Effects of Six-Week Resistance Training with or without Vibration on Metabolic Markers of Bone Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Exercise Protocols
2.3. Biochemical Analyses
2.4. Statistical Analysis
3. Results
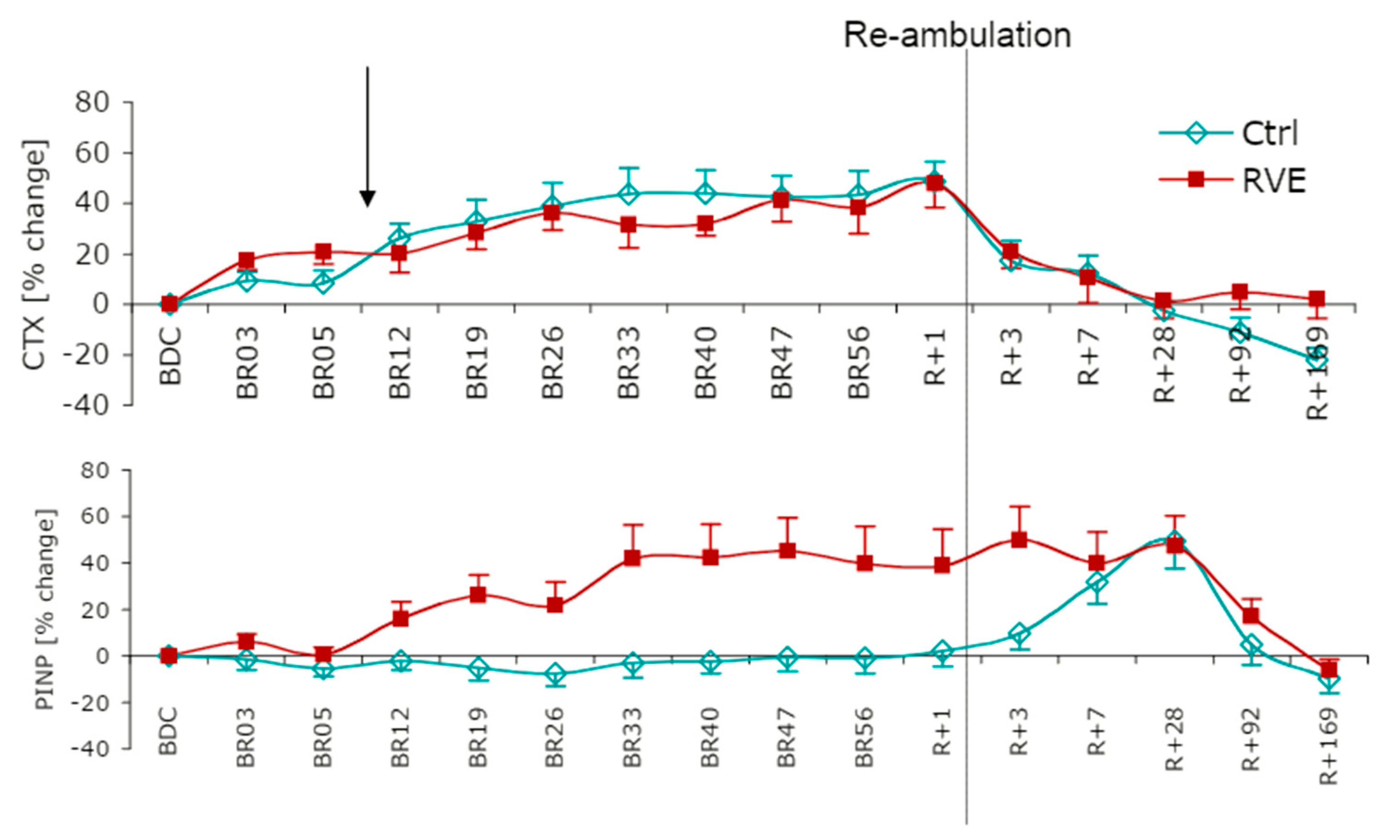
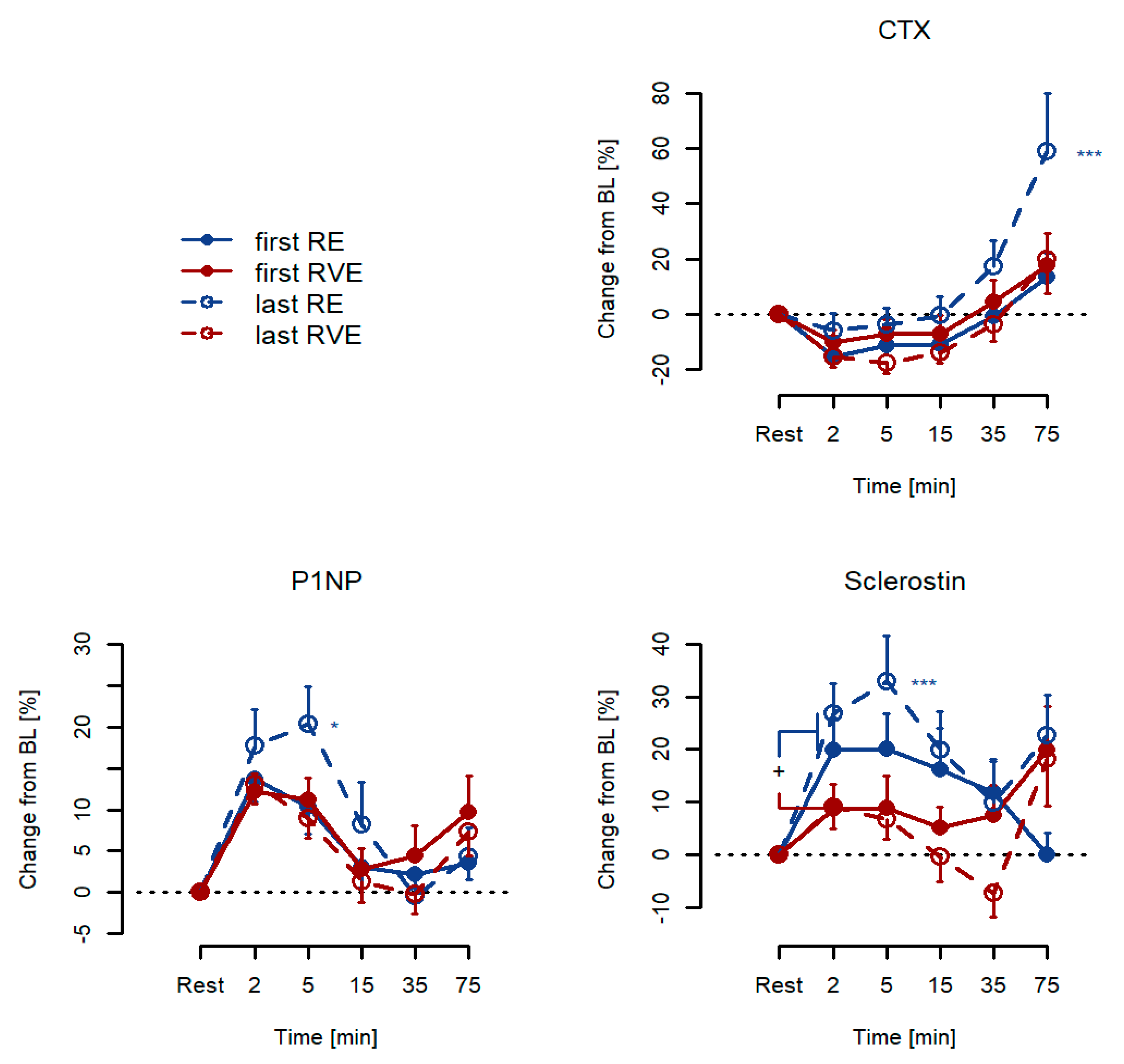
3.1. Chronic Responses: Resting Baseline Levels
3.2. Acute Responses: Percentage Changes from Resting Baseline Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rubin, C.T.; Lanyon, L.E. Kappa Delta Award Paper. Osteoregulatory Nature of Mechanical Stimuli: Function as a Determinant for Adaptive Remodeling in Bone. J. Orthop. Res. 1987, 5, 300–310. [Google Scholar] [CrossRef]
- Maïmoun, L.; Sultan, C. Effect of Physical Activity on Calcium Homeostasis and Calciotropic Hormones: A Review. Calcif. Tissue Int. 2009, 85, 277–286. [Google Scholar] [CrossRef]
- Shackelford, L.C.; LeBlanc, A.D.; Driscoll, T.B.; Evans, H.J.; Rianon, N.J.; Smith, S.M.; Spector, E.; Feeback, D.L.; Lai, D. Resistance Exercise as a Countermeasure to Disuse-Induced Bone Loss. J. Appl. Physiol. 2004, 97, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittweger, J.; Beller, G.; Armbrecht, G.; Mulder, E.; Buehring, B.; Gast, U.; Dimeo, F.; Schubert, H.; de Haan, A.; Stegeman, D.F.; et al. Prevention of Bone Loss during 56 Days of Strict Bed Rest by Side-Alternating Resistive Vibration Exercise. Bone 2010, 46, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.; Gollhofer, A.; Armbrecht, G.; Felsenberg, D.; Gruber, M. How to Prevent the Detrimental Effects of Two Months of Bed-Rest on Muscle, Bone and Cardiovascular System: An RCT. Sci. Rep. 2017, 7, 13177. [Google Scholar] [CrossRef] [PubMed]
- Pavy-Le Traon, A.; Heer, M.; Narici, M.V.; Rittweger, J.; Vernikos, J. From Space to Earth: Advances in Human Physiology from 20 Years of Bed Rest Studies (1986–2006). Eur. J. Appl. Physiol. 2007, 101, 143–194. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ohshima, H.; Mizuno, K.; Sekiguchi, C.; Fukunaga, M.; Kohri, K.; Rittweger, J.; Felsenberg, D.; Matsumoto, T.; Nakamura, T. Intravenous Pamidronate Prevents Femoral Bone Loss and Renal Stone Formation during 90-Day Bed Rest. J. Bone Miner. Res. 2004, 19, 1771–1778. [Google Scholar] [CrossRef]
