress in observations, and health effects of bioaerosols. Environ. Int. 2018, 18, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Machado, D.B.; Cislaghi, B.; Salman, O.M.; Karanikolos, M.; McKee, M.; Abbas, K.M.; Brady, O.J.; Larson, H.J.; Trias-Llimós, S. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar]
- Jang, A.S.; Yeum, C.H.; Son, M.H. Epidemiologic evidence of a relationship between airway hyperresponsiveness and exposure to polluted air. Allergy 2003, 58, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Gakidou, E.; Afshin, A.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Aboyans, V.; et al. Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990−2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef] [Green Version]
- Buiarelli, F.; Di Filippo, P.; Massimi, L.; Pomata, D.; Riccardi, C.; Simonetti, G.; Sonego, E. Ultrafine, fine and coarse airborne particle mass concentration in workplaces. Atmos. Pollut. Res. 2019, 10, 1685–1690. [Google Scholar] [CrossRef]
- Kumar, P.; Morawska, L.; Birmili, W.; Paasonen, P.; Hu, M.; Kulmala, M.; Harrison, R.M.; Norford, L.; Britter, R. Ultrafine particles in cities. Environ. Int. 2014, 66, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef]
- Kwon, H.S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef]
- Health Effects Institute. State of Global Air 2019: Air Pollution a Significant Risk Factor Worldwide; Health Effects Institute: Boston, MA, USA, 2019. [Google Scholar]
- United States Environmental Protection Agency. Guidelines for Developing an Air Quality (Ozone and PM2.5) Forecasting Program; Publication No.EPA-456/R-03–002; U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 2003. [Google Scholar]
- Schäfer, T.; Ring, J. Epidemiology of allergic diseases. Allergy 1997, 52, S14–S22. [Google Scholar] [CrossRef]
- Jang, A.S. Air Pollution: A Comprehensive Perspective; Haryanto, B., Ed.; Intech: Rijeka, Hrvatska, 2012; pp. 153–174. [Google Scholar]
- Leikauf, G.D.; Kim, S.H.; Jang, A.S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Al-Hegelan, M.; Robert, M.T.; Christian, C.; John, W.H. Ambient ozone and pulmonary innate immunity. Immunol. Res. 2011, 49, 173–191. [Google Scholar] [CrossRef] [Green Version]
- Michaudel, C.; Mackowiak, C.; Maillet, I.; Fauconnier, L.; Akdis, C.A.; Sokolowska, M. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J. Allergy Clin. Immunol. 2018, 142, 942–958. [Google Scholar] [CrossRef] [Green Version]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Perez, L.; Declercq, C.; Iñiguez, C.; Aguilera, I.; Badaloni, C.; Ballester, F.; Bouland, C.; Chanel, O.; Cirarda, F.B.; Forastiere, F.; et al. Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network). Eur. Respir. J. 2013, 42, 594–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Künzli, N.; Kaiser, R.; Medina, S.; Studnicka, M.; Chanel, O.; Filliger, P.; Herry, M.; Horak, F.; Puybonnieux-Texier, V.; Quénel, P.; et al. Public-health impact of outdoor and traffic-related air pollution: A European assessment. Lancet 2000, 356, 795–801. [Google Scholar] [CrossRef]
- Foster, W.M.; Costa, D.L.; Langenback, E.G. Ozone exposure alters tracheobronchial mucociliary function in humans. J. Appl. Physiol. 1987, 63, 996–1002. [Google Scholar] [CrossRef]
- Devlin, R.B.; McDonnell, W.F.; Mann, R.; Becker, S.; House, D.E.; Schreinemachers, D.; Koren, H.S. Exposure of humans to ambient levels of ozone for 6.6 h causes cellular and biochemical changes in the lung. Am. J. Respir. Cell Mol. Biol. 1991, 4, 72–81. [Google Scholar] [CrossRef]
- Gryparis, A.; Forsberg, B.; Katsouyanni, K.; Analitis, A.; Touloumi, G.; Schwartz, J.; Samoli, E.; Medina, S.; Anderson, H.R.; Niciu, E.M.; et al. Acute effects of ozone on mortality from the “air pollution and health: A European approach” project. Am. J. Respir. Crit. Care Med. 2004, 170, 1080–1087. [Google Scholar] [CrossRef]