- Armbrecht, G.; Belavý, D.L.; Gast, U.; Bongrazio, M.; Touby, F.; Beller, G.; Roth, H.J.; Perschel, F.H.; Rittweger, J.; Felsenberg, D. Resistive Vibration Exercise Attenuates Bone and Muscle Atrophy in 56 Days of Bed Rest: Biochemical Markers of Bone Metabolism. Osteoporos. Int. 2010, 21, 597–607. [Google Scholar] [CrossRef]
- Chapurlat, R.D.; Confavreux, C.B. Novel Biological Markers of Bone: From Bone Metabolism to Bone Physiology. Rheumatology 2016, 55, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Vasikaran, S.D.; Chubb, S.A. The Use of Biochemical Markers of Bone Turnover in the Clinical Management of Primary and Secondary Osteoporosis. Endocrine 2016, 52, 222–225. [Google Scholar] [CrossRef]
- Szulc, P.; Naylor, K.; Hoyle, N.R.; Eastell, R.; Leary, E.T. National Bone Health Alliance Bone Turnover Marker Project Use of CTX-I and PINP as Bone Turnover Markers: National Bone Health Alliance Recommendations to Standardize Sample Handling and Patient Preparation to Reduce Pre-Analytical Variability. Osteoporos. Int. 2017, 28, 2541–2556. [Google Scholar] [CrossRef]
- Bonewald, L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Dror, N.; Carbone, J.; Haddad, F.; Falk, B.; Klentrou, P.; Radom-Aizik, S. Sclerostin and bone turnover markers response to cycling and running at the same moderate-to-vigorous exercise intensity in healthy men. J. Endocrinol. Investig. 2021, 1–7. [Google Scholar] [CrossRef]
- Turner, C.H.; Warden, S.J.; Bellido, T.; Plotkin, L.I.; Kumar, N.; Jasiuk, I.; Danzig, J.; Robling, A.G. Mechanobiology of the Skeleton. Sci. Signal. 2009, 2, pt3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallas, S.L.; Bonewald, L.F. Dynamics of the Transition from Osteoblast to Osteocyte. Annu. N. Y. Acad. Sci. 2010, 1192, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Buchem, F.S.; Hadders, H.N.; Ubbens, R. An Uncommon Familial Systemic Disease of the Skeleton: Hyperostosis Corticalis Generalisata familiaris. Acta Radiol. 1955, 44, 109–120. [Google Scholar] [CrossRef]
- Sims, N.A.; Walsh, N.C. Intercellular Cross-Talk among Bone Cells: New Factors and Pathways. Curr. Osteoporos. Rep. 2012, 10, 109–117. [Google Scholar] [CrossRef]
- Kohrt, W.M. Osteoprotective Benefits of Exercise: More Pain, Less Gain? J. Am. Geriatr. Soc. 2001, 49, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Robling, A.G. Designing Exercise Regimens to Increase Bone Strength. Exerc. Sport Sci. Rev. 2003, 31, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Pavalko, F.M. Mechanotransduction and Functional Response of the Skeleton to Physical Stress: The Mechanisms and Mechanics of Bone Adaptation. J. Orthop. Sci. 1998, 3, 346–355. [Google Scholar] [CrossRef]
- Turner, C.H. Three Rules for Bone Adaptation to Mechanical Stimuli. Bone 1998, 23, 399–407. [Google Scholar] [CrossRef]
- Bemben, D.A.; Sharma-Ghimire, P.; Chen, Z.; Kim, E.; Kim, D.; Bemben, M.G. Effects of Whole-Body Vibration on Acute Bone Turnover Marker Responses to Resistance Exercise in Young Men. J. Musculoskelet. Neuronal Interact. 2015, 15, 23–31. [Google Scholar] [PubMed]
- Belavý, D.L.; Beller, G.; Armbrecht, G.; Perschel, F.H.; Fitzner, R.; Bock, O.; Börst, H.; Degner, C.; Gast, U.; Felsenberg, D. Evidence for an Additional Effect of Whole-Body Vibration Above Resistive Exercise Alone in Preventing Bone Loss during Prolonged Bed Rest. Osteoporos. Int. 2011, 22, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Beijer, Å.; Rosenberger, A.; Weber, T.; Zange, J.; May, F.; Schoenau, E.; Mester, J.; Bloch, W.; Rittweger, J. Randomized Controlled Study on Resistive Vibration Exercise (EVE Study): Protocol, Implementation and Feasibility. J. Musculoskelet. Neuronal Interact. 2013, 13, 147–156. [Google Scholar] [PubMed]
- Rosenberger, A.; Beijer, Å.; Johannes, B.; Schoenau, E.; Mester, J.; Rittweger, J.; Zange, J. Changes in muscle cross-sectional area, muscle force, and jump performance during 6 weeks of progressive whole-body vibration combined with progressive, high intensity resistance training. J. Musculoskelet. Neuronal Interact. 2017, 17, 38–49. [Google Scholar] [PubMed]
- Weber, T.; Beijer, Å.; Rosenberger, A.; Mulder, E.; Yang, P.; Schonau, E.; Bloch, W.; Rittweger, J. Vascular adaptations induced by 6 weeks WBV resistance exercise training. Clin. Physiol. Funct. Imaging 2013, 33, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Beijer, Å.; Rosenberger, A.; Bolck, B.; Suhr, F.; Rittweger, J.; Bloch, W. Whole-body vibrations do not elevate the angiogenic stimulus when applied during resistance exercise. PLoS ONE 2013, 8, e80143. [Google Scholar] [CrossRef]