- Foster, W.M.; Stetkiewicz, P.T. Regional clearance of solute from the respiratory epithelia: 18–20 h postexposure to ozone. J. Appl. Physiol. 1996, 81, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.M.; Freed, A.N. Regional clearance of solute from peripheral airway epithelia: Recovery after sublobar exposure to ozone. J. Appl. Physiol. 1999, 86, 641–646. [Google Scholar] [CrossRef] [PubMed]
- van Donkelaar, A.; Martin, R.V.; Brauer, M.; Hsu, N.C.; Kahn, R.A.; Levy, R.C.; Lyapustin, A.; Sayer, A.M.; Winker, D.M. Global Estimates of Fine Particulate Matter using a Combined Geophysical-Statistical Method with Information from Satellites, Models, and Monitors. Environ. Sci. Technol. 2016, 50, 3762–3772. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, H.J.; Lee, C.H.; Lee, C.H.; Lee, H.W. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Environ. Res. 2021, 194, 110703. [Google Scholar] [CrossRef]
- World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheswaran, D.; Zeng, Y.; Chan-Yeung, M.; Scott, J.; Osornio-Vargas, A.; Becker, A.B.; Kozyrskyj, A.L. Exposure to beta-(1,3)-D-glucan in house dust at age 7–10 is associated with airway hyperresponsiveness and atopic asthma by age 11–14. PLoS ONE 2014, 9, e98878. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, D.G.; Despres, V.; Frohlich-Nowoisky, J.; Psenner, R.; Ariya, P.A.; Posfai, M.; Ahern, H.E.; Moffett, B.F. Microbiology and atmospheric processes: Biological, physical and chemical characterization of aerosol particles. Biogeosciences 2009, 6, 721–737. [Google Scholar] [CrossRef] [Green Version]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar]
- Robertson, S.; Douglas, P.; Jarvis, D.; Marczylo, E. Bioaerosol exposure from composting facilities and health outcomes inworkers and in the community: A systematic review update. Int. J. Hyg. Environ. Health 2019, 222, 364–386. [Google Scholar] [CrossRef] [PubMed]
- Renaud-Picard, B.; Toussaint, J.; Leclercq, A.; Reeb, J.; Kessler, L.; Toti, F.; Kessler, R. Microparticules membranaires et maladies respiratoires [Membranous microparticles and respiratory disease]. Rev. Mal. Respir. 2017, 34, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Varmaghani, M.; Dehghani, M.; Heidari, E.; Sharifi, F.; Moghaddam, S.S.; Farzadfar, F. Global prevalence of chronic obstructive pulmonary disease: Systematic review and meta-analysis. East. Mediterr. Health J. 2019, 25, 47–57. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A. Acute respiratory effects of particulate air pollution. Annu. Rev. Public Health 1994, 15, 107–132. [Google Scholar] [CrossRef]
- Olivieri, D.; Scoditti, E. Impact of environmental factors on lung defences. Eur. Respir. Rev. 2005, 14, 51–56. [Google Scholar] [CrossRef]
- Bai, L.; Su, X.; Zhao, D.; Zhang, Y.; Cheng, Q.; Zhang, H.; Wang, S.; Xie, M.; Su, H. Exposure to traffic-related air pollution and acute bronchitis in children: Season and age as modifiers. J. Epidemiol. Community Health 2018, 72, 426–433. [Google Scholar] [CrossRef]
- Karimi, A.; Shirmardi, M.; Hadei, M.; Birgani, Y.T.; Neisi, A.; Takdastan, A.; Goudarzi, G. Concentrations and health effects of short- and long-term exposure to PM2.5, NO2, and O3 in ambient air of Ahvaz city, Iran (2014–2017). Ecotoxicol. Environ. Saf. 2019, 180, 542–548. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chang, S.C.; Lin, C.; Chen, Y.C.; Wang, Y.C. Comparing ozone metrics on associations with outpatient visits for respiratory diseases in Taipei Metropolitan area. Environ. Pollut. 2013, 177, 177–184. [Google Scholar] [CrossRef]
- Stojić, S.S.; Stanišić, N.; Stojić, A.; Šoštarić, A. Single and combined effects of air pollutants on circulatory and respiratory system-related mortality in Belgrade, Serbia. J. Toxicol. Environ. Health A 2016, 79, 17–27. [Google Scholar] [CrossRef]
- Yang, X.Y.; Wen, B.; Han, F.; Wang, C.; Zhang, S.P.; Wang, J.; Xu, D.Q.; Wang, Q. Acute Effects of Individual Exposure to Fine Particulate Matter on Pulmonary Function in Schoolchildren. Biomed. Environ. Sci. 2020, 33, 647–659. [Google Scholar] [PubMed]
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012, 782462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, F.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef]
- Faustini, A.; Stafoggia, M.; Colais, P.; Berti, G.; Bisanti, L.; Cadum, E.; Cernigliaro, A.; Mallone, S.; Scarnato, C.; Forastiere, F. Air pollution and multiple acute respiratory outcomes. Eur. Respir. J. 2013, 42, 304–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, K.E. Air pollution and heart disease. Rev. Cardiovasc. Med. 2006, 7, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhu, D.; Peng, D.; Zhao, Y. Air pollution and asthma attacks in children: A case-crossover analysis in the city of Chongqing, China. Environ. Pollut. 2017, 220, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, S.; Chen, M. Air pollutants and asthma patient visits: Indication of source influence. Sci. Total Environ. 2018, 625, 355–362. [Google Scholar] [CrossRef]
- Khreis, H.; Hoogh, K.; Nieuwenhuijsen, M.J. Fullchain health impact assessment of traffic-related air pollution and childhood asthma. Environ. Int. 2018, 114, 365–375. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Kweon, J. Hourly differences in air pollution on the risk of asthma exacerbation. Environ. Pollut. 2015, 203, 15–21. [Google Scholar] [CrossRef]
- Hehua, Z.; Qing, C.; Shanyan, G.; Qijun, W.; Yuhong, Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environ. Res. 2017, 159, 519–530. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review on human health perspective of air pollution with respect to allergies and asthma. Environ. Int. 2013, 59, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Veremchuk, L.V.; Tsarouhas, K.; Vitkina, T.I.; Mineeva, E.E.; Gvozdenko, T.A.; Antonyuk, M.V.; Rakitskii, V.N.; Sidletskaya, K.A.; Tsatsakis, A.M.; Golokhvast, K.S. Impact evaluation of environmental factors on respiratory function of asthma patients living in urban territory. Environ. Pollut. 2018, 235, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xiang, X.; Juan, J.; Sun, K.; Song, J.; Cao, Y.; Hu, Y. Fine particulate air pollution and hospital visits for asthma in Beijing, China. Environ. Pollut. 2017, 230, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Wu, J.; Tjoa, T.; Gullesserian, S.K.; Nickerson, B.; Gillen, D.L. Asthma morbidity and ambient air pollution: Effect modification by residential traffic-related air pollution. Epidemiology 2014, 25, 48–57. [Google Scholar] [CrossRef]
- Khreis, H.; Cirach, M.; Mueller, N.; de Hoogh, K.; Hoek, G.; Nieuwenhuijsen, M.J.; Rojas-Rueda, D. Outdoor air pollution and the burden of childhood asthma across Europe. Eur. Respir. J. 2019, 54, 1802194. [Google Scholar] [CrossRef] [PubMed]
- Pierangeli, I.; Nieuwenhuijsen, M.J.; Cirach, M.; Rojas-Rueda, D. Health equity and burden of childhood asthma-related to air pollution in Barcelona. Environ. Res. 2020, 22, 109067. [Google Scholar] [CrossRef]
- Bowatte, G.; Lodge, C.; Lowe, A.J.; Erbas, B.; Perret, J.; Abramson, M.J.; Matheson, M.; Dharmage, S.C. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: A systematic review and a meta-analysis of birth cohort studies. Allergy 2015, 70, 245–256. [Google Scholar] [CrossRef] [PubMed]
- GINA Reports. Global Initiative for Asthma-GINA. Available online: https://ginasthma.org/gina-reports/ (accessed on 13 June 2020).