- Beijer, Å.; Degens, H.; Weber, T.; Rosenberger, A.; Gehlert, S.; Herrera, F.; Kohl-Bareis, M.; Zange, J.; Bloch, W.; Rittweger, J. Microcirculation of skeletal muscle adapts differently to a resistive exercise intervention with and without superimposed whole-body vibrations. Clin. Physiol. Funct. Imaging 2015, 35, 425–435. [Google Scholar] [CrossRef]
- Baechle, T.R.; Earle, R.W.; Wathen, D. Essentials of Strength Training and Conditioning. Resistance Training; Human Kinetics Publishers: Champaign, IL, USA, 2000; pp. 395–425. [Google Scholar]
- Crawley, M.J. The R Book; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2007; 942p. [Google Scholar]
- Seeman, E. Bone Modeling and Remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 219–233. [Google Scholar] [CrossRef]
- Khosla, S. Pathogenesis of Age-Related Bone Loss in Humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1226–1235. [Google Scholar] [CrossRef] [Green Version]
- Sims, N.A.; Gooi, J.H. Bone Remodeling: Multiple Cellular Interactions Required for Coupling of Bone Formation and Resorption. Semin. Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Lanteri, P.; Colombini, A.; Mariotti, M.; Banfi, G. Sclerostin Concentrations in Athletes: Role of Load and Gender. J. Biol. Regul. Homeost. Agents 2012, 26, 157–163. [Google Scholar] [PubMed]
- Hinton, P.S.; Nigh, P.; Thyfault, J. Serum Sclerostin Decreases Following 12 Months of Resistance- or Jump-Training in Men with Low Bone Mass. Bone 2017, 96, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickering, M.E.; Simon, M.; Sornay-Rendu, E.; Chikh, K.; Carlier, M.C.; Raby, A.L.; Szulc, P.; Confavreux, C.B. Serum Sclerostin Increases After Acute Physical Activity. Calcif. Tissue Int. 2017, 101, 170–173. [Google Scholar] [CrossRef]
- Kouvelioti, R.; LeBlanc, P.; Falk, B.; Ward, W.E.; Josse, A.R.; Klentrou, P. Effects of High-Intensity Interval Running Versus Cycling on Sclerostin, and Markers of Bone Turnover and Oxidative Stress in Young Men. Calcif. Tissue Int. 2019, 104, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Fairfield, H.; Rosen, C.J.; Reagan, M.R. Connecting Bone and Fat: The Potential Role for Sclerostin. Curr. Mol. Biol. Rep. 2017, 3, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, W.M.; Wherry, S.J.; Wolfe, P.; Sherk, V.D.; Wellington, T.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Maintenance of Serum Ionized Calcium during Exercise Attenuates Parathyroid Hormone and Bone Resorption Responses. J. Bone Miner. Res. 2018, 33, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