- Thurston, G.D.; Balmes, J.R.; Garcia, E.; Gilliland, F.D.; Rice, M.B.; Schikowski, T.; Van Winkle, L.S.; Annesi-Maesano, I.; Burchard, E.G.; Carlsten, C.; et al. Outdoor Air Pollution and New-Onset Airway Disease. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2020, 17, 387–398. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Thompson, L.A.; Gross, H.E.; Shenkman, E.A.; DeWalt, D.A.; Huang, I.-C. Longitudinal Effect of Ambient Air Pollution and Pollen Exposure on Asthma Control: The Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric Asthma Study. Acad. Pediatr. 2019, 19, 615–623. [Google Scholar] [CrossRef]
- Mentz, G.; Robins, T.G.; Batterman, S.; Naidoo, R.N. Effect modifiers of lung function and daily air pollutant variability in a panel of schoolchildren. Thorax 2019, 74, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.M.; Phaneuf, D.J.; Barrett, M.A.; Su, J.G. Short-term impact of PM2.5 on contemporaneous asthma medication use: Behavior and the value of pollution reductions. Proc. Natl. Acad. Sci. USA 2019, 116, 5246–5253. [Google Scholar] [CrossRef] [Green Version]
- Pepper, J.R.; Barrett, M.A.; Su, J.G.; Merchant, R.; Henderson, K.; Van Sickle, D.; Balmes, J.R. Geospatial-temporal analysis of the impact of ozone on asthma rescue inhaler use. Environ. Int. 2020, 136, 105331. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Skrzypek, M.; Kowalski, M.; Cyrys, J. Effect of NOx and NO2 Concentration Increase in Ambient Air to Daily Bronchitis and Asthma Exacerbation, Silesian Voivodeship in Poland. Int. J. Environ. Res. Public Health 2020, 17, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Zhang, Y.; Lin, J.; Xia, G.; Zhang, W.; Knibbs, L.D.; Morgan, G.G.; Jalaludin, B.; Marks, G.; Abramson, M.; et al. Multi-city study on air pollution and hospital outpatient visits for asthma in China. Environ. Pollut. 2020, 257, 113638. [Google Scholar] [CrossRef]
- Zuo, B.; Liu, C.; Chen, R.; Kan, H.; Sun, J.; Zhao, J.; Wang, C.; Sun, Q.; Bai, H. Associations between short-term exposure to fine particulate matter and acute exacerbation of asthma in Yancheng, China. Chemosphere 2019, 237, 124497. [Google Scholar] [CrossRef]
- Garcia, E.; Berhane, K.T.; Islam, T.; McConnell, R.; Urman, R.; Chen, Z.; Gilliland, F.D. Association of Changes in Air Quality with Incident Asthma in Children in California, 1993–2014. JAMA 2019, 321, 1906–1915. [Google Scholar] [CrossRef] [Green Version]
- Chi, R.; Li, H.; Wang, Q.; Zhai, Q.; Wang, D.; Wu, M.; Liu, Q.; Wu, S.; Ma, Q.; Deng, F.; et al. Association of emergency room visits for respiratory diseases with sources of ambient PM2.5. J. Environ. Sci. 2019, 86, 154–163. [Google Scholar] [CrossRef]
- Alcala, E.; Brown, P.; Capitman, J.A.; Gonzalez, M.; Cisneros, R. Cumulative Impact of Environmental Pollution and Population Vulnerability on Pediatric Asthma Hospitalizations: A Multilevel Analysis of CalEnviroScreen. Int. J. Environ. Res. Public Health 2019, 16, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques Mejías, M.A.; Tomás Pérez, M.; Hernández, I.; López, I.; Quirce, S. Asthma Exacerbations in the Pediatric Emergency Department at a Tertiary Hospital: Association with Environmental Factors. J. Investig. Allergol. Clin. Immunol. 2019, 29, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Kash, B.A.; Xu, X.; Benden, M.; Roberts, J.; Carrillo, G. Association between Ambient Air Pollution and Hospital Length of Stay among Children with Asthma in South Texas. Int. J. Environ. Res. Public Health 2020, 17, 3812. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, J.; Zhang, H.; Shi, C.; Li, G.; Peng, Z.; Ma, J.; Zhou, Y.; Zhang, L. Short-Term Exposure to Ambient Air Pollution and Asthma Mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Meteran, H.; Thomsen, S.F.; Miller, M.R.; Hjelmborg, J.; Sigsgaard, T.; Backer, V. Impact of the spirometric definition on comorbidities in chronic obstructive pulmonary disease. Respir. Med. 2021, 184, 106399. [Google Scholar] [CrossRef]
- Blanchette, C.M.; Dalal, A.A.; Mapel, D. Changes in COPD demographics and costs over 20 years. J. Med. Econ. 2012, 15, 1176–1182. [Google Scholar] [CrossRef]
- Crisafulli, E.; Barbeta, E.; Ielpo, A.; Torres, A. Management of severe acute exacerbations of COPD: An updated narrative review. Multidiscip. Respir. Med. 2018, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Decramer, M.; Janssens, W.; Miravitlles, M. Chronic obstructive pulmonary disease. Lancet 2012, 379, 1341–1351. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Kennedy, S.M.; Chambers, R.; Du, W.; Dimich-Ward, H. Environmental and occupational exposures: Do they affect chronic obstructive pulmonary disease differently in women and men? Proc. Am. Thorac. Soc. 2007, 4, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.; Scholand, M.B. Smoking cessation and environmental hygiene. Med. Clin. N. Am. 2012, 96, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Halbert, R.J.; Natoli, J.L.; Gano, A.; Badamgarav, E.; Buist, A.S.; Mannino, D.M. Global burden of COPD: Systematic review and meta-analysis. Eur. Respir. J. 2006, 28, 523–532. [Google Scholar] [CrossRef]
- Jang, A.S.; Jun, Y.J.; Park, M.K. Effects of air pollutants on upper airway disease. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 13–17. [Google Scholar] [CrossRef]
- Wang, J.; Kang, X.; Huang, Z.Q.; Shen, L.; Luo, Q.; Li, M.Y.; Luo, L.P.; Tu, J.H.; Han, M.; Ye, J. Protease-Activated Receptor-2 Decreased Zonula Occlidens-1 and Claudin-1 Expression and Induced Epithelial Barrier Dysfunction in Allergic Rhinitis. Am. J. Rhinol. Allergy 2021, 35, 26–35. [Google Scholar] [CrossRef]
- Kortekaas Krohn, I.; Seys, S.F.; Lund, G.; Jonckheere, A.C.; Dierckx de Casterlé, I.; Ceuppens, J.L.; Steelant, B.; Hellings, P.W. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy 2020, 75, 1155–1164. [Google Scholar] [CrossRef]
- Zhou, L.B.; Zheng, Y.M.; Liao, W.J.; Song, L.J.; Meng, X.; Gong, X.; Chen, G.; Liu, W.X.; Wang, Y.Q.; Han, D.M.; et al. MUC1 deficiency promotes nasal epithelial barrier dysfunction in subjects with allergic rhinitis. J. Allergy Clin. Immunol. 2019, 144, 1716–1719.e5. [Google Scholar] [CrossRef] [PubMed]
- Goleva, E.; Berdyshev, E.; Leung, D.Y. Epithelial barrier repair and prevention of allergy. J. Clin. Investig. 2019, 129, 1463–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Van Crombruggen, K.; Gevaert, E.; Bachert, C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy 2016, 71, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.S.; Choi, I.S.; Koh, Y.I.; Park, C.S.; Lee, J.S. The relationship between alveolar epithelial proliferation and airway obstruction after ozone exposure. Allergy 2002, 57, 737–740. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.H.; Mortimer, K. Air Pollution and Noncommunicable Diseases A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest 2019, 155, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Sly, P.D.; Cormier, S.A.; Lomnicki, S.; Harding, J.N.; Grimwood, K. Environmentally Persistent Free Radicals Linking Air Pollution and Poor Respiratory Health? Am. J. Respir. Crit Care Med. 2019, 200, 1062–1063. [Google Scholar] [CrossRef]
- Thurston, G.D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R.D.; Cromar, K. A joint ERS/ATS policy statement what constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017, 49, 1600419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, A.S.; Choi, I.S.; Yang, S.Y.; Kim, Y.G.; Lee, J.H.; Park, S.W.; Park, C.S. Antioxidant responsiveness in BALB/c mice exposed to ozone. Respiration 2005, 72, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Moon, K.Y.; Bae, D.J.; Park, M.K.; Jang, A.S. The effects of pycnogenol on antioxidant enzymes in a mouse model of ozone exposure. Korean J. Intern. Med. 2013, 28, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwak, M.K.; Han, D.H.; Kim, S.H.; Jang, A.S. Ozone exposure suppresses proliferative response in mice skin. Korean J. Intern. Med. 2012, 27, 360–362. [Google Scholar] [CrossRef]