| Variable | RE Group (n = 13) | RVE Group (n = 13) | p-Value |
|---|---|---|---|
| Age, years | 23.4 (±1.4) | 24.3 (±3.3) | 0.518 |
| Body mass, kg | 75.0 (±4.7) | 74.7 (±6.9) | 0.081 |
| Height, m | 1.79 (±0.05) | 1.8 (±0.1) | 0.309 |
| BMI, kg/m2 | 23.4 (±1.4) | 23.5 (±2.1) | 0.113 |
| CMJ height, cm | 42.2 (±4.6) | 41.7 (±2.2) | 0.968 |
| First RE | First RVE | Last RE | Last RVE | p-Value [Group] | p-Value [Session] | p-Value [Group × Session] | |
|---|---|---|---|---|---|---|---|
| CTX, ng/L | 333.7 (±126.3) | 332.6 (±192.9) | 320.3 (±172.1) | 368.1 (±144.0) | 0.72 | 0.52 | 0.20 |
| P1NP, µg/L | 67.4 (±23.3) | 68.2 (±24.3) | 73.8 (±23.7) | 71.0 (±18.9) | 0.95 | 0.10 | 0.62 |
| Sclerostin, ng/L | 479.2 (±116.0) | 400.0 (±132.8) | 424.2 * (±104.2) | 396.4 * (±147.5) | 0.14 | 0.039 * | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, P.; Beijer, Å.; Rosenberger, A.; Schoenau, E.; Clemen, C.S.; Zange, J.; Rittweger, J. Effects of Six-Week Resistance Training with or without Vibration on Metabolic Markers of Bone Metabolism. Int. J. Environ. Res. Public Health 2021, 18, 9860. https://doi.org/10.3390/ijerph18189860
Lau P, Beijer Å, Rosenberger A, Schoenau E, Clemen CS, Zange J, Rittweger J. Effects of Six-Week Resistance Training with or without Vibration on Metabolic Markers of Bone Metabolism. International Journal of Environmental Research and Public Health. 2021; 18(18):9860. https://doi.org/10.3390/ijerph18189860
Chicago/Turabian StyleLau, Patrick, Åsa Beijer, André Rosenberger, Eckhard Schoenau, Christoph Stephan Clemen, Jochen Zange, and Jörn Rittweger. 2021. "Effects of Six-Week Resistance Training with or without Vibration on Metabolic Markers of Bone Metabolism" International Journal of Environmental Research and Public Health 18, no. 18: 9860. https://doi.org/10.3390/ijerph18189860
APA StyleLau, P., Beijer, Å., Rosenberger, A., Schoenau, E., Clemen, C. S., Zange, J., & Rittweger, J. (2021). Effects of Six-Week Resistance Training with or without Vibration on Metabolic Markers of Bone Metabolism. International Journal of Environmental Research and Public Health, 18(18), 9860. https://doi.org/10.3390/ijerph18189860