- Jang, A.S.; Choi, I.S.; Lee, J.H.; Park, C.S.; Park, C.S. Prolonged ozone exposure in an allergic airway disease model: Adaptation of airway responsiveness and airway remodeling. Respir. Res. 2006, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jang, A.S.; Choi, I.S.; Takizawa, H.; Rhim, T.; Lee, J.H.; Park, S.W.; Park, C.S. Additive effect of diesel exhaust particulates and ozone on airway hyperresponsiveness and inflammation in a mouse model of asthma. J. Korean Med. Sci. 2005, 20, 759–763. [Google Scholar] [CrossRef]
- Jang, A.S.; Choi, I.S.; Lee, J.U.; Park, S.W.; Lee, J.H.; Park, C.S. The NOS isoforms play different roles in airway inflammation after ozone exposure. Respir. Res. 2004, 5, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Jang, A.S.; Choi, I.S.; Kim, S.W.; Song, B.C.; Yeum, C.H.; Jung, J.Y. Airway obstruction after acute ozone exposure in BALB/c mice using barometric plethysmography. Korean J. Intern. Med. 2003, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.; Lee, M.R.; Kim, J.; Jung, A.; Park, J.S.; Jang, A.S.; Park, S.W.; Uh, S.T.; Choi, J.S.; et al. Winter season temperature drops and sulfur dioxide levels affect on exacerbation of refractory asthma in South Korea: A time-trend controlled case-crossover study using soonchunhyang asthma cohort data. J. Asthma 2012, 49, 679–687. [Google Scholar] [CrossRef]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Park, M.K.; Jang, A.S. Effect of TiO2 Nanoparticles on Inflammasome-Mediated Airway Inflammation and Responsiveness. Allergy Asthma Immunol. Res. 2017, 9, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, M.H.; Rhim, T.; Kim, K.H.; Jang, A.S.; Paik, Y.K.; Park, C.S. Proteomic identification of macrophage migration-inhibitory factor upon exposure to TiO2 particles. Mol. Cell Proteom. 2007, 6, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Kim, Y.E.; Shin, M.Y.; Kang, Y.; Bae, S.H.; Kim, M.J.; Rhim, T.; Park, C.S.; et al. Long-Term Effects of Diesel Exhaust Particles on Airway Inflammation and Remodeling in a Mouse Model. Allergy Asthma Immunol. Res. 2016, 8, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.G.; Park, M.K.; Lee, P.H.; Lee, S.H.; Hong, J.; Aung, M.M.M.; Moe, K.T.; Han, N.Y.; Jang, A.S. Effects of nanoparticles on neuroinflammation in a mouse model of asthma. Respir. Physiol. Neurobiol. 2020, 271, 103292. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.Y.; Park, M.K.; Leikauf, G.D.; Park, C.S.; Jang, A.S. Diesel exhaust particle-induced airway responses are augmented in obese rats. Int. J. Toxicol. 2014, 33, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Jang, A.S.; Lee, T.H.; Kim, Y.J.; Lee, E.J.; Kim, J.M.; Park, J.S.; Park, S.W.; Park, C.S. Particle stimulation dephosphorylates glutathione S-transferase π1 of epithelial cells. Toxicology 2011, 284, 12–18. [Google Scholar] [CrossRef]
- Song, H.M.; Jang, A.S.; Ahn, M.H.; Takizawa, H.; Lee, S.H.; Kwon, J.H.; Lee, Y.M.; Rhim, T.Y.; Park, C.S. Ym1 and Ym2 expression in a mouse model exposed to diesel exhaust particles. Environ. Toxicol. 2008, 23, 110–116. [Google Scholar] [CrossRef]
- Kang, C.M.; Jang, A.S.; Ahn, M.H.; Shin, J.A.; Kim, J.H.; Choi, Y.S.; Rhim, T.Y.; Park, C.S. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am. J. Respir. Cell Mol. Biol. 2005, 33, 290–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020. [about 1 screen]. Available online: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf (accessed on 6 October 2020).
- Davies, D.E. Epithelial barrier function and immunity in asthma. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. 5), S244–S251. [Google Scholar] [CrossRef]
- Cookson, W. The immunogenetics of asthma and eczema: A new focus on the epithelium. Nat. Rev. Immunol. 2004, 4, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Kast, J.I.; Wanke, K.; Soyka, M.B.; Wawrzyniak, P.; Akdis, D.; Kingo, K.; Rebane, A.; Akdis, C.A. The broad spectrum of interepithelial junctions in skin and lung. J. Allergy Clin. Immunol. 2012, 130, 544–547.e4. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Gunzel, D.; John, L.J.; Fromm, M. Perspectives on tight junction research. Ann. N. Y. Acad. Sci. 2012, 1257, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Epithelium dysfunction in asthma. J. Allergy Clin. Immunol. 2007, 120, 1233–1244. [Google Scholar] [CrossRef]
- Sweerus, K.; Lachowicz-Scroggins, M.; Gordon, E.; LaFemina, M.; Huang, X.; Parikh, M.; Kanegai, C.; Fahy, J.V.; Frank, J.A. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J. Allergy Clin. Immunol. 2017, 139, 72–81.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.G.; Lee, S.H.; Hong, J.; Lee, P.H.; Jang, A.S. Titanium dioxide particles modulate epithelial barrier protein, Claudin 7 in asthma. Mol. Immunol. 2021, 132, 209–216. [Google Scholar] [CrossRef]
- Inoue, H.; Akimoto, K.; Homma, T.; Tanaka, A.; Sagara, H. Airway Epithelial Dysfunction in Asthma: Relevant to Epidermal Growth Factor Receptors and Airway Epithelial Cells. J. Clin. Med. 2020, 9, 3698. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The airway epithelium in asthma. Nat. Med. 2012, 18, 684–692. [Google Scholar] [CrossRef]
- Grainge, C.L.; Davies, D.E. Epithelial Injury and Repair in Airways Diseases. Chest 2013, 144, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.C. New insights in oxidant biology in asthma. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 1), S35–S39. [Google Scholar]
- Schroer, K.T.; Gibson, A.M.; Sivaprasad, U.; Bass, S.A.; Ericksen, M.B.; Wills-Karp, M.; Lecras, T.; Fitzpatrick, A.M.; Brown, L.A.; Stringer, K.F.; et al. Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J. Allergy Clin. Immunol. 2011, 128, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Bucchieri, F.; Puddicombe, S.M.; Lordan, J.L.; Richter, A.; Buchanan, D.; Wilson, S.J.; Ward, J.; Zummo, G.; Howarth, P.H.; Djukanović, R.; et al. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am. J. Respir. Cell Mol. Biol. 2002, 27, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V.; Nariya, S.; Bhakta, N.R.; Beigelman, A.; Castro, M.; Dyer, A.M.; Israel, E.; Kraft, M.; Martin, R.J.; et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J. Allergy Clin. Immunol. 2017, 140, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.J.; Nariya, S.; Harris, J.M.; Lynch, S.V.; Choy, D.F.; Arron, J.R.; Boushey, H. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J. Allergy Clin. Immunol. 2015, 136, 874–884. [Google Scholar] [CrossRef] [Green Version]
- Goleva, E.; Jackson, L.P.; Harris, J.K.; Robertson, C.E.; Sutherland, E.R.; Hall, C.F.; Good, J.T., Jr.; Gelfand, E.W.; Martin, R.J.; Leung, D.Y. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e857810. [Google Scholar] [CrossRef] [Green Version]
- Loxham, M.; Davies, D.E. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J. Allergy Clin. Immunol. 2017, 139, 1736–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holgate, S.T. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol. Rev. 2011, 242, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Kim, B.G.; Park, M.K.; Hong, J.; Lee, Y.G.; Jang, A.S. The Impact of Diesel Exhaust Particles on Tight Junctional Proteins on Nose and Lung in a Mouse Model. Allergy Asthma Immunol. Res. 2021, 13, 350–352. [Google Scholar] [CrossRef]
- Lee, P.H.; Hong, J.; Jang, A.S. N-acetylcysteine decreases airway inflammation and responsiveness in asthma by modulating claudin 18 expression. Korean J. Intern. Med. 2020, 35, 1229–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Park, C.S.; Jang, A.S. Impact of ozone on claudins and tight junctions in the lungs. Environ. Toxicol. 2018, 33, 798–806. [Google Scholar] [CrossRef]
- Lee, P.H.; Kim, B.G.; Lee, S.H.; Lee, J.H.; Park, S.W.; Kim, D.J.; Park, C.S.; Leilkauf, G.D.; Jang, A.S. Alteration in Claudin-4 Contributes to Airway Inflammation and Responsiveness in Asthma. Allergy Asthma Immunol. Res. 2018, 10, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Moon, K.Y.; Lee, P.H.; Kim, B.G.; Park, C.S.; Leikauf, G.D.; Jang, A.S. Claudin 5 in a murine model of allergic asthma: Its implication and response to steroid treatment. J. Allergy Clin. Immunol. 2015, 136, 1694–1696. [Google Scholar] [CrossRef]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Baek, A.R.; Park, J.S.; Lee, J.; Park, S.W.; Kim, D.J.; Park, C.S.; Jang, A.S. Impact of the Endothelial Tight Junction Protein Claudin-5 on Clinical Profiles of Patients With COPD. Allergy Asthma Immunol. Res. 2018, 10, 533–542. [Google Scholar] [CrossRef]
- Smallcombe, C.C.; Harford, T.J.; Linfield, D.T.; Lechuga, S.; Bokun, V.; Piedimonte, G.; Rezaee, F. Titanium dioxide nanoparticles exaggerate respiratory syncytial virus-induced airway epithelial barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L481–L496. [Google Scholar] [CrossRef]
- Tatsuta, M.; Kan-o, K.; Ishii, Y.; Yamamoto, N.; Ogawa, T.; Fukuyama, S.; Ogawa, A.; Fujita, A.; Nakanishi, Y.; Matsumoto, K. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: Protective role of cathelicidin LL-37. Respir. Res. 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Zeglinski, M.R.; Turner, C.T.; Zeng, R.; Schwartz, C.; Santacruz, S.; Pawluk, M.A.; Zhao, H.; Chan, A.W.H.; Carlsten, C.; Granville, D.J. Soluble Wood Smoke Extract Promotes Barrier Dysfunction in Alveolar Epithelial Cells through a MAPK Signaling Pathway. Sci. Rep. 2019, 9, 10027. [Google Scholar] [CrossRef]
- Roscioli, E.; Hamon, R.; Lester, S.E.; Jersmann, H.P.A.; Reynolds, P.N.; Hodge, S. Airway epithelial cells exposed to wildfire smoke extract exhibit dysregulated autophagy and barrier dysfunction consistent with COPD. Respir. Res. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Jang, A.S.; Concel, V.J.; Bein, K.; Brant, K.A.; Liu, S.; Pope-Varsalona, H.; Dopico, R.A., Jr.; Di, Y.P.; Knoell, D.L.; Barchowsky, A.; et al. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2011, 44, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Hong, J.; Jang, A.S. Claudins, VEGF, Nrf2, Keap1, and Nonspecific Airway Hyper-Reactivity Are Increased in Mice Co-Exposed to Allergen and Acrolein. Chem. Res. Toxicol. 2019, 32, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am. J. Respir. Cell Mol. Biol. 2018, 58, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Uh, S.T.; Koo, S.M.; Kim, Y.; Kim, K.; Park, S.; Jang, A.S.; Kim, D.; Kim, Y.H.; Park, C.S. The activation of NLRP3-inflammsome by stimulation of diesel exhaust particles in lung tissues from emphysema model and RAW 264.7 cell line. Korean J. Intern. Med. 2017, 32, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, J.M.; Park, S.W.; Kim, K.S.; Lee, M.W.; Paik, S.; Jang, A.S.; Kim, D.J.; Uh, S.; Kim, Y.; et al. Attenuation of Cigarette Smoke-Induced Emphysema in Mice by Apolipoprotein A-1 Overexpression. Am. J. Respir. Cell Mol. Biol. 2016, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Uh, S.T.; Koo, S.M.; Jang, A.S.; Park, S.W.; Choi, J.S.; Kim, Y.H.; Park, C.S. Proteomic differences with and without ozone-exposure in a smoking-induced emphysema lung model. Korean J. Intern. Med. 2015, 30, 62–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-G.; Lee, P.-H.; Choi, S.-M.; An, M.-H.; Jang, A.-S. Effects of Air Pollutants on Airway Diseases. Int. J. Environ. Res. Public Health 2021, 18, 9905. https://doi.org/10.3390/ijerph18189905
Lee Y-G, Lee P-H, Choi S-M, An M-H, Jang A-S. Effects of Air Pollutants on Airway Diseases. International Journal of Environmental Research and Public Health. 2021; 18(18):9905. https://doi.org/10.3390/ijerph18189905
Chicago/Turabian StyleLee, Yun-Gi, Pureun-Haneul Lee, Seon-Muk Choi, Min-Hyeok An, and An-Soo Jang. 2021. "Effects of Air Pollutants on Airway Diseases" International Journal of Environmental Research and Public Health 18, no. 18: 9905. https://doi.org/10.3390/ijerph18189905




